Abstract
The dopamine transporter (DAT) and the enzyme catechol-O-methyltransferase (COMT) both terminate synaptic dopamine action. Here, we investigated the influence of two polymorphisms in the respective genes: DAT1 (SLC6A3) VNTR and COMT val158met (rs4680). Startle magnitudes to intense noise bursts as measured with the eye blink response were recorded during the presentation of pictures of three valence conditions (unpleasant, pleasant and neutral) and during baseline without additional pictorial stimulation in a sample of healthy older adults (N = 94). There was a significant Bonferroni corrected main effect of COMT genotype on the overall startle responses, with met/met homozygotes showing the highest and participants with the val/val genotype showing the lowest startle response, while participants with the val/met genotype displayed intermediate reactions. There was also a DAT1 VNTR main effect, which, after Bonferroni correction, still showed a tendency toward significance with carriers of at least one 9-repeat (R) allele showing smaller overall startle responses compared to 10R/10R homozygotes. Thus, older adult carriers of COMT variants, which result in lower enzyme activity and therefore probably enhanced dopamine signaling, showed stronger startle activity. Although the functional significance of DAT1 VNTR is less defined, our results point to a potential influence of SLC6A3 on startle magnitude.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Differences in emotional regulation are the result of complex gene–gene and gene–environment interactions (McClearn 2006). In addition, developmental as well as pathological changes across the life span impact the processing of emotional material (Diamond 2007; Lachman 2004). Since dopamine is a key regulator of several cognitive and affective processes (Nieoullon and Coquerel 2003), genetic variation that affects dopaminergic neurotransmission was examined in association studies linking polymorphisms to differences in emotional regulation and neuropsychiatric outcomes. Among the candidates for these studies are the catabolic enzyme catechol-O-methyltransferase (COMT) and the dopamine transporter (DAT) that both terminate synaptic dopamine action.
Catechol-O-methyltransferase facilitates metabolic degradation of released dopamine (Chen et al. 2004; Weinshilboum et al. 1999) and exists in two isoforms resulting from different translation start sites: soluble S-COMT, predominantly expressed in tissues such as liver, blood and kidney, and membrane-bound MB-COMT, mainly expressed in the brain, particularly in the prefrontal cortex (PFC) (Chen et al. 2004; Tenhunen et al. 1993; Karoum et al. 1994; Matsumoto et al. 2003). The gene encoding for COMT is located on chromosome 22q11 and contains several single nucleotide polymorphisms (SNPs). Among those is a G/A substitution (rs4680) at codon 158 in MB-COMT (codon 108 in S-COMT), resulting in the substitution of valine (val) to methionine (met). The val158met SNP influences the thermal stability and activity of COMT, which was reported to be 35–50% lower in the prefrontal cortex at 37°C in postmortem human tissue in met/met compared to val/val homozygotes (Chen et al. 2004), resulting probably in an enhanced dopamine signaling in met allele carriers (Tunbridge et al. 2004) and might therefore impact on cortical function.
The COMT val allele has been associated with deficient prefrontal activation during tasks measuring cognitive control and working memory in several fMRI and EEG studies (Blasi et al. 2005; Egan et al. 2001; Winterer et al. 2006), as well as with poorer performance on prefrontally mediated tasks (Joober et al. 2002; Malhotra et al. 2002; Barnett et al. 2007; Goldberg et al. 2003), although the val allele has also been suggested to be beneficial for tasks that demand cognitive flexibility (Bilder et al. 2004). Furthermore, some association studies found a greater risk for schizophrenia in val allele carriers, especially in the presence of other schizophrenia risk genes (Tunbridge et al. 2006; Nicodemus et al. 2007), although results were inconsistent (Munafo et al. 2005). In addition, investigations of antisaccadic eye movements as a marker for schizophrenia have yielded inconclusive results (Stefanis et al. 2004; Ettinger et al. 2008; Haraldsson et al. 2010). In contrast, the COMT met allele has been associated—although inconsistently—with self-report measures of negative emotionality including increased scores of neuroticism and reduced scores of extraversion (Reuter and Hennig 2005; Stein et al. 2005) and sensation seeking (Lang et al. 2007). COMT met allele carriers also have been found to be more prone to develop anxiety disorders (Enoch et al. 2003; Olsson et al. 2005; Woo et al. 2004). These findings are supported by neurophysiological studies with the met allele being associated with increased limbic and prefrontal activation in response to emotionally negative pictures in fMRI studies (Smolka et al. 2005; Drabant et al. 2006). Furthermore, these regions showed increased functional coupling in met/met homozygotes, with the magnitude of amygdala–orbitofrontal coupling being inversely correlated with novelty seeking (Drabant et al. 2006). Smolka et al. (2007) found additive and combined effects of COMT val158met and a polymorphism in the promoter region of the serotonin transporter gene (5-HTTLPR) on the processing of aversive stimuli, accounting together for 40% of the interindividual variance in the fMRI BOLD response. The findings of a recent meta-analysis on neuroimaging studies further underscore the significance of COMT val158met for emotional and executive cognitive paradigms (Mier et al. 2010).
In addition to fMRI data, COMT has been associated with certain features of startle response. Roussos et al. (2008) investigated the impact of COMT on prepulse inhibition (PPI) in a male student sample and found the highest PPI levels in met/met individuals, while val/val homozygotes had the smallest values and val/met showed intermediate levels. This finding was partly replicated by Quednow et al. (2009), again, in the male, but not in the female subsample of healthy adults: male met/met homozygotes displayed the highest PPI levels, although in this sample there was no difference between val/met and val/val genotypes. Furthermore, COMT has been shown to affect the emotional modulation of startle response at least in one study (Montag et al. 2008), although another study reported no influence of COMT on startle modulation or baseline startle (Pauli et al. 2010).
DAT facilitates dopamine reuptake into the presynaptic terminal and is a major target for various psychostimulants such as amphetamine and cocaine. The human gene coding for DAT (DAT1 or SLC6A3) is located on chromosome 5p15.3 and contains in its 3′-untranslated region (3′-UTR) a 40 bp variable number of tandem repeats (VNTR) polymorphism. The DAT1 9-repeat (9R) and 10R alleles are the most common variants, although 3–13 repeats have also been reported (Mignone et al. 2002; Dreher et al. 2009). DAT1 is mainly expressed in the striatum, midbrain and hippocampus, and to a much lesser extent in the prefrontal cortex (Diamond 2007; Dreher et al. 2009; Sesack et al. 1998). Concerning the functional significance of DAT1 VNTR, results are inconclusive. Fuke et al. (2001) reported enhanced gene expression in the presence of the 10R allele compared to the 7R or 9R allele. Consistently, Mill et al. (2002) found significantly higher DAT1 mRNA levels for the 10R compared to the 9R allele. However, the 9R allele has also been associated with higher levels of DAT1 expression in some studies (Miller and Madras 2002; Michelhaugh et al. 2001). In addition, results from human neuroimaging studies also reported either elevated (Jacobsen et al. 2000; van Dyck et al. 2005) or reduced (Heinz et al. 2000) striatal dopamine transporter binding for 9R, while other studies did not observe differences between 9R and 10R (Martinez et al. 2001; Lynch et al. 2003). Thus, it remains unclear whether the DAT1 9R or the 10R allele leads to a more active dopamine transporter. In association studies, the 10R allele has been linked to increased impulsivity and reduced inhibition (Congdon et al. 2009). In addition, a small but significant relationship with attention deficit hyperactivity disorder (ADHD) has been reported (Yang et al. 2007; Faraone et al. 2005). Recently, Hünnerkopf et al. (2007) reported a gene × gene interaction on personality traits: carriers of the DAT1 9R allele and the met allele of the val66met polymorphism of the gene encoding the brain-derived neurotrophic factor (BDNF) showed significantly lower scores in neuroticism and harm avoidance than non-carriers. In summary, COMT and DAT1 both have been suggested to affect dopaminergic neurotransmission and have been associated with differences in emotional regulation. However, to further understand the respective underlying pathways, the investigation of diverging endophenotypes of affective processes is warranted. Here, we report further evidence of the influence of COMT and DAT1 on the acoustic startle response (ASR) in a sample of older adults.
The ASR is evoked by sudden high-intensity noise bursts. The startle reflex is probably a protective mechanism involving contractions of facial, neck and skeletal muscles, eyelid closure and preparation of a flight/fight response (Davis et al. 1993; Koch 1999). The eyeblink component of the ASR can be measured by electromyographic (EMG) recordings from the orbicularis oculi muscle. The startle response can be further modulated by presenting the startling noise in the presence of affective stimuli: startle response is potentiated in the presence of unpleasant stimuli (fear-potentiated startle, FPS) and inhibited during the presentation of pleasant stimuli (pleasure-attenuated startle, PAS) (Lang et al. 1990; Vrana et al. 1988). While the startle modulation has thus been used as a measure of emotional processing in numerous studies, it has also been suggested that the startle stimulus itself is sufficient to induce a state of fear (Leaton and Cranney 1990), since intense noise has an aversive quality (Blumenthal et al. 2005). Moreover, the overall startle magnitude was found to be highly heritable (59–61%) in a twin study by Anokhin et al. (2007), while there was no evidence of heritability regarding the emotional startle modulation, suggesting that functional genetic variation might manifest its influence on the overall or baseline startle.
In addition to genetic variation, developmental and aging effects on emotionality, in general, and startle response, in particular, shall be outlined briefly. Several studies reported preferences for emotionally positive stimuli and/or a disengagement from negative material in older adults, which has been termed positivity effect (Mather and Carstensen 2003; Isaacowitz et al. 2006; Carstensen and Mikels 2005; Mikels et al. 2005; Murphy and Isaacowitz 2008). Nevertheless, a recent meta-analysis by Ruffman et al. (2008) found an age-related decline in emotional processing across all emotions and modalities and a general trend toward worsening of emotion recognition with age, which was not consistent with the positivity effect. Regarding startle reflex, older subjects were reported to be less responsive to startling noises (Ford et al. 1995), and considerably decreased ASR and increased latency were found in older individuals, while there was an inverted U-shaped function for PPI with the greatest levels at intermediate ages and no age effect on startle habituation (Ellwanger et al. 2003). However, while Ludewig et al. (2003) reported significantly lower startle magnitudes, they also found significantly more habituation in older individuals but no age effect on PPI. Thus, there is consistency regarding ASR reduction in older individuals, which is further supported by animal studies (Varty et al. 1998), but the effects of age on other features of startle response are less clear. Nevertheless, in summary, the available data suggest substantial differences in the processing of emotional information in older compared to younger adults. With regard to dopaminergic function, normal aging is marked by significant losses in pre- and postsynaptic biochemical markers of dopamine (for an overview see Rollo 2009; Bäckman et al. 2006). However, aging adults have been found to be remarkably heterogeneous regarding abilities under dopaminergic influence, such as working memory or executive functions (Bäckman et al. 2011). Thus, the influence of dopaminergic genetic variation on cognitive and affective processes has been suggested to be more pronounced in older compared to younger adults (Nagel et al. 2008).
Based on the considerations outlined above, we hypothesized that dopaminergic variation should impact on the overall startle, particularly in older adults (Nagel et al. 2008). Regarding COMT, we expected a stronger startle response in individuals with the met allele in a dosage-dependent manner, i.e., met/met > val/met > val/val. Since the functional significance of the DAT1 9R and 10R allele was less clear, we hypothesized different overall startle responses in carriers of the 9R allele compared to 10R/10R homozygotes.
Methods
Participants
All of our participants were of German/Middle European ancestry and originally consisted of 62 female and 40 male older adults. Of these, two participants did not complete the startle experiment and the data of two participants were lost due to technical problems. From the remaining sample, 94 participants were successfully genotyped for the COMT val158met SNP, leaving 58 female and 36 male older participants for the final COMT sample (mean age 61.13 years, SD = 2.57, range 54–68 years), and 92 participants were successfully genotyped for the DAT1 VNTR polymorphism, leaving 57 female and 35 male participants for the final DAT sample (mean age 61.11 years, SD = 2.53, range 54–68 years), respectively. All participants were non-smokers and reported to be in good health. They were screened for psychiatric or neurological disorders or treatment before participation. In addition, a semistructured interview was conducted to assess critical life events (data not reported here). As part of this interview, medical problems including psychiatric conditions were determined and participants who reported a history of mental illness were excluded. Participants were informed about the aims of the study before taking part. They gave written informed consent and were paid for participation. The study design was approved by the Ethics Committee of the German Psychological Association.
Materials and design
Acoustic startle probes were presented alone and during viewing of emotional pictures. The startle stimulus consisted of a single 50-ms burst of white noise (95 dB SPL with an instantaneous rise time) and was presented binaurally over Eartone A3 Audiometric Insert Earphones (Aearo Company, Indianapolis, IN, USA). Images used and modes of presentation are described in detail elsewhere (Armbruster et al. 2009). Briefly, 40 affective color pictures (16 unpleasant, 12 neutral and 12 pleasant) were selected from the International Affective Picture System (IAPS) (Lang et al. 1999), and eight additional unpleasant black and white pictures of angry or fearful faces were chosen from the Ekman series of facial affect (Ekman and Friesen 1976). Each picture was presented for 6 s and the images were grouped in four blocks of 12. For half of the participants, the order of the four blocks was reversed. During picture viewing, an acoustic startle probe was administered at 0.5, 2.5 or 4.5 s after picture onset. The timing of the startle probes was balanced across valence categories. A total of 12 images were presented without a startle probe and used as filler stimulus. Pictures were organized such that not more than two pictures of the same affective valence and not more than two pictures with the same startle onset time could occur consecutively. Otherwise, stimulus order was pseudo-randomized. Finally, 12 acoustic startle probes were delivered in the intertrial interval (ITI) to measure the baseline startle response.
Physiological data collection and reduction
The eyeblink component of startle response was measured by recording EMG activity over the orbicularis oculi muscle beneath the left eye, using two Ag–AgCl electrodes with 4-mm inner diameter. A ground electrode was attached to the left mastoid. Impedance level was kept below 10 kΩ. The raw EMG signal was amplified by a SynAmps amplifier (NeuroScan Inc., El Paso, TX, USA), sampled at 1,000 Hz, filtered (30–200 Hz band pass), rectified and integrated. Responses to startle probes were defined as EMG peak in a time window from 20 to 140 ms after probe presentation. Trials with excessive EMG artifacts were excluded.
Affective rating
Evaluative judgments of pleasure and arousal were measured using the Self-Assessment Manikin (SAM) (Lang 1980). The SAM valence scale shows a graphic figure with expressions ranging from happy to unhappy, and the SAM arousal scale displays a graphical representation of a figure with expressions ranging from calm and relaxed to excited. Ratings of valence and arousal were made on nine-point scales.
Procedure
After a telephone interview on basic inclusion criteria (e.g., age, health, or medication), participants were scheduled for a laboratory session. Upon arrival, they were given an overview of the study goals and protocol. All participants were instructed that they could decline to participate at any time. Then sensors were attached and the participants were instructed that a series of affective pictures would be presented and that each picture should be viewed for the entire presentation time, while occasional noises heard over earphones could be ignored. A total of 48 pictures were presented, separated by randomly generated variable intertrial intervals (ITIs) ranging from 11 to 24 s. During ITIs, a fixation cross was displayed. After the picture series was completed, participants were instructed on how to rate the images on valence and arousal using the computerized version of the Self-Assessment Manikin (SAM) rating method (Lang 1980). All images were presented in the same order a second time and subjective experience ratings were obtained. At a separate appointment, participants provided saliva, buccal cell or blood samples for later DNA extraction and genotyping. Participants were subsequently debriefed, paid for participation and thanked.
Genotyping
For genotyping, DNA was isolated either from EDTA blood samples, saliva samples using the Oragene DNA Extraction kits, or buccal cells using the BuccalAmp DNA Extraction kits and protocol. For COMT val158met, the polymorphic region was amplified by polymerase chain reactions (PCR) carried out in 25 μl volumes containing 30 ng genomic DNA, 0.4 mM primers, 50 mM KCl, 10 mM Tris/HCl (pH 8.3), 0.025% Tween 20, 0.025 mg/ml BSA, 1.5 mM magnesium chloride, 0.4 mM dNTP and 1U Taq polymerase with the following oligonucleotide primers: forward-5′ GGGGCCTACTGTGGCTACTC; reverse-5′ TTTTTCCAGGTCTGACAACG. After an initial denaturation for 5 min at 94°C, followed by 38 cycles of denaturing at 94°C for 45 s, annealing at 58.4°C for 45 s and extension of 72°C for 45 s were performed, followed by a final extension step of 72°C for 5 min. PCR products were digested with NlaIII. The undigested PCR product (114 bp) carried the G variant (val) while the digested product with two fragments of 96 and 13 bp contained the A allele (met).
For DAT VNTR, PCRs were carried out in 25 μl volumes containing 30 ng genomic DNA, 0.4 mM primers, 50 mM KCl, 10 mM Tris/HCl (pH 8.3), 0.025% Tween 20, 0.025 mg/ml BSA, 1.5 mM magnesium chloride, 0.4 mM dNTP and 1U Taq polymerase with the following primers: forward-5′ TGTGGTGTAGGGAACGGCCTGAG; reverse-5′ CTTCCTGGAGGTCACGGCTCAAGG. PCR conditions were as follows: initial denaturation for 3 min at 95°C, 38 cycles of 45 s at 95°C, 45 s at 67.5°C, 45 s at 72°C, and final extension at 72°C for 3 min. The resulting products for COMT and DAT1 were separated on agarose gels containing ethidium bromide and bands were visualized under UV light.
Statistical analysis
All analyses were performed using SPSS for Windows 16.0 (SPSS Inc., Chicago, IL, USA). The 48 startle variables (36 for emotionally modulated startle and 12 for baseline startle condition) were log-transformed because of the highly skewed distribution of the raw startle variables and the resulting deviation from the normal distribution (Kolmogorov–Smirnov tests, P < 0.20). The average startle magnitudes in the four conditions (baseline, unpleasant, neutral, pleasant) were computed, tested for univariate normality (Kolmogorov–Smirnov-tests, P ≥ 0.424) and then entered into two repeated measures analyses of variance (ANOVA) with condition as a within-subject factor, COMT and DAT genotype as between-subject factors and sex as covariate. Logarithmized startle magnitudes in the four conditions were highly intercorrelated (all r ≥ 0.946; all P ≤ 0.01). Thus, a full Bonferroni correction to account for multiple testing since four conditions were investigated would have been too conservative. Therefore, the equivalent numbers of independent variables were calculated based on their intercorrelation (Li and Ji 2005; Nyholt 2004) using the matSpD program (http://gump.qimr.edu.au/general/daleN/matSpD/). The significance threshold required to keep the type I error rate at 5% was estimated to be 0.0404. In addition, since we investigated two (independent) polymorphisms, alpha was further adjusted to 0.0202 (Bonferroni correction: 0.0404/2). Greenhouse–Geisser corrected degrees of freedom were used where appropriate.
Results
Genotype frequencies
The genotype frequencies of the COMT val158met were 34.0% (n = 32) for val/val, 44.7% (n = 42) for val/met and 21.3% (n = 20) for met/met. The percentages of the DAT1 VNTR genotypes were 55.4% (n = 51) for 10R/10R, 38.0% (n = 35) for 9R/10R and 6.5% (n = 6) for 9R/9R. While at least some studies have reported differential DAT1 expression in the 10R and 9R allele carriers (Fuke et al. 2001; Mill et al. 2002), the overall results remain inconclusive. Nevertheless, carriers of 9R allele (9R/9R and 9R/10R) were grouped together (9+ group) and compared to 10R/10R homozygotes (9− group) to divide participants into approximately equal-sized subsamples, with the 9+ group consisting of 41 subjects and the 9− group consisting of 51. Age and sex did not differ by COMT val158met or DAT1 VNTR genotype (age: ANOVAs, P ≥ 0.300; sex: χ2 tests, P ≥ 0.195). The genotypes were in Hardy–Weinberg equilibrium (COMT: χ2 = 0.78, P = 0.374; DAT1: χ2 = 2.22, P = 0.998).
Affective picture ratings
As some of the valence and arousal ratings were not normally distributed (Kolmogorov–Smirnov-tests, P < 0.20), the medians of the valence and arousal ratings for the different picture categories were compared using the nonparametric Wilcoxon tests for paired samples. The median valence ratings for unpleasant, neutral and pleasant pictures were 2.42, 5.29 and 6.11, respectively, and the median arousal ratings were 3.55, 1.58 and 2.81, respectively. All two-way comparisons for valence and arousal were highly significant (all P ≤ 0.001).
COMT val158met genotype groups did not differ in valence ratings of negative and neutral pictures and arousal ratings of any picture category (nonparametric Mann–Whitney tests, all P ≥ 0.210). However, there was an effect of COMT on the valence ratings of positive emotional pictures that showed a tendency toward significance (P = 0.098): COMT val/val homozygotes rated positive pictures as slightly more pleasant. DAT1 VNTR genotype groups did not differ in valence and arousal ratings of any emotional image category (all P ≥ 0.201).
Acoustic startle
Analysis of variance showed a significant main effect of valence condition on the startle magnitude (F 2.7,245.6 = 13.38, P < 0.001, η2 = 0.13). Within-subject contrast analyses revealed that the presentation of pleasant pictures resulted in significant pleasure attenuation of the startle (PAS; pleasant vs. neutral condition: F 1,90 = 27.34, P < 0.001, η2 = 0.233). However, there was no significant fear potentiation (FPS; negative vs. neutral condition: P = 0.112). Contrary to our expectations, the startle magnitudes in the baseline condition was slightly higher than in the neutral condition (P = 0.005) and in the negative condition (P = 0.064). However, this was mainly due to the male participants, since there was a significant sex × condition interaction (P = 0.014) with only the male subsample showing a baseline startle that was higher than the startle response in the neutral and negative condition. However, in males, these effects did not reach significance due to sample size (N = 36; P = 0.068 and P = 0.107, respectively).
Impact of COMT genotype on the startle response
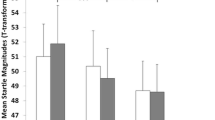
There was a significant COMT val158met genotype effect on average startle magnitudes across conditions (F 1,90 = 4.22, P = 0.018, η2 = 0.086), with met/met carriers showing the highest and participants with the val/val genotype showing the lowest startle response, while participants with the val/met genotype exhibited intermediate reactions. This effect remained significant after Bonferroni correction. There was no effect of COMT genotype on the emotional modulation of the startle reflex as indicated by the absence of a genotype × condition interaction effect (P = 0.29). Table 1 presents the mean startle magnitudes and standard errors of means for the four conditions and Fig. 1 shows the COMT genotype group differences.
In a further step, habituation of startle response was analyzed by investigating the startle magnitude over the four experimental blocks. There was a main effect of block (F 2.1,187.2 = 19.17, P < 0.001, η2 = 0.176) showing substantial habituation of the startle magnitude over the course of the experiment. However, there was no influence of COMT genotype on habituation, since there was no significant block × genotype interaction effect (P = 0.22).
Impact of DAT1 VNTR genotype on the startle response
DAT1 VNTR had a significant main effect on average startle magnitudes across conditions (F1,89 = 4.94, P = 0.029, η2 = 0.053) with participants with at least one 9R allele showing smaller overall startle magnitudes than 10R/10R homozygotes. After correction for multiple testing, this effect was still marginally significant. There was no COMT × DAT1 interaction effect on the average startle magnitude (P = 0.683). Concerning emotional modulation of startle response, there was a DAT1 genotype × condition interaction effect that showed a tendency toward significance (P = 0.090). However, this effect did not survive Bonferroni correction. Similarly to COMT, there was no effect of DAT1 VNTR on the habituation of the startle reflex, since we found no genotype × block interaction effect (P = 0.19). Table 2 presents the mean startle magnitudes and standard errors of means for the four conditions. Figure 2 illustrates the DAT1 VNTR genotype differences.
Discussion
We found that differences in the functioning of the dopamine catabolic enzyme COMT and in dopamine transporter function influence the overall startle magnitude in all three valence conditions, as well as baseline startle, in a sample of older adults. Concerning the COMT val158met polymorphism, participants with the low expressing met/met genotype exhibited the strongest startle responses, while val/val homozygotes showed the smallest response and val/met heterozygotes had intermediate reactions. With regard to the dopamine transporter, participants with at least one copy of the 9R allele of the DAT1 VNTR polymorphism showed smaller startle responses than 10R/10R homozygotes. After Bonferroni correction, this effect still had tendency toward significance. Both genetic effects had considerable effect sizes (η2 = 0.086 and η2 = 0.053, respectively). There was no evidence that any of the genetic groups showed differences in startle enhancement during the presentation of unpleasant pictures (FPS) or in startle attenuation during the presentation of pleasant pictures (PAS). While FPS has been used as a measure of fear and anxiety processing in numerous studies, it has also been suggested that the startle stimulus itself is sufficient to induce a state of fear (Leaton and Cranney 1990), since intense noise has an aversive quality (Blumenthal et al. 2005). In addition, there was no influence of COMT or DAT1 on the startle habituation. Our results are in line with results of a recent twin study (Anokhin et al. 2007) that found that the absolute startle magnitude showed high heritability (59–61%), while there was no evidence of genetic influences on the startle modulation across emotional valence conditions. Consistently, recent results from our laboratory also point to the genetic effects of polymorphism in the transcriptional control region of the serotonin transporter gene on the baseline startle, but not on emotional startle modulation (Armbruster et al. 2009; Brocke et al. 2006).
Overall, the investigation of the association of COMT and startle response has so far yielded mixed results. Findings from animal studies suggest similar effects on the ASR as in our human sample: COMT knockout mice, which represent an extreme version of the less functional met allele, showed increased ASR compared to normal wild types, while transgenic COMT val mice, showing a comparable functionality to the human val allele, displayed decreased ASR (Papaleo et al. 2008). In humans, contrary to our findings, COMT has been found to influence affective startle modulation in a young female sample (Montag et al. 2008), with met/met homozygotes showing stronger FPS compared to carriers of the val allele. While we found no effect of COMT genotype on FPS but on the overall startle, the pattern was nevertheless similar: met/met homozygotes showed the strongest and val/val homozygotes the smallest startle response, with val/met genotypes exhibiting intermediate reactions. However, Pauli et al. (2010) found no effect of COMT on the ASR or on the emotional startle modulation. Additionally, COMT was also reported to impact PPI with male val/val homozygotes having significantly lower PPI levels compared to met allele carriers (Roussos et al. 2008; Quednow et al. 2009). Incidentally, in the study by Roussos et al. (2008), at least at the descriptive level, val/val homozygotes also had smaller overall startle magnitudes than val/met heterozygotes, while met/met homozygotes showed the highest scores although this effect fell short of reaching significance. It must be noted that there are considerable methodological differences between the studies cited above that may account for the different results. We investigated the effects of COMT in a sample of older adults (mean age 61.11 ± 2.53 years), while the sample of Montag et al. (2008) consisted of young women (mean age 22.11 ± 3.29 years). The sample of Pauli et al. (2010) comprised adults (mean age ≈35 ± 10.2 years), while Roussos et al. (2008) investigated young men (mean age 26.2 ± 4.0 years) and Quednow et al. (2009) investigated young adults (mean age 26.2 ± 5.8 years). In addition, the startle paradigms used were different (i.e., ASR vs. PPI; startle stimulus: db SPL, stimulus duration and onset, IAPS pictures).
In general, the COMT met allele has been associated with deficits in emotional regulation, especially anxiety-related traits and disorders (Woo et al. 2004; Olsson et al. 2005; Enoch et al. 2003; Stein et al. 2005). In neuroimaging studies, carriers of the met allele also showed increased prefrontal activation in emotional tasks (overview in Mier et al. 2010). This enhanced vulnerability to anxiety may be counterbalanced with better cognitive performance, while val allele carriers show modestly reduced executive cognitive performance under most conditions but appear to be more stress resilient (Goldman et al. 2005). Since both alleles have been conserved in the population and both have been associated with beneficial as well as with disadvantageous traits, a warrior/worrier model has been proposed (Goldman et al. 2005) suggesting a trade-off between specific advantages and costs. The results of our startle experiment point in the same direction: carriers of the COMT met allele (worriers) showed an allele dosage-dependent stronger overall startle response.
Concerning DAT1 VNTR, while we found a stronger overall startle response in 10R/10R homozygotes, an effect that after Bonferroni correction still showed a trend toward significance, at least one other study reported no effect of DAT1 genotype on the ASR or the affect-modulated startle (Pauli et al. 2010). Again, it has to be noted that there are methodological differences between the studies that may at least in part account for the divergent results. The 10R allele has been associated with increased impulsivity (Congdon et al. 2009) and ADHD, although there are also non-replications and contradictory results (Rommelse et al. 2008; Yang et al. 2007; Faraone et al. 2005). In addition, analyses of its functional significance have yielded inconsistent findings: the 10R allele (Fuke et al. 2001; Mill et al. 2002) and the 9R allele (Michelhaugh et al. 2001; Miller and Madras 2002) have both been found to be associated with higher levels of DAT expression. Furthermore, there have been studies reporting no effect (Lynch et al. 2003; Martinez et al. 2001). Although its functional significance is not completely understood, DAT1 VNTR remains an interesting candidate gene for differences in emotional regulation. The dopamine transporter has a 1,000-fold higher affinity for dopamine than COMT and provides the best mechanism for dopamine reuptake (Diamond 2007; Chen et al. 2004). However, DATs are mainly found in the striatum and to a much lesser extent in the PFC, while COMT appears to be most important for dopamine clearance in the PFC (Tunbridge et al. 2006; Diamond 2007) where it accounts for more than 60% of the dopamine degradation (Karoum et al. 1994), suggesting different neuronal mechanisms by which these two polymorphisms may influence the startle reflex.
Both COMT and DAT1 are expressed in brain regions that are involved in emotional processing, as well as in cognitive functions, and have been reported to influence cognitive processes: COMT has been found to impact on working memory and cognitive flexibility (Bilder et al. 2004; Barnett et al. 2007; Joober et al. 2002; Malhotra et al. 2002) and DAT1 has been associated with inhibition, impulsivity and ADHD (Congdon et al. 2009; Faraone et al. 2005; Yang et al. 2007). Since the startle magnitude, as well as FPS and PAS, has been found to be influenced by attention (Filion et al. 1998; Alhadad et al. 2008), it cannot be ruled out that COMT and DAT1 exert their influence via these processes.
Interestingly, we did not find any influence of dopaminergic polymorphisms on the overall startle response in healthy younger adults who underwent the same experiment (unpublished data), but clear effects of genetic variation impacting serotonergic function (Armbruster et al. 2009; Brocke et al. 2006; Armbruster et al. 2010). The levels of dopamine have been found to decrease significantly with age (Diamond 2007; Bäckman et al. 2006; Rollo 2009) and the effects of genetic variation in the dopaminergic system on emotional regulation might thus be more pronounced in older adults (Nagel et al. 2008), explaining why we found the influence of COMT and DAT1 only in the older sample. However, younger and older samples do not only represent different developmental periods, but also different birth cohorts. For instance, most participants of our older sample were born during the last few months or shortly after the end of World War II. Since adverse prenatal and early postnatal conditions have been found to influence the development of the central nervous system independently and in interaction with genetic variations (e.g., Barr et al. 2004; Caspi et al. 2003), cohort effects might also at least be partly responsible for the different genetic influence patterns.
Furthermore, our older participants showed comparatively small startle magnitudes, which is in line with results from several studies reporting that older individuals were less responsive to startling stimuli and had substantially decreased ASR compared to younger adults (Ford et al. 1995; Ellwanger et al. 2003; Ludewig et al. 2003). This effect has also been documented in animal studies (Varty et al. 1998). The effects of aging on emotional startle modulation are less consistent. Our sample showed a strong PAS in the positive compared to the neutral condition as well as to baseline, while there was no FPS in the negative condition. However, negative pictures were rated as clearly more unpleasant than neutral pictures by both sexes. Contrary to our expectations, the baseline startle was even slightly elevated compared to the neutral and negative condition, although this was mainly due to the male subsample. Contrasting results were reported by Smith et al. (2005), who found a significant FPS but no PAS in older adults, although methodological differences (i.e., chosen IAPS pictures, startle stimulus intensity and onset) might account for the divergent results.
Nevertheless, our results regarding emotional startle regulation raise the question whether the paradigm was not suited to evoke modulating effects, particularly with regard to FPS. We used the same paradigm in two different samples of young adults and found a clear PAS, as well as a significant enhanced startle in the negative condition, when compared to baseline (Armbruster et al. 2009, 2010; Brocke et al. 2006). One possible explanation for these differences between younger and older participants is the so called positivity effect. Healthy older adults have been reported to show preferences in visual attention toward positive and away from negative emotional material (Mather and Carstensen 2003; Isaacowitz et al. 2006). Their performance in memory tasks also appears to be influenced by positivity effects (Murphy and Isaacowitz 2008). Thus, it has been suggested that processing of emotional information in older adults might be focused on positive and/or disengaged from negative stimuli (Carstensen and Mikels 2005; Mikels et al. 2005) which would be in line with our findings. However, a recent meta-analysis reported an age-related decline regarding emotional processing across all emotions and modalities and a general trend for worsening of emotion recognition with age (Ruffman et al. 2008), inconsistent with a positivity effect. In addition, recent fMRI studies report different activation patterns in healthy older compared to younger adults while processing emotional stimuli (Fischer et al. 2005; Mather et al. 2004). In summary, available evidence points to substantial age-related differences in the processing of emotional stimuli, which may account for the lack of FPS in our sample.
There are several limitations to our study. The sample size was comparatively small for a genetic association study. Additionally, the comparably strict sample selection procedure (e.g., excluding participants who reported to be smokers, or suffer from physical or mental disorders) may have reduced sample representativeness and led to a restriction of variability of startle response. This restriction of variability might have in turn obscured possible associations, which, however, could have been also obscured by additional confounding factors. To tackle this dilemma, we chose to exclude known or likely confounding factors and to take the risk that some effects might not reach significance due to restriction of variability rather than the opposite. Clearly though, future studies should examine whether the present results hold true in more heterogeneous populations.
Taken together, the findings of our study provide evidence that the overall startle magnitude in older adults is sensitive to genetic variation of dopaminergic function with carriers of allelic variants that lead to less efficient dopamine clearance, mainly in the PFC (COMT met/met), showing stronger startle responses. Similarly, participants with a genetic variation in the DAT1 gene (10R/10R) that has been associated with lower DAT availability in the striatum and, therefore, less efficient dopamine reuptake in some studies (Michelhaugh et al. 2001; Miller and Madras 2002; van Dyck et al. 2005) showed a tendency toward stronger overall startle responses. Although analyses of the functional significance of the DAT1 VNTR have been inconsistent, it remains an interesting candidate for differences in emotional regulation. Our results yield further support to the notion that DAT may influence the processing of affective stimuli. However, the underlying biological pathways and the exact role of COMT and DAT in the various regions of the brain that impact on this kind of emotional regulation need further investigation.
References
Alhadad SS, Lipp OV, Purkis HM (2008) Modality-specific attentional startle modulation during continuous performance tasks: a brief time is sufficient. Psychophysiology 45(6):1068–1078. doi:10.1111/j.1469-8986.2008.00705.x
Anokhin AP, Golosheykin S, Heath AC (2007) Genetic and environmental influences on emotion-modulated startle reflex: a twin study. Psychophysiology 44(1):106–112. doi:10.1111/j.1469-8986.2006.00486.x
Armbruster D, Moser DA, Strobel A, Hensch T, Kirschbaum C, Lesch KP, Brocke B (2009) Serotonin transporter gene variation and stressful life events impact processing of fear and anxiety. Int J Neuropsychopharmacol 12(3):393–401. doi:10.1017/S1461145708009565
Armbruster D, Mueller A, Strobel A, Kirschbaum C, Lesch KP, Brocke B (2010) Influence of functional tryptophan hydroxylase 2 gene variation and sex on the startle response in children, young adults, and older adults. Biol Psychology 83(3):214–221. doi:10.1016/j.biopsycho.2009.12.010
Bäckman L, Nyberg L, Lindenberger U, Li SC, Farde L (2006) The correlative triad among aging, dopamine, and cognition: current status and future prospects. Neurosci Biobehav Rev 30(6):791–807. doi:10.1016/j.neubiorev.2006.06.005
Bäckman L, Lindenberger U, Li SC, Nyberg L (2011) Linking cognitive aging to alterations in dopamine neurotransmitter functioning: recent data and future avenues. Neurosci Biobehav Rev 34:670–677. doi:S0149-7634(09)00205-X
Barnett JH, Jones PB, Robbins TW, Muller U (2007) Effects of the catechol-o-methyltransferase Val158Met polymorphism on executive function: a meta-analysis of the Wisconsin Card Sort test in schizophrenia and healthy controls. Mol Psychiatry 12(5):502–509. doi:10.1038/sj.mp.4001973
Barr CS, Newman TK, Schwandt M, Shannon C, Dvoskin RL, Lindell SG, Taubman J, Thompson B, Champoux M, Lesch KP, Goldman D, Suomi SJ, Higley JD (2004) Sexual dichotomy of an interaction between early adversity and the serotonin transporter gene promoter variant in rhesus macaques. Proc Natl Acad Sci USA 101(33):12358–12363. doi:10.1073/pnas.0403763101
Bilder RM, Volavka J, Lachman HM, Grace AA (2004) The catechol-o-methyltransferase polymorphism: relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology 29(11):1943–1961. doi:10.1038/sj.npp.1300542
Blasi G, Mattay VS, Bertolino A, Elvevag B, Callicott JH, Das S, Kolachana BS, Egan MF, Goldberg TE, Weinberger DR (2005) Effect of catechol-o-methyltransferase val158met genotype on attentional control. J Neurosci 25(20):5038–5045. doi:10.1523/JNEUROSCI.0476-05.2005
Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, van Boxtel A (2005) Committee report: guidelines for human startle eyeblink electromyographic studies. Psychophysiology 42(1):1–15. doi:10.1111/j.1469-8986.2005.00271.x
Brocke B, Armbruster D, Muller J, Hensch T, Jacob CP, Lesch KP, Kirschbaum C, Strobel A (2006) Serotonin transporter gene variation impacts innate fear processing: acoustic startle response and emotional startle. Mol Psychiatry 11(12):1106–1112. doi:10.1038/sj.mp.4001908
Carstensen LL, Mikels JA (2005) At the intersection of emotion and cognition—aging and the positivity effect. Curr Direct Psychol Sci 14(3):117–121. doi:10.1111/j.0963-7214.2005.00348.x
Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R (2003) Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 301(5631):386–389. doi:10.1126/science.1083968
Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, Kolachana BS, Hyde TM, Herman MM, Apud J, Egan MF, Kleinman JE, Weinberger DR (2004) Functional analysis of genetic variation in catechol-o-methyltransferase (comt): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet 75(5):807–821. doi:10.1086/425589
Congdon E, Constable RT, Lesch KP, Canli T (2009) Influence of SLC6A3 and comt variation on neural activation during response inhibition. Biol Psychol 81(3):144–152. doi:10.1016/j.biopsycho.2009.03.005
Davis M, Falls WA, Campeau S, Kim M (1993) Fear-potentiated startle: a neural and pharmacological analysis. Behav Brain Res 58(1–2):175–198. doi:10.1016/0166-4328(93)90102-V
Diamond A (2007) Consequences of variations in genes that affect dopamine in prefrontal cortex. Cereb Cortex 17(Suppl 1):i161–i170. doi:10.1093/cercor/bhm082
Drabant EM, Hariri AR, Meyer-Lindenberg A, Munoz KE, Mattay VS, Kolachana BS, Egan MF, Weinberger DR (2006) Catechol o-methyltransferase val158met genotype and neural mechanisms related to affective arousal and regulation. Arch Gen Psychiatry 63(12):1396–1406. doi:10.1001/archpsyc.63.12.1396
Dreher JC, Kohn P, Kolachana B, Weinberger DR, Berman KF (2009) Variation in dopamine genes influences responsivity of the human reward system. Proc Natl Acad Sci USA 106(2):617–622. doi:10.1073/pnas.0805517106
Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR (2001) Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA 98(12):6917–6922. doi:10.1073/pnas.111134598
Ekman P, Friesen WV (1976) Pictures of facial affect. Consulting Psychologists Press, Palo Alto
Ellwanger J, Geyer MA, Braff DL (2003) The relationship of age to prepulse inhibition and habituation of the acoustic startle response. Biol Psychol 62(3):175–195. doi:10.1016/S0301-0511(02)00126-6
Enoch MA, Xu K, Ferro E, Harris CR, Goldman D (2003) Genetic origins of anxiety in women: a role for a functional catechol-o-methyltransferase polymorphism. Psychiatr Genet 13(1):33–41. doi:10.1097/01.ypg.0000054709.85338.c3
Ettinger U, Kumari V, Collier DA, Powell J, Luzi S, Michel TM, Zedomi O, Williams SC (2008) Catechol-o-methyltransferase (comt) val158met genotype is associated with bold response as a function of task characteristic. Neuropsychopharmacology 33(13):3046–3057. doi:10.1038/sj.npp.1301658
Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, Sklar P (2005) Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry 57(11):1313–1323. doi:10.1016/j.biopsych.2004.11.024
Filion DL, Dawson ME, Schell AM (1998) The psychological significance of human startle eyeblink modification: a review. Biol Psychol 47(1):1–43. doi:10.1016/S0301-0511(97)00020-3
Fischer H, Sandblom J, Gavazzeni J, Fransson P, Wright CI, Backman L (2005) Age-differential patterns of brain activation during perception of angry faces. Neurosci Lett 386(2):99–104. doi:10.1016/j.neulet.2005.06.002
Ford JM, Roth WT, Isaacks BG, White PM, Hood SH, Pfefferbaum A (1995) Elderly men and women are less responsive to startling noises: N1, P3 and blink evidence. Biol Psychol 39(2–3):57–80. doi:10.1016/0301-0511(94)00959-2
Fuke S, Suo S, Takahashi N, Koike H, Sasagawa N, Ishiura S (2001) The VNTR polymorphism of the human dopamine transporter (DAT1) gene affects gene expression. Pharmacogenomics J 1(2):152–156
Goldberg TE, Egan MF, Gscheidle T, Coppola R, Weickert T, Kolachana BS, Goldman D, Weinberger DR (2003) Executive subprocesses in working memory: relationship to catechol-o-methyltransferase val158met genotype and schizophrenia. Arch Gen Psychiatry 60(9):889–896. doi:10.1001/archpsyc.60.9.889
Goldman D, Oroszi G, Ducci F (2005) The genetics of addictions: uncovering the genes. Nat Rev Genet 6(7):521–532. doi:10.1038/nrg1635
Haraldsson HM, Ettinger U, Magnusdottir BB, Sigmundsson T, Sigurdsson E, Ingason A, Petursson H (2010) Catechol-o-methyltransferase val 158 met polymorphism and antisaccade eye movements in schizophrenia. Schizophr Bull 36(1):157–164. doi:10.1093/schbul/sbn064
Heinz A, Goldman D, Jones DW, Palmour R, Hommer D, Gorey JG, Lee KS, Linnoila M, Weinberger DR (2000) Genotype influences in vivo dopamine transporter availability in human striatum. Neuropsychopharmacology 22(2):133–139. doi:10.1016/S0893-133X(99)00099-8
Hünnerkopf R, Strobel A, Gutknecht L, Brocke B, Lesch KP (2007) Interaction between BDNF Val66Met and dopamine transporter gene variation influences anxiety-related traits. Neuropsychopharmacology 32(12):2552–2560. doi:10.1038/sj.npp.1301383
Isaacowitz DM, Wadlinger HA, Goren D, Wilson HR (2006) Is there an age-related positivity effect in visual attention? A comparison of two methodologies. Emotion 6(3):511–516. doi:10.1037/1528-3542.6.3.511
Jacobsen LK, Staley JK, Zoghbi SS, Seibyl JP, Kosten TR, Innis RB, Gelernter J (2000) Prediction of dopamine transporter binding availability by genotype: a preliminary report. Am J Psychiatry 157(10):1700–1703
Joober R, Gauthier J, Lal S, Bloom D, Lalonde P, Rouleau G, Benkelfat C, Labelle A (2002) Catechol-o-methyltransferase val-108/158-met gene variants associated with performance on the Wisconsin Card Sorting Test. Arch Gen Psychiatry 59(7):662–663
Karoum F, Chrapusta SJ, Egan MF (1994) 3-methoxytyramine is the major metabolite of released dopamine in the rat frontal cortex: Reassessment of the effects of antipsychotics on the dynamics of dopamine release and metabolism in the frontal cortex, nucleus accumbens, and striatum by a simple two pool model. J Neurochem 63(3):972–979. doi:10.1046/j.1471-4159.1994.63030972.x
Koch M (1999) The neurobiology of startle. Prog Neurobiol 59(2):107–128. doi:10.1016/S0301-0082(98)00098-7
Lachman ME (2004) Development in midlife. Annu Rev Psychol 55:305–331. doi:10.1146/annurev.psych.55.090902.141521
Lang PJ (1980) Behavioral treatment and bio-behavioral assessment: computer applications. In: Sidowski JB, Johnson JH, Williams TA (eds) Technology in mental health care delivery systems. Ablex, Norwood, pp 119–137
Lang PJ, Bradley MM, Cuthbert BN (1990) Emotion, attention, and the startle reflex. Psychol Rev 97:377–395
Lang PJ, Bradley MM, Cuthbert BN (1999) International affective picture system (IAPS): instruction manual and affective ratings. Technical report a-4, Center for Research in Psychophysiology, University of Florida, Gainesville
Lang UE, Bajbouj M, Sander T, Gallinat J (2007) Gender-dependent association of the functional catechol-o-methyltransferase val158met genotype with sensation seeking personality trait. Neuropsychopharmacology 32(9):1950–1955. doi:10.1038/sj.npp.1301335
Leaton RN, Cranney J (1990) Potentiation of the acoustic startle response by a conditioned stimulus paired with acoustic startle stimulus in rats. J Exp Psychol Anim Behav Process 16(3):279–287
Li J, Ji L (2005) Adjusting multiple testing in multilocus analyses using the eigenvalues of a correlation matrix. Heredity 95(3):221–227. doi:10.1038/sj.hdy.6800717
Ludewig K, Ludewig S, Seitz A, Obrist M, Geyer MA, Vollenweider FX (2003) The acoustic startle reflex and its modulation: effects of age and gender in humans. Biol Psychol 63(3):311–323. doi:10.1016/S0301-0511(03)00074-7
Lynch DR, Mozley PD, Sokol S, Maas NM, Balcer LJ, Siderowf AD (2003) Lack of effect of polymorphisms in dopamine metabolism related genes on imaging of TRODAT-1 in striatum of asymptomatic volunteers and patients with Parkinson’s disease. Mov Disord 18(7):804–812. doi:10.1002/mds.10430
Malhotra AK, Kestler LJ, Mazzanti C, Bates JA, Goldberg T, Goldman D (2002) A functional polymorphism in the COMT gene and performance on a test of prefrontal cognition. Am J Psychiatry 159(4):652–654
Martinez D, Gelernter J, Abi-Dargham A, van Dyck CH, Kegeles L, Innis RB, Laruelle M (2001) The variable number of tandem repeats polymorphism of the dopamine transporter gene is not associated with significant change in dopamine transporter phenotype in humans. Neuropsychopharmacology 24(5):553–560. doi:10.1016/S0893-133X(00)00216-5
Mather M, Carstensen LL (2003) Aging and attentional biases for emotional faces. Psychol Sci 14(5):409–415. doi:10.1111/1467-9280.01455
Mather M, Canli T, English T, Whitfield S, Wais P, Ochsner K, Gabrieli JD, Carstensen LL (2004) Amygdala responses to emotionally valenced stimuli in older and younger adults. Psychol Sci 15(4):259–263. doi:10.1111/j.0956-7976.2004.00662.x
Matsumoto M, Weickert CS, Beltaifa S, Kolachana B, Chen J, Hyde TM, Herman MM, Weinberger DR, Kleinman JE (2003) Catechol o-methyltransferase (COMT) MRNA expression in the dorsolateral prefrontal cortex of patients with schizophrenia. Neuropsychopharmacology 28(8):1521–1530. doi:10.1038/sj.npp.1300218
McClearn GE (2006) Contextual genetics. Trends Genet 22(6):314–319. doi:10.1016/j.tig.2006.04.005
Michelhaugh SK, Fiskerstrand C, Lovejoy E, Bannon MJ, Quinn JP (2001) The dopamine transporter gene (SLC6A3) variable number of tandem repeats domain enhances transcription in dopamine neurons. J Neurochem 79(5):1033–1038. doi:10.1046/j.1471-4159.2001.00647.x
Mier D, Kirsch P, Meyer-Lindenberg A (2010) Neural substrates of pleiotropic action of genetic variation in COMT: a meta-analysis. Mol Psychiatry 15(9):918–927. doi:10.1038/mp.2009.36
Mignone F, Gissi C, Liuni S, Pesole G (2002) Untranslated regions of mRNAs. Genome Biol 3 (3):REVIEWS0004. doi:10.1186/gb-2002-3-3-reviews0004
Mikels JA, Larkin GR, Reuter-Lorenz PA, Cartensen LL (2005) Divergent trajectories in the aging mind: changes in working memory for affective versus visual information with age. Psychol Aging 20(4):542–553. doi:10.1037/0882-7974.20.4.542
Mill J, Asherson P, Browes C, D’Souza U, Craig I (2002) Expression of the dopamine transporter gene is regulated by the 3′ UTR VNTR: evidence from brain and lymphocytes using quantitative RT-PCR. Am J Med Genet 114(8):975–979. doi:10.1002/ajmg.b.10948
Miller GM, Madras BK (2002) Polymorphisms in the 3′-untranslated region of human and monkey dopamine transporter genes affect reporter gene expression. Mol Psychiatry 7(1):44–55. doi:10.1038/sj/mp/4000921
Montag C, Buckholtz JW, Hartmann P, Merz M, Burk C, Hennig J, Reuter M (2008) COMT genetic variation affects fear processing: psychophysiological evidence. Behav Neurosci 122(4):901–909. doi:10.1037/0735-7044.122.4.901
Munafo MR, Bowes L, Clark TG, Flint J (2005) Lack of association of the COMT (val158/108 met) gene and schizophrenia: a meta-analysis of case–control studies. Mol Psychiatry 10(8):765–770. doi:10.1038/sj.mp.4001664
Murphy NA, Isaacowitz DM (2008) Preferences for emotional information in older and younger adults: a meta-analysis of memory and attention tasks. Psychol Aging 23(2):263–286. doi:10.1037/0882-7974.23.2.263
Nagel IE, Chicherio C, Li SC, von Oertzen T, Sander T, Villringer A, Heekeren HR, Backman L, Lindenberger U (2008) Human aging magnifies genetic effects on executive functioning and working memory. Front Hum Neurosci 2:1–8. doi:10.3389/neuro.09.001.2008
Nicodemus KK, Kolachana BS, Vakkalanka R, Straub RE, Giegling I, Egan MF, Rujescu D, Weinberger DR (2007) Evidence for statistical epistasis between catechol-o-methyltransferase (COMT) and polymorphisms in RGS4, G72 (DAOA), GRM3, and DISC1: influence on risk of schizophrenia. Hum Genet 120(6):889–906. doi:10.1007/s00439-006-0257-3
Nieoullon A, Coquerel A (2003) Dopamine: a key regulator to adapt action, emotion, motivation and cognition. Curr Opin Neurol 16(Suppl 2):S3–S9
Nyholt DR (2004) A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. Am J Hum Genet 74(4):765–769. doi:10.1086/383251
Olsson CA, Anney RJ, Lotfi-Miri M, Byrnes GB, Williamson R, Patton GC (2005) Association between the COMT val158met polymorphism and propensity to anxiety in an Australian population-based longitudinal study of adolescent health. Psychiatr Genet 15(2):109–115. doi:00041444-200506000-00007
Papaleo F, Crawley JN, Song J, Lipska BK, Pickel J, Weinberger DR, Chen J (2008) Genetic dissection of the role of catechol-o-methyltransferase in cognition and stress reactivity in mice. J Neurosci 28(35):8709–8723. doi:10.1523/JNEUROSCI.2077-08.2008
Pauli P, Conzelmann A, Mucha RF, Weyers P, Baehne CG, Fallgatter AJ, Jacob CP, Lesch KP (2010) Affect-modulated startle reflex and dopamine D4 receptor gene variation. Psychophysiology 47(1):25–33. doi:10.1111/j.1469-8986.2009.00923.x
Quednow BB, Schmechtig A, Ettinger U, Petrovsky N, Collier DA, Vollenweider FX, Wagner M, Kumari V (2009) Sensorimotor gating depends on polymorphisms of the serotonin-2A receptor and catechol-o-methyltransferase, but not on neuregulin-1 Arg38Gln genotype: a replication study. Biol Psychiatry 66(6):614–620. doi:10.1016/j.biopsych.2009.05.007
Reuter M, Hennig J (2005) Association of the functional catechol-o-methyltransferase val158met polymorphism with the personality trait of extraversion. Neuroreport 16(10):1135–1138. doi:00001756-200507130-00020
Rollo CD (2009) Dopamine and aging: intersecting facets. Neurochem Res 34(4):601–629. doi:10.1007/s11064-008-9858-7
Rommelse NN, Altink ME, Arias-Vasquez A, Buschgens CJ, Fliers E, Faraone SV, Buitelaar JK, Sergeant JA, Franke B, Oosterlaan J (2008) A review and analysis of the relationship between neuropsychological measures and DAT1 in ADHD. Am J Med Genet B Neuropsychiatr Genet 147B(8):1536–1546. doi:10.1002/ajmg.b.30848
Roussos P, Giakoumaki SG, Rogdaki M, Pavlakis S, Frangou S, Bitsios P (2008) Prepulse inhibition of the startle reflex depends on the catechol o-methyltransferase val158met gene polymorphism. Psychol Med 38(11):1651–1658. doi:10.1017/S0033291708002912
Ruffman T, Henry JD, Livingstone V, Phillips LH (2008) A meta-analytic review of emotion recognition and aging: Implications for neuropsychological models of aging. Neurosci Biobehav Rev 32(4):863–881. doi:10.1016/j.neubiorev.2008.01.001
Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI (1998) Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J Neurosci 18(7):2697–2708
Smith DP, Hillman CH, Duley AR (2005) Influences of age on emotional reactivity during picture processing. J Gerontol B Psychol Sci Soc Sci 60(1):P49–P56. doi:10.1093/geronb/60.1.P49
Smolka MN, Schumann G, Wrase J, Grusser SM, Flor H, Mann K, Braus DF, Goldman D, Buchel C, Heinz A (2005) Catechol-o-methyltransferase val158met genotype affects processing of emotional stimuli in the amygdala and prefrontal cortex. J Neurosci 25(4):836–842. doi:10.1523/JNEUROSCI.1792-04.2005
Smolka MN, Buhler M, Schumann G, Klein S, Hu XZ, Moayer M, Zimmer A, Wrase J, Flor H, Mann K, Braus DF, Goldman D, Heinz A (2007) Gene–gene effects on central processing of aversive stimuli. Mol Psychiatry 12(3):307–317. doi:10.1038/sj.mp.4001946
Stefanis NC, Van Os J, Avramopoulos D, Smyrnis N, Evdokimidis I, Hantoumi I, Stefanis CN (2004) Variation in catechol-o-methyltransferase val158 met genotype associated with schizotypy but not cognition: A population study in 543 young men. Biol Psychiatry 56(7):510–515. doi:10.1016/j.biopsych.2004.06.038
Stein MB, Fallin MD, Schork NJ, Gelernter J (2005) COMT polymorphisms and anxiety-related personality traits. Neuropsychopharmacology 30(11):2092–2102. doi:10.1038/sj.npp.1300787
Tenhunen J, Salminen M, Jalanko A, Ukkonen S, Ulmanen I (1993) Structure of the rat catechol-o-methyltransferase gene: Separate promoters are used to produce mRNAs for soluble and membrane-bound forms of the enzyme. DNA Cell Biol 12(3):253–263. doi:10.1089/dna.1993.12.253
Tunbridge EM, Bannerman DM, Sharp T, Harrison PJ (2004) Catechol-o-methyltransferase inhibition improves set-shifting performance and elevates stimulated dopamine release in the rat prefrontal cortex. J Neurosci 24(23):5331–5335. doi:10.1523/JNEUROSCI.1124-04.2004
Tunbridge EM, Harrison PJ, Weinberger DR (2006) Catechol-o-methyltransferase, cognition, and psychosis: Val158met and beyond. Biol Psychiatry 60(2):141–151. doi:10.1016/j.biopsych.2005.10.024
van Dyck CH, Malison RT, Jacobsen LK, Seibyl JP, Staley JK, Laruelle M, Baldwin RM, Innis RB, Gelernter J (2005) Increased dopamine transporter availability associated with the 9-repeat allele of the SLC6A3 gene. J Nucl Med 46(5):745–751
Varty GB, Hauger RL, Geyer MA (1998) Aging effects on the startle response and startle plasticity in Fischer F344 rats. Neurobiol Aging 19(3):243–251. doi:10.1016/S0197-4580(98)00053-0
Vrana SR, Spence EL, Lang PJ (1988) The startle probe response: a new measure of emotion? J Abnorm Psychol 97(4):487–491. doi:10.1037/0021-843X.97.4.487
Weinshilboum RM, Otterness DM, Szumlanski CL (1999) Methylation pharmacogenetics: Catechol o-methyltransferase, thiopurine methyltransferase, and histamine N-methyltransferase. Annu Rev Pharmacol Toxicol 39:19–52. doi:10.1146/annurev.pharmtox.39.1.19
Winterer G, Musso F, Vucurevic G, Stoeter P, Konrad A, Seker B, Gallinat J, Dahmen N, Weinberger DR (2006) COMT genotype predicts bold signal and noise characteristics in prefrontal circuits. Neuroimage 32(4):1722–1732. doi:10.1016/j.neuroimage.2006.05.058
Woo JM, Yoon KS, Choi YH, Oh KS, Lee YS, Yu BH (2004) The association between panic disorder and the l/l genotype of catechol-o-methyltransferase. J Psychiatr Res 38(4):365–370. doi:10.1016/j.jpsychires.2004.01.001
Yang B, Chan RC, Jing J, Li T, Sham P, Chen RY (2007) A meta-analysis of association studies between the 10-repeat allele of a Vntr polymorphism in the 3’-Utr of dopamine transporter gene and attention deficit hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet 144B(4):541–550. doi:10.1002/ajmg.b.30453
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (KI 537/20-1, 20-3) and SFB 581, KFO 125 and SFB TTR 58 to K.P.L. We would like to thank Nicole Steigerwald and Nicole Döring for their excellent technical assistance in DNA sample processing and genotyping, and U. Buhss for excellent work in processing and analyzing the EMG data.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Armbruster, D., Mueller, A., Strobel, A. et al. Variation in genes involved in dopamine clearance influence the startle response in older adults. J Neural Transm 118, 1281–1292 (2011). https://doi.org/10.1007/s00702-011-0625-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-011-0625-6