Abstract
There is considerable evidence showing that the neurodegenerative processes that lead to sporadic Parkinson’s disease (PD) begin many years before the appearance of the characteristic motor symptoms and that impairments in olfactory, cognitive and motor functions are associated with time-dependent disruption of dopaminergic neurotransmission in different brain areas. Midkine is a 13-kDa retinoic acid-induced heparin-binding growth factor involved in many biological processes in the central nervous system such as cell migration, neurogenesis and tissue repair. The abnormal midkine expression may be associated with neurochemical dysfunction in the dopaminergic system and cognitive impairments in rodents. Here, we employed adult midkine knockout mice (Mdk−/−) to further investigate the relevance of midkine in dopaminergic neurotransmission and in olfactory, cognitive and motor functions. Mdk/− mice displayed pronounced impairments in their olfactory discrimination ability and short-term social recognition memory with no gross motor alterations. Moreover, the genetic deletion of midkine decreased the expression of the enzyme tyrosine hydroxylase in the substantia nigra reducing partially the levels of dopamine and its metabolites in the olfactory bulb and striatum of mice. These findings indicate that the genetic deletion of midkine causes a partial loss of dopaminergic neurons and depletion of dopamine, resulting in olfactory and memory deficits with no major motor impairments. Therefore, Mdk−/− mice may represent a promising animal model for the study of the early stages of PD and for testing new therapeutic strategies to restore sensorial and cognitive processes in PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder that affects approximately 1% of the population older than 50 years (Mayeux 2003). Classically, PD is considered to be a motor system disease, and its diagnosis is based on the presence of a set of cardinal motor signs (e.g., rigidity, bradykinesia, tremor and postural reflex disturbance). Unfortunately, the patients only fulfill these clinical criteria when 60–70% of the neurons of the substantia nigra (SN) are degenerated and the striatal dopamine (DA) content is reduced by 80% (Riederer and Wuketich 1976; Meissner et al. 2004). Recent evidence suggests that areas in the central nervous system (CNS) processing olfactory information are affected at the early stages of PD, even before the development of its classical symptoms (Doty et al. 1988; Muller et al. 2002; Braak et al. 2004). Consequently, olfactory dysfunction might be an early indicator of PD, and the development of specific olfactory testing may represent an important tool in the clinical diagnosis of early stages of this disease (Doty et al. 1995).
Beyond the olfactory symptoms, in the early stages of PD, subtle cognitive impairments can be observed, consisting mainly of executive dysfunction with secondary visuospatial and mnemonic disturbances (Dubois and Pillon 1997; Bosboom et al. 2004). In about 20–40% of patients, these problems may eventually proceed to dementia, which constitutes an important risk factor for caregiver distress, decreased quality of life and nursing home placement. Even non-demented PD patients have been reported to present visuospatial working memory deficits (Dubois and Pillon 1997; Stebbins et al. 1999; Lewis et al. 2003). Furthermore, although there are reports of declarative (or episodic) memory impairments in PD (Bondi and Kaszniak 1991), they are less severe in comparison to other neurodegenerative disorders such as Alzheimer’s disease (Bondi and Kaszniak 1991; Bosboom et al. 2004).
Animal models are an invaluable tool for studying the pathogenesis and progression of human diseases, as well as for testing new therapeutic intervention strategies. To date, most studies performed with animal models of PD have focused on their ability to induce nigrostriatal dopaminergic pathway damage and motor alterations associated with advanced phases of PD (for review see Gerlach and Riederer 1996; Beal 2001). Because PD is accompanied by alterations in a variety of functions, including olfactory dysfunction (Doty et al. 1988; Muller et al. 2002) and memory deficits (Dubois and Pillon 1997; Stebbins et al. 1999; Lewis et al. 2003), evaluating whether the proposed animal models of PD alter any of these functions seems important. Until recently, no well-accepted model of the early phase of PD was available in literature. This view has been re-evaluated following the findings that the infusion of the proneurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) by intranasal route (Prediger et al. 2006, 2009; Moreira et al. 2010) or directly into the rat SN (Da Cunha et al. 2001) causes a partial loss of DA neurons and depletion of striatal DA levels that result in olfactory and memory deficits with no gross motor impairments.
On the other hand, midkine is a multifunctional peptide that constitutes a novel heparin-binding growth factor family together with pleiotrophin (Muramatsu 2002). As neurotrophic factors, they have been implicated in development, regeneration and pathology of CNS (for review see Muramatsu 2010). Of high interest, Marchionini et al. (2007) reported that midkine and pleiotrophin, their receptors syndecan-3 and receptor protein tyrosine phosphatase β/ζ (RPTP β/ζ), and associated intracellular signaling molecules are highly expressed in the striatum during nigrostriatal development. Moreover, previous studies have demonstrated that midkine-deficient (Mdk−/−) mice exhibit a hypodopaminergic state (i.e., decreased levels of DA and its receptors in the striatum) together with working memory and prepulse inhibition deficits (Nakamura et al. 1998; Ohgake et al. 2009). Additionally, midkine is up-regulated under several pathological conditions in the adult brain, such as in the serum and senile plaques of patients with Alzheimer’s disease (Yasuhara et al. 1993; Kadomatsu and Muramatsu 2004), in ischemic brain regions (Kadomatsu and Muramatsu 2004) and in the serum of patients with schizophrenia (Shimizu et al. 2003). Despite the recent findings that pleiotrophin was up-regulated in the degenerating SN of PD patients (Marchionini et al. 2007) and in the striatum of rats with partial lesions of the nigrostriatal pathway (Ferrario et al. 2008), no evidence for the direct involvement of midkine in PD has to date been documented. Therefore, in the present study we employed adult midkine knockout (Mdk/−) mice, behavioral tests and neurochemical analysis to evaluate the relevance of midkine in the olfactory, cognitive and motor functions and dopaminergic neurotransmission.
Materials and methods
Animals
Experiments were conducted using male wild-type C57BL/6 and Mdk−/− mice 3–4 months old, weighing 25–30 g. They were kept in groups of five animals per cage and were maintained in a room under controlled temperature (23 ± 1°C). They were subjected to a 12-h light/dark cycle (lights on 7:00 a.m.) with free access to food and water. Wild-type C57BL/6 and Mdk−/− mice were obtained from the Faculty of Psychological and Physical Sciences, Aichi Gakuin University (Aichi, Japan). Deletion of the coding sequence for midkine was achieved according to the methodology described previously by Nakamura et al. (1998). The heterozygous mice had been backcrossed for 11 generations to a C57BL/6 genetic background. All mice were experimentally naïve, and two separate groups of mice were used for experiments [one for olfactory discrimination, social recognition memory tests and HPLC, and one for activity chambers test and tyrosine hydroxylase (TH) immunohistochemistry]. All animal procedures were carried out in compliance with the European Directive No.: 86/609/EEC and the guidelines of the local institutional animal care and use committee. All efforts were made to minimize the number of animals used and their suffering.
Behavioral tests
Olfactory discrimination
The olfactory discrimination ability of mice was assessed with an olfactory discrimination test that had been previously evaluated in our laboratory (Prediger et al. 2005a, 2010). The task was based on the fact that rodents usually disclose preference for places impregnated with their own odor (familiar compartments) and not for places with other non-familiar odors. Briefly, each mouse was placed for 5 min in a cage divided in two equal areas separated by an open door, where it could choose between one compartment with fresh sawdust (non-familiar compartment) and another with unchanged sawdust (familiar compartment) that the same mouse had occupied for 3 days before the test. Performance was monitored with a ViewPoint video-tracking system (Life Sciences, Montreal, Canada) and the following parameters were registered: time (s) and distance (cm) traveled in each compartment (familiar versus non-familiar) and total distance (cm) traveled.
Social recognition
Short-term social memory was assessed with the social recognition task described by Dantzer et al. (1987) and previously evaluated in our laboratory (Prediger et al. 2005a, b, 2010. The test was scored by the same rater in an observation room, where the mice had been habituated for at least 1 h before the beginning of the test. Juvenile C57BL/6 mice (25–30 days old) (Cente d’Elevage René Janvier, Le Genest St Ile, France) were kept in groups of ten per cage and served as social stimuli for the adult mice. All juveniles were isolated in individual cages for 20 min prior to the beginning of the experiment. The social recognition task consisted of two successive presentations (5 min each), separated by a short period of time, where the juvenile mouse was placed in the home cage of the adult mouse and the time spent by the adult in investigating the juvenile (nosing, sniffing, grooming or pawing) was recorded. At the end of the first presentation, the juvenile was removed and kept in an individual cage during the delay period and re-exposed to the same adult mouse after 30 min. In this paradigm, if the delay period is less than 40 min, the adult rodents display recognition of the juvenile, as indicated by a significant reduction in the social investigation time during the second presentation (Dantzer et al. 1987; Prediger et al. 2005a, b, 2010). However, when the same juvenile is re-exposed after a longer time (more than 60 min) after the first presentation, the adult mouse no longer recognizes this juvenile, i.e., the social investigation time in the second presentation is similar to that observed during the first one. Thus, a 30-min interval between two presentations of the same conspecific juvenile was used to demonstrate possible deficits in the social recognition memory of Mdk−/− mice.
Spontaneous locomotor activity
To assess possible effects of the genetic deletion of midkine on locomotor activity, the animals were tested in activity chambers as described previously (Prediger et al. 2010). The activity chambers (20 × 20 × 20 cm), with a steel grid floor, equipped with three parallel horizontal infrared beams positioned 3 cm above the floor and spaced evenly along the longitudinal axis, had a digital counter which recorded photocell beam interruptions. The items of data obtained were expressed as signals corresponding to spontaneous movements for 15 min.
Neurochemistry
Measurements of monoamine levels by high-performance liquid chromatography
For determining DA, 3,4-dihydroxyphenylacetic acid (DOPAC), homovallinic acid (HVA), noradrenaline (NA) and 5-hydroxytryptamine (5-HT) contents in brain, some wild-type and Mdk−/− mice were killed by decapitation. Brains were removed immediately and the structures, olfactory bulb, striatum, prefrontal cortex and hippocampus, were dissected, weighed and sonicated for 5 s in 10 volumes (v/wt) of 0.1 N perchloric acid/0.05% disodium EDTA/0.05% sodium metabisulfite. DA, DOPAC, HVA, NA and 5-HT were extracted, and 10-μl samples were injected onto a Beckman Ultrasphere 5-μm IP column (Beckman) (Hamon et al. 1988; Prediger et al. 2010). Eluted DA, DOPAC, HVA, NA and 5-HT were quantified electrochemically (at 0.65 V) and concentrations were calculated in nanograms per gram of tissue.
Immunohistochemistry
Some wild-type and Mdk−/− mice were killed and the posterior part of the brain, including the ventral mesencephalon, was dissected and fixed by immersion in 4% formaldehyde in 0.1 M phosphate buffer (pH 7.4) for 1 day and then placed in 0.1 M PBS containing 30% sucrose for 24 h before being frozen at −30°C in isopentane until further processing. This material was used to make a stereological analysis of TH-positive neurons in the SN. The evaluation of TH-positive neurons in the substantia nigra pars compacta (SNpc) was performed as previously reported (Prediger et al. 2010). Briefly, serial coronal 20-μm thick sections were cut on a cryostat (Leica SM2000 R) and identified according to the mouse brain atlas of Franklin and Paxinos (1997). Sections between coordinates 1.26 and −0.08 mm with respect to the interaural axis contained the SN ventral tegmental area complex. One of every tenth section was used for immunostaining. Free-floating sections were incubated overnight at 4°C with anti-TH monoclonal antibody (1:300, catalog MAB318, Millipore/Chemicon International, Technology, Billerica, MA, USA) following 30 min pre-incubation in 0.1 M PBS containing 5% normal goat serum to block the nonspecific binding and 0.15% Triton X-100. After incubation with primary antibodies, the sections were washed with PBS and incubated with the biotinylated secondary antibody (anti-rabbit IgG 1:250 for 2 h), then processed using an avidin–biotin-peroxidase complex (1:100, Vectastain, Elite ABC kit, Vector Laboratories, Burlingame, CA). Finally, the antigen–antibody complexes were visualized by reaction with 3-3′-diaminobenzidine (DAB) and H2O2 (0.5 mg/mL and 0.025%, respectively) in 25 mM TBS.
Analysis of tyrosine hydroxylase immunoreactivity
For quantification of TH-positive cells in SNpc, digital images were acquired by a digital camera coupled to a Leitz microscope displaying the structure on a video monitor. Brain structures were identified according to the atlas of Franklin and Paxinos (1997). Settings for image acquisition were identical for tissues from wild-type and Mdk−/− groups. We obtained six images SNpc for each animal. TH immunoreactivity was assessed using a computer-based image analyzer (Visioscan 2000, Biocom, les Ulis, France). For spatial mapping and counting of individually stained neurons, the sections were placed in the microscope field so that the midline of the brain was always oriented along the same line. Cells were counted on one side of the brain. The contour of the SNpc was first delineated manually at low magnification. Then, the stained neurons within the area were counted at higher magnification, marked on the digitized image of each section and filed in the computer for archiving purposes. The number of TH-immunoreactive neurons was estimated by counting positive cells in six sections encompassing rostral, medial and caudal levels of the SNpc. Values obtained were then plotted with respect to the cumulative distance between the sections, and the surface area under this curve gave an estimate of the total number of neurons in the SNpc from its rostral to caudal extent, as previously described (Hirsch et al. 1992; Prediger et al. 2010).
Statistical analysis
The values are expressed as mean ± SEM (n equals the number of mice included in each analysis). Differences between wild-type and Mdk−/− groups were analyzed using unpaired Student’s t test. The accepted level of significance for the tests was P ≤ 0.05. All tests were performed using the Statistica® software package (StatSoft Inc., Tulsa, OK, USA).
Results
Effects of genetic deletion of midkine on the olfactory discrimination in mice
The results of the effects of genetic deletion of midkine on the olfactory discrimination ability of mice are illustrated in Fig. 1. Unpaired Student’s t test revealed that wild-type mice were able to discriminate between the familiar and the non-familiar compartments, spending much more time (t = 5.70, P ≤ 0.0001) (Fig. 1a) and traveling high distances (t = 3.71, P ≤ 0.01) (Fig. 1b) in the familiar compartment. However, Mdk−/− mice presented disruption in their olfactory discrimination ability, spending similar time (t = 1.77, P = 0.11) (Fig. 1a) and traveling a similar distance (t = 1.06, P = 0.32) (Fig. 1b) in the familiar and non-familiar compartments. These effects on the olfactory discrimination test are not related with motor impairments in Mdk−/− mice, since no alterations in total distance were observed (t = −0.45, P = 0.66) (Fig. 1c).
The effects of genetic deletion of midkine on odor discrimination ability of mice. Midkine knockout (Mdk−/−) and C57BL6 wild-type mice (n = 8 animals in each group) were placed for 5 min in a cage, divided into two identical compartments, where they could choose between one compartment with fresh sawdust (non-familiar; gray) and another with unchanged sawdust that the same mouse had occupied for 72 h before the test (familiar; white). Data are expressed as the mean ± SEM of the a time (s) and b distance (cm) traveled in each compartment and c total distance. * P ≤ 0.05 compared to the familiar compartment of the same group (unpaired Student’s t test)
Effects of genetic deletion of midkine on the short-term social recognition memory in mice
Figure 2 summarizes the effects of genetic deletion of midkine on the short-term social recognition memory of mice. Unpaired Student’s t test revealed that wild-type mice spent significantly less time (t = 9.66, P ≤ 0.0001) investigating the familiar juvenile mouse in the second exposure. On the other hand, Mdk−/−mice spent as much time investigating the juvenile mouse during the second encounter as they did in the first exposure (t = 0.26, P = 0.80), reflecting a clear impairment of the juvenile’s recognition ability (Fig. 2).
The effects of genetic deletion of midkine on short-term social memory of mice. Data are expressed as the mean ± SEM of the investigation time (s) of adult midkine knockout (Mdk−/−) and C57BL6 wild-type mice (n = 8 animals in each group) in the first (white) and second presentation (gray) when the same juvenile was exposed for 5 min with an interval of 30 min. * P ≤ 0.05 compared to the first presentation of the same group (unpaired Student’s t test)
Effects of genetic deletion of midkine on locomotor activity in mice
As illustrated in Fig. 3, the exploratory behavior evaluated by number of crossings (t = 0.24, P = 0.80) and rearing (t = 1.60, P = 0.13) in the activity chambers during 15 min was not significantly affected by genetic deletion of midkine in mice (Fig. 3).
Analysis of neurochemical alterations induced by genetic deletion of midkine in mice
With the purpose of determining the relationship between the olfactory and short-term social memory alterations induced by the genetic deletion of midkine in mice and neurochemical alterations in dopaminergic, noradrenergic and serotonergic neurotransmission, the levels of DA, DOPAC, HVA, NA and 5-HT in the olfactory bulb, striatum, prefrontal cortex and hippocampus were measured in wild-type and Mdk−/− mice.
As illustrated in Fig. 4, unpaired Student’s t test revealed that the genetic deletion of midkine promoted a significant depletion of DA and its metabolites in the olfactory bulb (DA: t = 2.32, P ≤ 0.05; DOPAC: t = 2.24, P ≤ 0.05; HVA: t = 2.45, P ≤ 0.05) and striatum (DA: t = 2.14, P ≤ 0.05; DOPAC: t = 2.77, P ≤ 0.05; HVA: t = 3.58, P ≤ 0.01) of mice. On the other hand, the levels of DA and its metabolites in the hippocampus and prefrontal cortex were not significantly altered by genetic deletion of midkine (Table 1).
The effects of genetic deletion of midkine on the levels of dopamine (DA) and its metabolites 3,4-dihydroxyphenylacetic acid (DOPAC) and homovallinic acid (HVA) in the a olfactory bulb and b striatum of mice. The values represent the mean ± SEM monoamine levels (ng/g wet tissue) of midkine knockout (Mdk−/−) and C57BL6 wild-type mice (n = 4–8 animals in each group). * P ≤ 0.05 compared to the wild-type group (unpaired Student’s t test)
Additionally, NA concentration was increased in the hippocampus of Mdk−/− mice (t = 3.42, P ≤ 0.01), whereas no significant alterations of NA were seen in the olfactory bulb, striatum and prefrontal cortex. Moreover, no significant differences in the 5-HT levels between wild-type and Mdk−/− mice were observed in any of the brain structures investigated (Table 1).
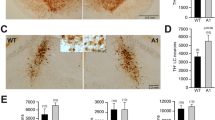
Figure 5 shows representative photomicrographs of TH immunohistochemistry in the ventral mesencephalom of wild-type (Fig. 5a) and Mdk−/− (Fig. 5b) mice. As can be seen, immunohistochemistry revealed a reduced number of TH-positive neurons in the SNpc (about 30%) of Mdk−/− mice in comparison to wild-type animals (t = 3.57, P ≤ 0.01) (Fig. 5c).
The effects of genetic deletion of midkine on tyrosine hydroxylase (TH)-positive cells in the substantia nigra pars compacta (SNpc) of mice. Representative images of TH immunostaining in the ventral mesencephalon containing SNpc of a C57BL6 wild-type and b midkine knockout (Mdk−/−) mice. Scale bar 200 μm. c Graphic representation of the average TH immunostaining analysis. The values represent the mean ± SEM of TH-positive cells per mm2 of four animals in each group. * P ≤ 0.05 compared to the wild-type group (unpaired Student’s t test)
Discussion
The present findings demonstrate that most of the behavioral impairments presented by Mdk−/− mice appear analogous to those observed during the early phase of PD, where sensory and memory deficits appear with no major motor alterations. Moreover, the deletion of midkine decreased about 30% the levels of the enzyme TH in SNpc, resulting in partial depletion of DA and its metabolites in the olfactory bulb and striatum of mice.
The three main strategic developments that have led to progress in the medical management of PD have focused on improvements in dopaminergic therapies (including those aimed at managing or preventing the onset of motor complications), the identification of non-dopaminergic drugs for symptomatic improvement and the discovery of compounds to modify the course of PD (Schapira et al. 2006). Despite extensive research, to date, there is no proven therapy to prevent cell death or to restore affected neurons to a normal state (Dawson and Dawson 2002). Preclinical studies on laboratory animals have provided several candidate neuroprotective drugs (e.g., DA agonists, monoamine oxidase type-B inhibitors, caffeine, co-enzyme Q10, growth factors, antiapoptotic agents and glutamate inhibitors), but clinical end points are readily confounded by any symptomatic effect of the study intervention and thus do not provide an unequivocal measure of disease progression that can be used to determine if a drug has a neuroprotective effect (Schapira and Olanow 2004; Schapira et al. 2006). It can be hypothesized that the low clinical efficacy of several neuroprotective agents is due to a late diagnosis of PD. Frequently, new potential agents are tested when the patient already shows cardinal motor signs. Unfortunately, the patients only fulfill these clinical criteria when 60–70% of the neurons of the SN are degenerated and the striatal DA content is reduced by 80% (Riederer and Wuketich 1976; Meissner et al. 2004). Therefore, for a better response, seems imperative to evaluate new candidate neuroprotective agents in early pre-motor stages of PD.
The development of new neuroprotective therapies in PD depends on the existence of representative animal models to facilitate the evaluation of new pharmacological agents and therapeutic strategies before they are applied in clinical trials (Gerlach et al. 2003). As stated in the introduction, early preclinical stages of PD are accompanied by alterations in a variety of functions (including olfactory and cognitive) and the management of the non-motor symptoms of PD remains a challenge (Chaudhuri et al. 2006; Prediger 2010). However, few studies have addressed consistently these preclinical symptoms in animal models of PD. In this context, we have recently proposed a new experimental model of PD consisting of a single i.n. administration of MPTP in rats (Prediger et al. 2006, 2009; Moreira et al. 2010) and mice (Prediger et al. 2010). Rodents treated intranasally with MPTP suffered progressive impairments in olfactory, cognitive and motor functions associated with time-dependent disruption of dopaminergic neurotransmission in different brain structures, which appears to be correlated with different stages of the human PD. Here, we demonstrated that Mdk−/− mice may represent a new genetic model featuring partial depletion of mesencephalic DA neurons, and olfactory and short-term memory impairments characteristics of the preclinical phase of PD.
Midkine is a member of a novel heparin-binding growth factor family together with pleiotrophin that has emerged as an important modulator in the CNS (for review see Muramatsu 2010). For instance, midkine, pleiotrophin and their receptors are highly expressed in the striatum during nigrostriatal development (Marchionini et al. 2007) and midkine was found to promote the survival of mouse mesencephalic neurons in culture (Kikuchi et al. 1993), which suggest that midkine exerts a neurotrophic effect on dopaminergic neurons. The first study with Mdk−/− mice suggested that these animals have no gross anatomical and behavioral abnormalities (Nakamura et al. 1998). However, more recent studies have pointed that Mdk−/− mice exhibit a hypodopaminergic state (i.e., decreased levels of DA and its receptors in the striatum) together with working memory and prepulse inhibition deficits (Nakamura et al. 1998; Ohgake et al. 2009).
In the present study, we evaluated whether the olfactory discrimination ability and short-term social recognition memory in adult mice are affected by the genetic deletion of midkine. Mdk−/− mice exhibited a clear disruption in their olfactory discrimination ability. The present olfactory discrimination task, previously standardized in our laboratory (Prediger et al. 2005a, 2010), was based on some initial observations of Carr et al. (1976) who had shown that mature male rodents significantly prefer their own odor to no odor at all. We suggest that the inability of Mdk−/− mice to discriminate between the familiar and the non-familiar compartments really reflects a deficit in olfactory discrimination, and not a simple locomotor impairment, since no alterations in the total distance traveled in the two compartments as well as the number of crossings and rearing in the activity cages were observed. Moreover, in the social recognition task, adult Mdk−/− mice spent as much time investigating the familiar juvenile mouse during the second presentation as they did on the first encounter, suggesting an impairment in the ability to recognize the juvenile after a short period of time (30 min).
The observed behavioral deficits induced by the genetic deletion of midkine were correlated with a significant reduction of the levels of DA and its metabolites in the olfactory bulb of mice. Our results are in accordance with several lines of evidence that strongly suggest the involvement of DA in olfactory processing. The olfactory bulb of mammals contains a large population of dopaminergic interneurons, principally periglomerular and external tufted cells, which are important for the odor information processing (Halasz and Shepherd 1983). Most data indicate that these dopaminergic cells constitute the entire DA content in the bulb, although there is a report of a minor projection from the ventral tegmental area (Gall et al. 1987). One systemic injection of a dopaminergic agonist can reduce odor detection (Doty and Risser 1989) and can abolish the odor-induced metabolic activation pattern in the olfactory bulb (Sallaz and Jourdan 1992). Moreover, DA appears to be necessary for olfactory memory because its release increases during olfactory learning (Coopersmith et al. 1991), whereas DA receptor antagonists (Weldon et al. 1991; Prediger et al. 2004, 2005b) or treatments that reduce the dopaminergic neurotransmission such as MPTP (Dluzen and Kreutzberg 1993; Prediger et al. 2010) and reserpine (Prediger et al. 2004, 2005b) inhibit olfactory memory.
In this context, we have previously demonstrated that the administration of the preferential DA D2 receptor antagonist haloperidol or the selective DA D2 receptor antagonist sulpiride, but not the DA D1 receptor antagonist SCH23390, disrupts the social recognition memory of adult rats (Prediger et al. 2004). Furthermore, Ennis et al. (2001) have demonstrated that DA D2 receptor agonists reduce the olfactory nerve-evoked synaptic response in mitral/tufted and juxtaglomerular cells, while these responses are enhanced by DA D2 receptor antagonists. There is also evidence of changes in DA levels and DA D2 receptor densities, indicating that the dopaminergic system in the olfactory bulb can be affected by olfactory experience (Coopersmith et al. 1991; Guthrie et al. 1991). Together, these data indicate that the activation of DA D2 receptors induce a temporary inhibition of reciprocal synapses (between mitral and granule cells) in the accessory olfactory bulb of adult rodents, which seems to be a critical step in olfactory memory formation (for review see Brennan and Keverne 1997), facilitating the acquisition of the juvenile olfactory information. Therefore, the decrease of DA levels in the olfactory bulb induced by the genetic deletion of midkine seems to be responsible, at least in part, by the olfactory and short-term social memory impairments observed in Mdl−/− mice. Further research is needed to clarify whether the densities of DA receptors are also altered in the olfactory bulb of Mdl−/− mice.
It must be argued that previous studies have shown the importance of other monoamines (beyond DA) for social recognition ability in rodents. In fact, the olfactory bulb of rodents receives a prominent noradrenergic input from the locus coeruleus (Shipley et al. 1985). Indeed, the social memory for a conspecific juvenile can be enhanced or disrupted, respectively, by a drug-induced elevation or depletion of NA in the CNS of adult rodents (Griffing and Taylor 1995; Dluzen et al. 1998). Furthermore, Letty et al. (1997) have demonstrated the improvement of social recognition memory by 5-HT in rats through the activation of 5-HT4 receptors. Although we cannot exclude completely a possible interaction between midkine with other monoamines (NA and 5-HT), high-performance liquid chromatography (HPLC) analysis indicated that their levels were not significantly altered in the investigated brain areas consecutive to the genetic deletion of midkine in mice.
As mentioned before, Nakamura et al. (1998) reported previously working memory deficits in Mdk−/− mice. There is compelling evidence that the prefrontal cortex plays a critical role in working memory (Passingham and Sakai 2004). Even though most of the dopaminergic afferents to the prefrontal cortex arise from the ventral tegmental area, some of them come from the central area of the SN (Albanese and Bentivoglio 1982). Thus, the prefrontal cortex processes working memory information through cortico-basal parallel loops that are also modulated by dopaminergic projections from the SN (Alexander et al. 1986). Of high importance, DA depletion in the prefrontal cortex is observed in the brain of early-stage PD patients (Zgaljardic et al. 2003; Bruck et al. 2005). For this reason, the depletion of DA in the prefrontal cortex consecutive to the genetic deletion of midkine in mice could explain their impairment in performing the working memory tests. Surprisingly, we failed to demonstrate significant alterations in the levels of DA and its metabolites in the prefrontal cortex of Mdk−/− mice. Therefore, at this moment, it is not easy to explain the precise underlying mechanisms by which the genetic deletion of midkine disrupts the working memory in mice, and this constitutes a very interesting field that requires additional research.
In accordance with recent findings (Ohgake et al. 2009), in the present study we demonstrated significant dysfunction of the nigrostriatal dopaminergic pathway in Mdk−/− mice. HPLC analysis revealed significant depletion of DA (25% low) and its metabolites DOPAC (30% low) and HVA (35% low) in the striatum of Mdk−/− mice when compared to C57BL/6 wild-type mice. Moreover, immunohistochemistry revealed a reduced number of TH-positive neurons in the SNpc (about 30%) of Mdk−/− mice in comparison to wild-type animals. The present results together with early findings (Kikuchi et al. 1993; Marchionini et al. 2007) suggest that midkine exerts a ubiquitous neurotrophic effect on dopaminergic neurons and may therefore be useful for neuroprotection or reconstruction of the DA system. Although the potential of midkine to confer protection against the loss of dopaminergic neurons in experimental models of PD remains to be established, it is important to emphasize that the neuroprotection by midkine extends beyond PD and the dopaminergic system. Midkine accumulates in senile plaques of Alzheimer’s disease (Yasuhara et al. 1993) and protects the cells from Aβ-induced cytotoxicity (Yu et al. 1993). Midkine is expressed in the surrounding zone of the brain infarction (Yoshida et al. 1995). Intraventricular administration of midkine (Yoshida et al. 2001) or midkine gene transfer (Ishikawa et al. 2009) protects against neuronal death following transient forebrain ischemia. Therefore, midkine confers neuroprotection in diverse brain regions and against a variety of brain noxious stimuli.
In conclusion, our findings indicate that the genetic deletion of midkine in mice causes a partial loss of dopaminergic neurons in the SNpc and depletion of DA and its metabolites in the olfactory bulb and striatum, resulting in olfactory and memory deficits with no major motor impairments. Therefore, Mdk−/− mice may represent a promising animal model for the study of the early stages of PD and for testing new therapeutic strategies to restore sensorial and cognitive processes in PD.
References
Albanese A, Bentivoglio M (1982) The organization of dopaminergic and nondopaminergic mesencephalocortical neurons in the rat. Brain Res 238:421–425
Alexander GE, DeLong MR, Strick PL (1986) Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9:357–381
Beal MF (2001) Experimental models of Parkinson’s disease. Nat Rev Neurosci 2:325–334
Bondi MW, Kaszniak AW (1991) Implicit and explicit memory in Alzheimer’s disease and Parkinson’s disease. J Clin Exp Neuropsychol 13:339–358
Bosboom JL, Stoffers D, Wolters ECh (2004) Cognitive dysfunction and dementia in Parkinson’s disease. J Neural Transm 111:1303–1315
Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K (2004) Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res 318:121–134
Brennan PA, Keverne EB (1997) Neural mechanisms of mammalian olfactory learning. Prog Neurobiol 51:457–481
Bruck A, Aalto S, Nurmi E, Bergman J, Rinne JO (2005) Cortical 6-[18F]fluoro-L-dopa uptake and frontal cognitive functions in early Parkinson’s disease. Neurobiol Aging 26:891–898
Carr WJ, Yee L, Gable D, Marasco E (1976) Olfactory recognition of conspecifics by domestic Norway rats. J Comp Physiol Psychol 90:821–828
Chaudhuri KR, Healy DG, Schapira AH, National Institute for Clinical Excellence (2006) Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol 5:235–245
Coopersmith R, Weihmuller FB, Kirstein CL, Marshall JF, Leon M (1991) Extracellular dopamine increase in neonatal olfactory bulb during odor preference training. Brain Res 564:149–153
Da Cunha C, Gevaerd MS, Vital MA, Miyoshi E, Andreatini R, Silveira R, Takahashi RN, Canteras NS (2001) Memory disruption in rats with nigral lesions induced by MPTP: a model for early Parkinson’s disease amnesia. Behav Brain Res 124:9–18
Dantzer R, Bluthe RM, Koob GF, Le Moal M (1987) Modulation of social memory in male rats by neurohypophyseal peptides. Psychopharmacology 91:363–368
Dawson TM, Dawson VL (2002) Neuroprotective and neurorestorative strategies for Parkinson’s disease. Nat Neurosci 5:1058–1061
Dluzen DE, Kreutzberg JD (1993) 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) disrupts social memory/recognition processes in the male mouse. Brain Res 609:98–102
Dluzen DE, Muraoka S, Landgraf R (1998) Olfactory bulb norepinephrine depletion abolishes vasopressin and oxytocin preservation of social recognition responses in rats. Neurosci Lett 254:161–164
Doty RL, Risser JM (1989) Influence of the D2 dopamine receptor agonist quinpirole on the odor detection performance of rat before and after spiperone administration. Psychopharmacology 98:310–315
Doty RL, Deems DA, Stellar S (1988) Olfactory dysfunction in parkinsonism: a general deficit unrelated to neurologic signs, disease stage, or disease duration. Neurology 38:1237–1244
Doty RL, Bromley SM, Stern MB (1995) Olfactory testing as an aid in the diagnosis of Parkinson’s disease: development of optimal discrimination criteria. Neurodegeneration 4:93–97
Dubois B, Pillon B (1997) Cognitive deficits in Parkinson’s disease. J Neurol 244:2–8
Ennis M, Zhou FM, Ciombor KJ, Aroniadou-Anderjaska V, Hayar A, Borrelli E, Zimmer LA, Margolis F, Shipley MT (2001) Dopamine D2 receptor-mediated presynaptic inhibition of olfactory nerve terminals. J Neurophysiol 86:2986–2997
Ferrario JE, Rojas-Mayorquín AE, Saldaña-Ortega M, Salum C, Gomes MZ, Hunot S, Raisman-Vozari R (2008) Pleiotrophin receptor RPTP-zeta/beta expression is up-regulated by L-DOPA in striatal medium spiny neurons of parkinsonian rats. J Neurochem 107:443–452
Franklin KBJ, Paxinos G (1997) The mouse brain in stereotaxic coordinates. Academic Press, San Diego
Gall CM, Hendry SH, Seroogy KB, Jones EG, Haycock JW (1987) Evidence of coexistence of GABA and dopamine in neurons of the rat olfactory bulb. J Comp Neurol 266:307–318
Gerlach M, Riederer P (1996) Animal models of Parkinson’s disease: an empirical comparison with the phenomenology of the disease in man. J Neural Transm 103:987–1041
Gerlach M, Foley P, Riederer P (2003) The relevance of preclinical studies for the treatment of Parkinson’s disease. J Neurol 250:31–34
Griffing MG, Taylor GT (1995) Norepinephrine modulation of social memory: evidence for a time-dependent functional recovery of behavior. Behav Neurosci 109:466–473
Guthrie KM, Pullara JM, Marshall JF, Leon M (1991) Olfactory deprivation increases dopamine D2 receptors density in the rat olfactory bulb. Synapse 8:61–70
Halasz N, Shepherd GM (1983) Neurochemistry of the vertebrate olfactory bulb. Neuroscience 10:619–759
Hamon M, Fattaccini CM, Adrien J, Gallissot MC, Martin P, Gozlan H (1988) Alterations of central serotonin and dopamine turnover in rats treated with ipsapirone and other 5-hydroxytryptmaine 1A agonists with potential anxiolytic properties. J Pharmacol Exp Ther 246:745–752
Hirsch EC, Lejeune O, Colliot G, Corkidi G, Tajani M (1992) Computer methods in nuclei cartography. Methods Neurosci 10:62–79
Ishikawa E, Ooboshi H, Kumai Y, Takada J, Nakamura K, Ago T, Sugimori H, Kamouchi M, Kitazono T, Ibayashi S, Iida M (2009) Midkine gene transfer protects against focal brain ischemia and augments neurogenesis. Neurol Sci 285:78–84
Kadomatsu K, Muramatsu T (2004) Midkine and pleiotrophin in neural development and cancer. Cancer Lett 204:127–143
Kikuchi S, Muramatsu H, Muramatsu T, Kim SU (1993) Midkine, a novel neurotrophic factor, promotes survival of mesencephalic neurons in culture. Neurosci Lett 160:9–12
Letty S, Child R, Dumuis A, Pantaloni A, Bockaert J, Rondouin G (1997) 5-HT4 receptors improve social olfactory memory in the rat. Neuropharmacology 36:681–687
Lewis SJG, Dove A, Robbins TW, Barker RA, Owen AM (2003) Cognitive impairments in early Parkinson’s disease are accompanied by reductions in activity in frontostriatal neural circuitry. J Neurosci 23:6351–6356
Marchionini DM, Lehrmann E, Chu Y, He B, Sortwell CE, Becker KG, Freed WJ, Kordower JH, Collier TJ (2007) Role of heparin binding growth factors in nigrostriatal dopamine system development and Parkinson’s disease. Brain Res 1147:77–88
Mayeux R (2003) Epidemiology of neurodegeneration. Annu Rev Neurosci 26:81–104
Meissner W, Hill MO, Tison F, Gross CE, Bezard E (2004) Neuroprotective strategies for Parkinson’s disease: conceptual limits of animal models and clinical trials. Trends Pharmacol Sci 25:249–253
Moreira EL, Rial D, Aguiar AS Jr, Figueiredo CP, Siqueira JM, Dalbó S, Horst H, Oliveira J, Mancini G, dos Santos TS, Villarinho JG, Pinheiro FV, Marino-Neto J, Ferreira J, De Bem AF, Latini A, Pizzolatti MG, Ribeiro-do-Valle RM, Prediger RD (2010) Proanthocyanidin-rich fraction from Croton celtidifolius Baill confers neuroprotection in the intranasal 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) rat model of Parkinson’s disease. J Neural Transm 117:1337–1351
Muller A, Reichmann H, Livermore A, Hummel T (2002) Olfactory function in idiopathic Parkinson’s disease (IPD): results from cross-sectional studies in IPD patients and long-term follow-up of de novo IPD patients. J Neural Transm 109:805–811
Muramatsu T (2002) Midkine and pleiotrophin: two related proteins involved in development, survival, inflammation and tumorigenesis. J Biochem 132:359–371
Muramatsu T (2010) Midkine, a heparin-binding cytokine with multiple roles in development, repair and diseases. Proc Jpn Acad Ser B Phys Biol Sci 86:410–425
Nakamura E, Kadomatsu K, Yuasa S, Muramatsu H, Mamiya T, Nabeshima T, Fan QW, Ishiguro K, Igakura T, Matsubara S, Kaname T, Horiba M, Saito H, Muramatsu T (1998) Disruption of the midkine gene (Mdk) resulted in altered expression of a calcium binding protein in the hippocampus of infant mice and their abnormal behaviour. Genes Cells 3:811–822
Ohgake S, Shimizu E, Hashimoto K, Okamura N, Koike K, Koizumi H, Fujisaki M, Kanahara N, Matsuda S, Sutoh C, Matsuzawa D, Muramatsu H, Muramatsu T, Iyo M (2009) Dopaminergic hypofunctions and prepulse inhibition deficits in mice lacking midkine. Prog Neuropsychopharmacol Biol Psychiatry 33:541–546
Passingham D, Sakai K (2004) The prefrontal cortex and working memory: physiology and brain imaging. Curr Opin Neurobiol 14:163–168
Prediger RD (2010) Effects of caffeine in Parkinson’s disease: from neuroprotection to the management of motor and non-motor symptoms. J Alzheimers Dis 20(Suppl 1):S205–S220
Prediger RD, Batista LC, Miyoshi E, Takahashi RN (2004) Facilitation of short-term social memory by ethanol in rats is mediated by dopaminergic receptors. Behav Brain Res 153:149–157
Prediger RD, Batista LC, Takahashi RN (2005a) Caffeine reverses age-related deficits in olfactory discrimination and social recognition memory in rats. Involvement of adenosine A1 and A2A receptors. Neurobiol Aging 26:957–964
Prediger RD, Da Cunha C, Takahashi RN (2005b) Antagonistic interaction between adenosine A2A and dopamine D2 receptors modulates the social recognition memory in reserpine-treated rats. Behav Pharmacol 16:209–218
Prediger RD, Batista LC, Medeiros R, Pandolfo P, Florio JC, Takahashi RN (2006) The risk is in the air: intranasal administration of MPTP to rats reproducing clinical features of Parkinson’s disease. Exp Neurol 202:391–403
Prediger RD, Rial D, Medeiros R, Figueiredo CP, Doty RL, Takahashi RN (2009) An intranasal MPTP (1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine) rat model of Parkinson’s disease. Ann N Y Acad Sci 1170:629–636
Prediger RD, Aguiar AS Jr, Rojas-Mayorquin AE, Figueiredo CP, Matheus FC, Ginestet L, Chevarin C, Bel ED, Mongeau R, Hamon M, Lanfumey L, Raisman-Vozari R (2010) Single intranasal administration of 1-methyl-4-phenyl-1, 2, 3,6-tetrahydropyridine in C57BL/6 mice models early preclinical phase of Parkinson’s disease. Neurotoxic Res 17:115–129
Riederer P, Wuketich S (1976) Time course of nigrostriatal degeneration in Parkinson’s disease. A detailed study of influential factors in human brain amine analysis. J Neural Transm 38:277–301
Sallaz M, Jourdan F (1992) Apomorphine disrupts odour-induced patterns of glomerular activation in the olfactory bulb. Neuroreport 3:833–836
Schapira AH, Olanow CW (2004) Neuroprotection in Parkinson’s disease: mysteries, myths and misconceptions. JAMA 291:358–364
Schapira AH, Bezard E, Brotchie J, Calon F, Collingridge GL, Ferger B, Hengerer B, Hirsch E, Jenner P, Le Novere N, Obeso JA, Schwarzschild MA, Spampinato U, Davidai G (2006) Novel pharmacological targets for the treatment of Parkinson’s disease. Nat Rev Drug Discov 5:845–854
Shimizu E, Hashimoto K, Salama RH, Watanabe H, Komatsu N, Okamura N, Koike K, Shinoda N, Nakazato M, Kumakiri C, Okada S, Muramatsu H, Muramatsu T, Iyo M (2003) Two clusters of serum midkine levels in drug-naive patients with schizophrenia. Neurosci Lett 344:95–98
Shipley MT, Halloran FJ, de la Torre J (1985) Surprisingly rich projection from locus coeruleus to the olfactory bulb in the rat. Brain Res 329:294–299
Stebbins GT, Gabrieli JD, Masciari F, Monti L, Goetz CG (1999) Delayed recognition memory in Parkinson’s disease: a role for working memory? Neuropsychologia 37:503–510
Weldon DA, Travis ML, Kennedy DA (1991) Posttraining D1 receptor blockade impairs odor conditioning in neonatal rats. Behav Neurosci 105:450–458
Yasuhara O, Muramatsu H, Kim SU, Muramatsu T, Maruta H, McGeer PL (1993) Midkine, a novel neurotrophic factor, is present in senile plaques of Alzheimer disease. Biochem Biophys Res Commun 192:246–251
Yoshida Y, Goto M, Tsutsui J, Ozawa M, Sato E, Osame M, Muramatsu T (1995) Midkine is present in the early stage of cerebral infarct. Dev Brain Res 85:25–30
Yoshida Y, Ikematsu S, Moritoyo T, Goto M, Tsutsui J, Sakuma S, Osame M, Muramatsu T (2001) Intraventricular administration of the neurotrophic factor midkine ameliorates hippocampal delayed neuronal death following transient forebrain ischemia in gerbils. Brain Res 894:46–55
Yu GS, Hu J, Nakagawa H (1993) Inhibition of beta-amyloid cytotoxicity by midkine. Neurosci Lett 254:125–128
Zgaljardic DJ, Borod JC, Foldi NS, Mattis P (2003) A review of the cognitive and behavioral sequelae of Parkinson’s disease: relationship to frontostriatal circuitry. Cogn Behav Neurol 16:193–210
Acknowledgments
This work was supported by grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), the Coordenacão de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), the Programa de Apoio aos Núcleos de Excelência (PRONEX) and the Fundação de Apoio a Pesquisa do Estado de Santa Catarina (FAPESC), all of Brazil. ASA Jr was supported by a scholarship from CNPq-Brazil. AERM was supported by CONACYT (México cod. 76101, 93485). RDSP and EDB were supported by research fellowships from CNPq-Brazil. RDSP, EDB and RRV were supported by CAPES-COFECUB (France/Brazil; 491/2005 and 681/2010) and FAPESP-INSERM (55092-9/2008). The authors have no financial or personal conflicts of interest related to this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
R. D. S. Prediger and A. E. Rojas-Mayorquin contributed equally to this work.
Rights and permissions
About this article
Cite this article
Prediger, R.D.S., Rojas-Mayorquin, A.E., Aguiar, A.S. et al. Mice with genetic deletion of the heparin-binding growth factor midkine exhibit early preclinical features of Parkinson’s disease. J Neural Transm 118, 1215–1225 (2011). https://doi.org/10.1007/s00702-010-0568-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-010-0568-3