Abstract
Epidemiological studies indicate that the intake of Mediterranean-style diet is inversely associated with risk of stroke, cardiovascular diseases, and cancer. Spirulina is widely used nutritional supplement rich in proteins and antioxidants. Evidence demonstrates that the impaired energy metabolism and the excessive generation of reactive oxygen radicals contribute to the brain injury associated with cerebral ischemia. In the present study, the protective effect of Spirulina was investigated in transient middle cerebral artery occlusion (MCAO)-induced focal cerebral ischemia–reperfusion injury in rats. Male albino rats were divided into six groups: control, sham-operated group, ischemic control group, and Spirulina-pretreated groups (45, 90 and 180 mg/kg/p.o.). Spirulina was administered once a day, for 7 days. The rats were subjected to a 2-h right MCAO via the intraluminal filament technique and 22 h of reperfusion. Pretreatment with Spirulina significantly reduced the histological changes and neurological deficits. Spirulina at a dose of 180 mg/kg significantly reversed the elevated brain malondialdehyde (MDA) content and restored the decreased activities of brain superoxide dismutase (SOD), catalase (CAT), and reduced glutathione (GSH) indicating that Spirulina has the protective potential against cerebral ischemia injury and its protective effects may be due to its antioxidant property.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke is one of the most common cause of death worldwide (Murray and Lopez 1997), is currently the leading cause of death in the western world, ranking after heart disease and before cancer (Donnan et al. 2008). It causes 10% of deaths worldwide, until now there is no effective neuroprotective therapy for the treatment of stroke. Thus, there is an urgent need to develop therapeutic strategies for the management of stroke.
Ischaemic stroke reduces blood supply, leading to energy shortage-induced imbalance of ionic gradients across membrane and accumulates calcium, sodium, and reduces pH, which in turn alters membrane transport, mitochondrial function, activates calcium-dependent enzymatic reactions, including DNA-breaking enzymes and generates excessive free radicals and triggers lipid peroxidation and ultimately cell apoptosis. Primary concern in cerebral ischemia is to restore blood flow and reperfusion. Reperfusion is necessary for alleviating the ischemic status, but excessive generation of reactive oxygen species (ROS) is leading cause of reperfusion injury (Gringo 1997; Nakashima et al. 1999). Thus, antioxidants or free radical scavenging agents are demonstrated to have protective effect in experimental stroke (Hwang et al. 2004; Bhandari and Ansari 2008; Shah et al. 2005; Thiyagarajan and Sharma 2004; Yanpallewar et al. 2004; Hosseinzadeh et al. 2007). Epidemiological studies indicate that the intake of Mediterranean-style diet is inversely associated with risk of stroke, cardiovascular diseases, and cancer. Spirulina is widely used nutritional supplement rich in proteins and antioxidants. Of late, there is overwhelming interest on natural products due to better acceptance. Spirulina is a blue green algae, rich in proteins, vitamins, minerals, essential amino acids, and essential fatty acids. Spirulina contains 60–70% protein by weight and contains a rich source of vitamins, especially vitamin B12 and minerals such as manganese, zinc, copper, iron, and gamma linolenic acid (an essential fatty acid). In addition, it has wide range of antioxidants such as superoxide dismutase (SOD), provitamin-A (beta-carotene), vitamin C, E, selenium and phycocyanin, and flavonoids which were proved to have antioxidant potential in many in vivo and in vitro tests (Careri et al. 2001; Reddy et al. 2000; Mitchell et al. 1990; Miranda et al. 1998; Premkumar et al. 2001; Upasani et al. 2001); dietary supplementation of Spirulina was reported to reduce ischemic brain damage (Wang et al. 2005).
Middle cerebral artery occlusion is the most commonly used model for ischemia (Belayev et al. 1995). The advantage of middle cerebral artery occlusion model is its reproducibility, and ischemic injury observed is similar to that found in humans (Gupta and Briyal 2004). The present study was undertaken to evaluate the neuroprotective potential of Spirulina in middle cerebral artery occlusion-induced focal cerebral ischemia in rats.
Materials and methods
Plant material
Spirulina maxima was generously supplied as gift sample in the form of spray-dried powder by Sunova Pharmaceuticals Limited, Chennai, India. The composition of spray-dried powder of S. maxima/100 g is proteins 65% (includes phycocyanin 15%), lipids 6%, minerals 8%, carbohydrates 15%, vitamins 0.75%, β-carotene 0.20%, xanthophylls 0.25%, chlorophyll 1%, moisture 3.80%.
Animals
Male albino rats weighing 150–200 g were chosen to avoid fluctuations due to estrous cycle. The rats were housed in polypropylene cages under 12 h light/dark cycle, fed with standard laboratory chow (Hindustan Lever Limited, Mumbai) and water ad libitum. Animals were acclimatized to the laboratory conditions prior to experimentation; all the experiments were carried out according to the study protocol approved by the Institutional Animal Ethical Committee.
Middle cerebral artery occlusion
The left middle cerebral artery occlusion (MCAO) was performed using intraluminal filament model and the method described by Longa et al. (1989). In brief, the rats were anesthetized with Thiopentone Sodium (40 mg/kg/i.p.); a 4-0 nylon monofilament with a blunt end was introduced into the external carotid artery (ECA) and advanced into the lumen of internal carotid artery (ICA) (17–20 mm) until a slight resistance was felt; such resistance indicated the passing of filament beyond the proximal segment of the anterior cerebral artery (ACA); at this point, the intraluminal suture blocks the origin of the MCA and occludes all sources of blood flow from the internal carotid artery, anterior, and the posterior cerebral artery. 2 h after the induction of ischemia, the filament was slowly withdrawn and the animals were returned to their cages for a period of 24 h; the body temperature was maintained at 37°C with a thermostatically controlled infrared lamp. In sham rats, the ECA was surgically prepared for the insertion of the filament, but the filament was not inserted.
Animal protocol
Male albino rats were randomly divided into 6 groups; each group consisted of 12 rats, fed with drug or vehicle for 7 days prior to experiment. The first group served as control and received 1% tween 80 orally. The second group served as sham control and received 1% tween 80 orally. The third group served as ischemic control and received 1% tween 80 orally. The fourth, fifth, and sixth groups were treated with Spirulina 45, 90, 180 mg/kg/p.o., respectively, for 7 days prior to middle cerebral artery occlusion. Then six rats in each group were decapitated to obtain brain tissue samples for biochemical analysis and brain tissues of the remaining six rats in each group were fixed in a fixative solution.
Neurological deficit
Neurological deficit in the vehicle- and drug-pretreated groups were determined after 24 h of reperfusion. Neurological findings were scored on a 5-point scale (Longa et al. 1989) as follows: no neurological deficit = 0, failure to extend right paw fully = 1, circling to right = 2, falling to right = 3, did not walk spontaneously and had decreased levels of consciousness = 4. The above procedures were performed in a blinded manner.
Biochemical estimations
24 h after induction of ischemia the animals were killed by cervical dislocation and their brains were dissected out quickly. The infarcted area was homogenized in 50 mM phosphate buffer (pH 7.0) containing 0.1 mM EDTA to give 5% w/v homogenate. The homogenate was centrifuged at 10,000 rpm for 10 min at 0°C in cold centrifuge and the resulting supernatant was used for biochemical estimations.
Superoxide dismutase
SOD activity was measured according to the method of Misra and Fridovich (1972), at room temperature. 100 μl of supernatant was added to 880 μl of 0.05 M carbonate buffer (pH 10.2, containing 0.1 mM EDTA), and 20 μl of 30 mM epinephrine (in 0.05% acetic acid) was added to the mixture, and the optical density values were measured at 480 nm for 4 min on a Systronics UV–Visible Spectrophotometer; activity was expressed as the amount of enzyme that inhibits the oxidation of epinephrine by 50% which is equal to 1 unit.
Catalase
Catalase activity was measured by a slightly modified version of Aebi (1984), at room temperature. 100 μl of supernatant was added to 10 μl of 100% ethanol and placed in ice bath for 30 min. Tubes were brought to room temperature and then 10 μl of Triton X-100 was added. In a cuvette containing 200 μl of phosphate buffer and 50 μl of above mixture, 250 μl of 0.066 M H2O2 in phosphate buffer was added. The decrease in optical density was measured at 240 nm for 60 s in Systronics UV–Visible Spectrophotometer. The molar extinction coefficient of 43.6 M/cm was used to determine CAT activity which is equal to the moles of H2O2 degraded/mg protein/min.
Reduced glutathione
Reduced glutathione levels were measured according to the method of Ellman (1959), at room temperature. 0.75 ml of supernatant was mixed with 0.75 ml of 4% sulphosalicylic acid and then centrifuged at 1,200 rpm for 5 min at 4°C. From this 0.5 ml of supernatant was taken and added to 4.5 ml of 0.01 M DTNB, and absorbance was measured at 412 nm by using a Systronics UV–Visible Spectrophotometer.
Estimation of lipid peroxidation
MDA levels were measured according to the method of Ohkawa et al. (1979) at room temperature. 200 μl of supernatant was added to 50 μl of 8.1% sodium dodecyl sulphate, vortexed, and incubated for 10 min at room temperature. 375 μl of thiobarbituric acid (0.6%) was added and placed in a boiling water bath for 60 min and then the samples were allowed to cool at room temperature. A mixture of 1.25 ml of butanol:pyridine (1.5:1) was added, vortexed, and centrifuged at 1,000 rpm for 5 min. The optical density values of the colored layer was measured at 532 nm on a Hitachi U-2000 spectrophotometer against reference blank and the values were expressed in nM of MDA formed for mg protein/min.
Histopathology
Coronal brain sections from control and experimental groups of focal ischemia were fixed in a mixture of formaldehyde (4%), glacial acetic acid, and methanol (1:1:8, v/v). Brain slices were cut into 4- to 5-mm thickness and embedded in paraffin blocks; brain sections of 4- to 6-μm thickness were stained with hematoxylin and eosin.
Statistical analysis
All the data expressed as mean ± SEM statistical significance were determined by one-way analysis of variance (ANOVA) followed by Dunnet’s ‘T’ test, using Graphpad Instat version 3. In all tests, the criterion for statistical significance was P < 0.05.
Results
Effect of Spirulina on neurological deficit
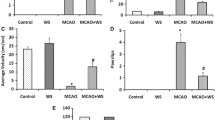
The neurological score after 24 h of reperfusion is given in Table 1. The neurological score of ischemic control group was significantly high as compared with control group, indicating induction of ischemia. Pretreatment with Spirulina (90, 180 mg/kg) significantly reduced the neurological deficit (P < 0.05, P < 0.001).
Effect of Spirulina on brain MDA, SOD, CAT and GSH levels
The effect of Spirulina on brain MDA, SOD, CAT, and GSH levels in MCA occlusion-reperfusion rats is shown in Table 2. The content of brain MDA in the ischemic hemisphere was significantly increased (P < 0.001) in ischemic control group as compared with the control group. Pretreatment with Spirulina (45 mg/kg/p.o.) did not significantly decrease the MDA content (P > 0.05) compared with ischemic control group. Pretreatment with Spirulina (90, 180 mg/kg/p.o.) significantly reversed the elevated MDA level (P < 0.05, P < 0.001) as compared with the ischemic control group.
The SOD levels in the ischemic hemisphere were significantly decreased (P < 0.001) in ischemic control group as compared with the control group. Pretreatment with Spirulina (45 mg/kg/p.o.) did not significantly alter the SOD levels (P > 0.05) compared with ischemic control group. Pretreatment with Spirulina (90, 180 mg/kg/p.o.) significantly reversed the decreased SOD levels (P < 0.05, P < 0.001) as compared with the ischemic control group.
The catalase activity in the ischemic hemisphere was significantly decreased (P < 0.001) in ischemic control group as compared with the control group. Pretreatment with Spirulina (45, 90 and 180 mg/kg/p.o.) significantly elevated the decreased catalase levels (P < 0.001) compared with ischemic control group.
The reduced glutathione levels in the ischemic hemisphere were significantly decreased (P < 0.001) in ischemic control group as compared with the control group. Pretreatment with Spirulina (90 and 180 mg/kg/p.o.) significantly increased the reduced glutathione levels (P < 0.001) compared with ischemic control group.
Effect of Spirulina on histopathological studies
Figures 1 and 2 show brain sections of control and sham control animals and depicts normal cytoarchitecture of brain tissue and neuronal cells (N) with normal nucleus. Figure 3 shows MCA occlusion, hypo perfusion-induced marked congestion of blood vessels (HCBV), excessive degeneration of neuronal cells (DN), excessive vaculation, and necrosis of neural cells. Pretreatment with Spirulina 45, 90 mg/kg shows mild congestion, mild degeneration of neuronal cells, and slight congestion and vaculations, respectively, and Spirulina 180 mg/kg shows neuronal cells with normal nucleus. Figures 4, 5, and 6 show that Spirulina pretreatment dose dependently reversed the congestion of blood vessels, congestion and necrotic degeneration of neuronal cells compared with ischemic control.
Discussion
In the present study, pretreatment with Spirulina significantly reversed the MCA occlusion-induced focal cerebral ischemia in rats. Neurological deficit symptoms and histopathological changes confirm the evidence of the brain damage. Focal cerebral ischemia causes cerebral cell death, resulting in neuronal deficit symptoms and cerebral infarction (Park et al. 2009). The severity of neurological deficits and area of infarction represent the degree of brain injury. Numerous reports indicate the involvement of oxidative stress in focal cerebral ischemia (Thiyagarajan and Sharma 2004; Zang and Yang 2006; Shah et al. 2005). Elevated ROS with concommitent decrease in plasma antioxidant activity during cerebral ischemia and reperfusion, induced neurodegeneration associated with higher lesion volumes and neurological impairment in stroke patients (Leinonen et al. 2000). Oxygen free radicals are known to contribute to ischemic brain damage (Baker et al. 1998; Cross et al. 1987; Globus et al. 1995), superoxide and hydroxyl radicals (Nakashima et al. 1999) formation was confirmed by an increase in hydroxylated salicylate in post-ischemic insult (Cao et al. 1998; Morimoto et al. 1996).
In the present study GSH, CAT, and SOD levels were decreased and MDA level was increased, indicating the involvement of oxidative stress in MCAO induced reperfusion injury. The antioxidant status of the tissue affected by ischemia/reperfusion is of great importance for the primary endogenous defense against the free radical-induced injury. As observed in our study, decrease in protective enzymes (SOD, CAT, and GSH) lead to increase in lipid peroxidation products.
SOD dismutases superoxide radicals to form hydrogen peroxide, which in turn is decomposed to water and oxygen by glutathione peroxidase and catalase, thereby preventing the formation of hydroxyl radicals (Yao et al. 1998). Therefore, these enzymes act co-operatively at different sites in the metabolic pathway of free radicals. Failure of this antioxidant defense leads to oxidative damage and initiation of lipid peroxidation (Parikh et al. 2003). In particular, evidence exists that the SOD activity in serum is reduced in stroke patients and ischemia significantly increases the brain superoxide ion (Saybasili et al. 2001, 2002), and that the replacement of antioxidant activity is beneficial in the acute treatment of cerebral ischemia (Spranger et al. 1997). Cerebral ischemia up regulates inducible nitric oxide synthase (iNOS), producing excessive nitric oxide and it reacts with superoxide to form peroxynitrite, which directly hydroxylate and nitrate the aromatic residues of amino acids and nucleotides in cytosol and nucleus (Beckman et al. 1992; Moreno and Pryor 1992), resulting in cellular machinery dysfunction and neuronal loss. Focal cerebral ischemia increases MDA and decreases GSH, CAT, and SOD levels (Thiyagarajan and Sharma 2004; Zang and Yang 2006).
In the present study depletion of GSH levels was observed in ischemic rats. Reduced glutathione (GSH) is one of the primary endogenous antioxidant defense systems in the brain, which scavenges hydrogen peroxide and lipid peroxides (Coyle and Puttfarcken 1993) and the decline in GSH levels reflects oxidative stress (Bains and Shaw 1997). Depletion of GSH in ischemia reperfusion injury is attributed to several factors such as cleavage of GSH to cysteine, decrease in the synthesis of GSH and the formation of mixed disulfides, causing their cellular stores to be depleted (Shivakumar et al. 1995). Excessive generation of ROS results in the lipid peroxidation of the cell membrane and subsequent damage is reflected by accumulation of MDA, a byproduct of lipid peroxidation (Halliwell 1991). Lipid peroxidation is one of the major consequences of free radical mediated injury to the brain. The peroxidation of the membrane phospholipids (PUFA) continues either until the exhaustion of the substrate or termination of chain propagation by antioxidants. Lipid peroxidation products are widely accepted group of oxidative stress markers, especially MDA levels detected as a stable derivative chromophore of Thiobarbituric acid adducts, is used as a potentially reliable and sensitive marker of reperfusion injury (Cano et al. 2003, Soska et al.1997). Several studies demonstrated higher MDA levels in stroke patients (Re et al. 1997; Bolokadze et al. 2004; Sharpe et al. 1994; Polidori et al. 2002; Gariballa et al. 2002). Clinical severity and patient’s outcome is well correlated with MDA levels (Polidori et al. 2002; Gariballa et al. 2002). Online with previous studies in the present study, ischemic rats showed significant increase in MDA levels indicating reperfusion injury by ROS.
Our findings, viz. significantly elevated LPO and depleted protective antioxidant enzymes (GSH, CAT, and SOD) in ischemia/reperfusion groups, are thus in agreement with other reports on the role of oxidative stress in cell injury in general and Cerebrovascular disease in particular (Shah et al. 2005; Viglino et al. 1988; Jenner 1994; Farbiszewski et al. 1995; Delanty and Dichter 1998). In the present study, Spirulina exhibited marked protection against MCAO induced ischemia/reperfusion as evidenced by significant reversal of enzymatic alterations produced by such insult.
Rutin, sodium selenite, curcumin, isoliquiritigenin (flavanoid), Acorus calamus rhizomes extract, Thymoquinone, Nigella sativa seed oil, and Korean ginseng tea showed their protective effect on MCAO-induced reperfusion injury due to their antioxidant property (Guptha et al. 2003; Zang and Yang 2006, 2004; Shukla et al. 2006; Hosseinzadeh et al. 2007; Shah et al. 2005; Thiyagarajan and Sharma 2004).
Search for agents providing protection against lipid peroxidation and enhancing anti-oxidant enzyme defense system is a rational approach for therapy of cerebrovascular ailments. Natural products (i.e. medicinal plants) with intrinsic antioxidant property constitute an ideal choice for maximum therapeutic effects with minimal risk of iatrogenic adverse effects. Dietary intake of antioxidants and vegetables lower the risk of cerebral infarction in male patients (Hirvonen et al. 2000). Recently, several dietary supplements have been reported to have strong antioxidant effects and reduce neurological deficits in aged animals (Bickford et al. 2000; Gemma et al. 2002), and pretreatment with antioxidant chemicals reduce ischemic brain injury (Clark et al. 2001; Fujimura et al. 2000; Yang et al. 2001). Dietary supplementation with spinach, blue berries, and Spirulina for 4 weeks reduced the infarct volume and neuronal apoptosis by altering capase 3 activity (Wang et al. 2005).
β-Carotene acts as an antioxidant by quenching singlet oxygen, scavenging free radicals, and breaking chain during lipid peroxidation (Foote and Denny 1968; Foote et al. 1970; Krinsky and Denekem 1982; Gerster 1993). Luxia et al. (1996) reported that β-carotene reduces cell damage, especially the damage to DNA molecules, thus playing a role in the repair and regeneration process of damaged cells. Vitamin E traps lipid peroxyl (LOO.) and other free radicals and maintains GSH and ascorbic acid levels in damaged tissue (Duval and Poelman 1994; Kulkarni and Byczkowski 1994). Phycocyanin pigment is 20 times more potent than ascorbic acid (Romay and Gonzalez 2000) and it significantly inhibited hydroxyl, alkoxyl, peroxyl radical-induced lipid peroxidation in rat liver microsomes (Bhat and Madyastha 2000, 2001). In addition, the pigment also scavenged super oxides, peroxy dinitrate thereby reduced peroxy dinitrate-induced oxidative damage to DNA (Romay et al. 1998; Bhat and Madyastha 2000). A component of phycocyanin, Phycocyanobilin is a more potent antioxidant than α-tocopherol on molar basis (Hirata et al. 2000). Spirulina has active components such as β-carotene (Prescott 1978; Sheshadri et al. 1991), vitamin C and E (Mathew et al. 1995), SOD enzyme (Henrikson 1989), selenium (Henrikson 1989), and brilliant blue polypeptide pigment phycocyanin (Shimamatsu 1989), which contribute to its antioxidant potential and thereby act as neuroprotective agent in ischemic reperfusion injury.
In spite of a relatively short period of ischemia (2 h), a significantly higher extent of damage in the ischemia reperfusion group was recorded in comparison with the control group. Neurodegeneration is also confirmed by the histopathological differences between different treatment groups. Spirulina pretreatment dose dependently decreased the ischemia–reperfusion-induced lipid peroxidation and improved the neurological score. Pretreatment with Spirulina 90, 180 mg/kg/p.o. significantly reversed and restored the levels of SOD, CAT and reduced GSH to near normal. Spirulina at a dose of 180 mg/kg managed to protect quite effectively the integrity of brain tissue and limited the extent of lipid peroxidation as evidenced by lipid peroxidation and histopathological studies as well. The normal human dose of Spirulina is 500–2,000 mg/day; 180 mg/kg rat dose is equivalent to 2,000 mg/day human dose. The present study confirms the neuroprotective effect of Spirulina due to its antioxidant potential even after short duration of pretreatment for 1 week and is online with the previous study of dietary supplementation of Spirulina for 4 weeks (Wang et al. 2005). Further, in the previous dietary supplementation study, the effective dose of Spirulina was not established; moreover, neuro protective effect of Spirulina was attributed to decreased activity of capase 3.
In conclusion, these findings suggest a potential role of Spirulina in stroke and gain importance in view of the fact that stroke is at present the third leading cause of death worldwide, and CVD constitute second most frequent cause of projected deaths. Spirulina offered significant neuroprotection in middle cerebral artery occlusion-induced focal cerebral ischemia, and this may be attributed to lower the levels of lipid peroxidation and enhancement of endogenous nonenzymatic and enzymatic antioxidants. Spirulina is cocktail of antioxidants and these may act co-operatively at various stages of free radical generation and protect the neurons from focal cerebral ischemia reperfusion injury. However, further work on infarct size measurement in rats is required for clinical use of Spirulina.
References
Aebi H (1984) Catalase. Methods Enzymol 105:125–126
Bains JS, Shaw CA (1997) Neurodegenerative disorders in humans: the role of glutathione in oxidative stress-mediated neuronal death. Brain Res Rev 25:335–338
Baker K, Marcus CB, Huffman K, Kruk H, Malfori B, Doctrow SR (1998) Synthetic combined superoxide dismutase/catalase mimetics are protective as a delayed treatment in a rat stroke model: a key role for reactive oxygen species in ischemic brain injury. J Pharmacol Exp Ther 284:215–221
Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA (1992) Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA 87:1620–1624
Belayev L, Busto R, Zhao W, Ginsberg MD (1995) HU-211, a novel noncompetitive N-methyl-d aspartate antagonist, improves neurological deficit and reduces infarct volume after reperfusion injury in the rat. Stroke 26:2313–2320
Bhandari U, Ansari NM (2008) Protective effect of aqueous extract of Embelia ribes Burm fruits in mid cerebral ischemia in rats. Indian J Pharmacol 40:215–220
Bhat VB, Madyastha KM (2000) C-phycocyanin: a potent peroxylradical scavenger in vivo and in vitro. Biochem Biophys Res Commun 275(1):20–25
Bhat VB, Madyastha KM (2001) Scavenging of peroxynitrite by phycocyanin and phycocyanobilin from Spirulina plantensis: protection against oxidative damage to DNA. Biochem Biophys Res Commun 285:262–266
Bickford PC, Gould T, Briederick L, Chadman K, Pollock A, Young D, Shukitt-Hale B, Joseph J (2000) Antioxidant-rich diets improve cerebellar physiology and motor learning in aged rats. Brain Res 866:211–217
Bolokadze N, Lobjanidze I, Momtselidze N, Solomonia R, Shakarishvili R (2004) Blood rheological properties and lipid peroxidation in cerebral and systemic circulation of neurocritical patients. Clin Hemorheol Microcirc 30:99–105
Cano CP, Bermudez VP, Atencio HE, Medina MT, Anilsa A (2003) Increased serum malondialdehyde and decreased nitric oxide within 24 hours of thrombotic stroke onset. Am J Ther 10:473–476
Cao W, Carney JM, Duchon A, Floyd RA, Chevion M (1998) Oxygen free radical involvement in ischemia and reperfusion injury to brain. Neurosci Lett 88:233–238
Careri M, Furlattini L, Mangia A, Musc M, Anklam E, Theobald A, Holst Von (2001) Supercritical fluid extraction for liquid chromatographic determination of carotenoids in spirulina pacifica algae: a chemometric approach. J Chromatogr A 912:61–71
Clark WM, Rinker LG, Lessov NS, Lowery SL, Cipolla MJ (2001) Efficacy of antioxidant therapies in transient focal ischemia in mice. Stroke 32:1000–1004
Coyle JT, Puttfarcken P (1993) Oxidative stress, glutamate and neurodegenerative disorders. Science 262:689–695
Cross CE, Halliwell B, Borish ET, Pryor WA, Ames BN, Saus RL (1987) Oxygen radicals and human disease. Ann Intern Med 107:526–545
Delanty LN, Dichter MA (1998) Oxidative injury in the nervous system. Arch Neurol Scand 98:145–153
Donnan GA, Fisher M, Macleod M, Davis SM (2008) Stroke. Lancet 371
Duval C, Poelman M (1994) Scavenger effect of vitamin E and derivatives on free radicals generated by photo irradiated phenomelanin. J Pharm Sci 84:107–110
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Farbiszewski R, Bielawski K, Bielawska A, Sobaniec W (1995) Spermine protects in vivo the anti-oxidant enzymes in transiently hypoperfused rat brain. Acta Neurobiol Expermentalis 55:253–258
Foote CF, Denny RW (1968) Chemistry of singlet oxygen. VII. Quenching by b-carotene. J Am Chem Soc 90:6233–6235
Foote CF, Chang YC, Denny RW (1970) Chemistry of singlet oxygen. X. Carotenoids quenching parallels biological protection. J Am Chem Soc 92:5216–5219
Fujimura M, Morita-Fujimura Y, Noshita N, Sugawara T, Kawase M, Chan PH (2000) The cytosolic antioxidant copper/zinc-superoxide dismutase prevents the early release of mitochondrial cytochrome c in ischemic brain after transient focal cerebral ischemia in mice. J Neurosci 20:2817–2824
Gariballa SE, Hutchin TP, Sinclair AJ (2002) Antioxidant capacity after acute ischaemic stroke. QJM 95:685–690
Gemma C, Mesches MH, Sepesi B, Choo K, Holmes DB, Bickford PC (2002) Diets enriched in foods with high antioxidant activity reverse age-induced decreases in cerebellar-adrenergic function and increases in proinflammatory cytokines. J Neurosci 22:6114–6120
Gerster H (1993) Anticarcinogenic effect of common carotenoids. Int J Vitam Nutr Res 63:93–121
Globus MY, Alonsa O, Dietrich WD, Busto R, Ginseberg MD (1995) Glutamate release and free radical production following brain injury: effect of posttraumatic hypothermia. J Neurochem 65:1704–1711
Gringo JM (1997) Reperfusion injury. Transplant proc 29:59–61
Gupta YK, Briyal S (2004) Animal models of cerebral ischemia for evaluation of drugs. Indian J Physiol Pharmacol 48:379–394
Guptha R, Manjeet S, Ajay S (2003) Neuroprotective effect of antioxidants on ischemia-reperfusion induced cerebral injury. Pharmacol Res 48(2):209–215
Halliwell B (1991) Reactive oxygen species in living system, source, biochemistry and role in human disease. Am J Med 99:145–225
Henrikson R (1989) Earth food spirulina. Cited from Recolina Limited, Ronore Enterprises Inc., Launa Beach, CA, pp 27–65
Hirata T, Tanaki M, Ooiki M, Tsunomura T, Sagachuchi M (2000) Antioxidant activities of Phycocyanobilin prepared from Spirulina platensis. J Appl Phycol 12:435–439
Hirvonen T, Virtamo J, Korhonen P, Albanes D, Pietinen P (2000) Intake of flavonoids, carotenoids, vitamins C and E, and risk of stroke in male smokers. Stroke 31:2301–2306
Hosseinzadeh H, Parvadeh S, Nassiri M, Hamid R, Sadgnia Ziaee T (2007) Effect of Thymoquinone and Nigella Sativa Seed oil on lipid peroxidation level during global cerebral ischemia reperfusion injury in rat hippocampus. Phyto Med 14(9):621–627
Hwang IK, Yoo K-Y, Kim DS, Jeong Y-K, Kim JD, Shin H-K, Lim SS, Yoo I-D, Kang T-C, Kim D-w, Moon W-K, Won MH (2004) Neuroprotective effects of grape seed extract on neuronal injury by inhibiting DNA damage in the gerbil hippocampus after transient forebrain ischemia. Life Sci 75:1989–2001
Jenner P (1994) Oxidative damage in neurodegenerating diseases. Lancet 344:796–798
Krinsky NI, Denekem SM (1982) Interaction of oxygen and oxyradicals with carotenoids. J Natl Cancer Inst 69:205–210
Kulkarni AP, Byczkowski JZ (1994) Introduction to biochemical toxicology, 2nd edn. Appleton and Lange, Connecticut, pp 103–105
Leinonen JS, Ahonen JP, Lonnrot K, Jechonen M, Dastidar P, Molnar J, Alho H (2000) Low plasma antioxidant activity is associated with high lesion volume and neurological impairment in stroke. Stroke 31:33–39
Longa EZ, Weinstein PR, Carlson S, Cummins R (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20:84–91
Luxia AS, Monica S, Ornella C, Plizzala B, Laura R, Livia B, Anio M, Ennio P (1996) Effect of b-carotene on cell cycle progression of human fibroblasts. Mutagen 17:2395–2401
Mathew WB, Sanakranarayana R, Nair P, Varghese C, Somanathan T, Amma P, Amma N, Nair M (1995) Evaluation of chemoprevention of oral cancer with Spirulina fusiformis. Nutr Cancer 24:197–202
Miranda MS, Cintra RG, Barros SM, Macini-Fitho J (1998) Antioxidant activity of the microalgae Spirulina maxima. Braz J Med Biol Res 31:1075–1079
Misra HP, Fridovich J (1972) The role of Superoxide anion in the auto oxidation of epinephrine and simple assay for superoxide dismutase. J Biol chem 247:3170–3175
Mitchell GV, Grundel E, Jenkins M, Blakely SR (1990) Effects of graded dietary levels of Spirulina maxima on vitamins A and E in male rats. J Nutr 120:1235–1240
Moreno JJ, Pryor WA (1992) Inactivation of alpha 1-proteinase inhibitor by peroxynitrite. Chem Res Toxicol 5:425–431
Morimoto T, Globus MY, Busto R, Martinez E, Ginsberg MD (1996) Simultaneous measurement of salicylate hydroxylation and glutamate release in the penumbral cortex following transient middle cerebral artery occlusion in rats. J Cereb Blood Flow Metab 16:92–99
Murray CJ, Lopez AD (1997) Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet 349:1269–1276
Nakashima M, Niwa M, Iwai T, Uematsu T (1999) Involvement of free radicals in cerebral vascular reperfusion injury evaluated in a transient focal cerebral ischemia model of rat. Free Radic Biol Med 26:722–729
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animals and tissue by thiobarbituric acid reaction. Anal Biochem 95:351–358
Parikh V, Mohammad Khan M, Mahadik SP (2003) Differential effects of antipsychotics on expression of antioxidant enzymes and membrane lipid peroxidation in rat brain. J Psychiatr Res 37:43–51
Park SJ, Nam KW, Lee HJ, Cho EY, Koo U, Mar W (2009) Neuroprotective effects of an alkaloid free ethyl acetate extract from the root of Sophora flavescens Ait. Against focal cerebral ischemia in rats. Phytomedicine 16(11):1042–1051
Polidori MC, Cherubini A, Stahl W, Senin U, Sies H (2002) Plasma carotenoid and malondialdehyde levels in ischemic stroke patients: relationship to early outcome. Free Radic Res 36:265–268
Premkumar K, Pachiappan A, Abraham SK, Santhiya ST, Gopinath PM, Ramesh A (2001) Effect of Spirulina fusiformis on cyclophosphamide and mitomycin-C induced genotoxicity and oxidative stress in mice. Fitoterapia 72:906–911
Prescott GW (1978) How to know the fresh water algae? 3rd edn. Wn.C., Brown Company Publishers, Iowa, p 182
Re G, Azzimondi G, Lanzarini C, Bassein L, Vaona I (1997) Plasma lipoperoxidative markers in ischaemic stroke suggest brain embolism. Eur J Emerg Med 4:5–9
Reddy CM, Bhat VB, Kiranmai G, Reddy MN, Reddanna P, Madyastha KM (2000) Selective inhibition of cyclooxygense-2 by C-phycocyanin, a biliprotein from Spirulina platensis. Biochem Biophys Res Commun 277:599–603
Romay C, Gonzalez R (2000) Phycocyanin is an antioxidant protector of human erythrocytes against lysis by peroxyl radicals. J Pharmacol 52:367–368
Romay C, Armesto J, Remirez D, Gonzalez R, Ledon N, Garcia I (1998) Antioxidant and anti-inflammatory properties of C-phycocyanin from blue-green algae. Inflamm Res 47:36–41
Saybasili H, Yuksel M, Haklar G, Yalcin AS (2001) Effect of mitochondrial electron transport chain inhibitors on superoxide radical generation in rat hippocampal and striatal slices. Antioxid Redox signal 3:1099–1104
Saybasili H, Yuksel M, Haklar G, Yalcin AS (2002) Depolarization induced superoxide formation in rat hippocampal slices. Neurochem Res 27:473–476
Shah ZA, Gilani RA, Sharma P, Vohora SB (2005) Cerebroprotective effect of Korean ginseng tea against global and focal models of ischemia in rats. J Ethnopharmacol 101:299–307
Sharpe PC, Mulholland C, Trinick T (1994) Ascorbate and malondialdehyde in stroke patients. Ir J Med Sci 163:488–491
Sheshadri CV, Umesh BV, Manoharan R (1991) β-Carotene studies in spirulina. Bio resource Technol 38:111–113
Shimamatsu S (1989) Cited from RECON. Recon Limited, A rainbow of colorful pigments, Bangalore, pp 13–14
Shivakumar BR, Kolluri SV, Ravindranath V (1995) Glutathione and protein thiol homeostasis in brain during reperfusion after cerebral ischemia. J Pharmacol Exp Ther 274:1167–1173
Shukla PK, Khanna VK, Ali MM, Mourya R, Khan MY, Srimal RC (2006) Neuroprotective effect of Acorus Calamus against middle cerebral artery occlusion induced ischemia in rats. Hum Exp Toxicol 25:187–194
Soska V, Olsovsky J, Zechmeister A, Lojek A, Bouda J, Garcia Escamilla RM (1997) Free oxygen radicals and lipoperoxides in type 2 diabetic patients. Revisions Mexicana Pathologists Clinicians 44:62–66
Spranger M, Krempien S, Schwab S, Donneberg S, Hacke W (1997) Superoxide dismutase activity in serum of patients with acute cerebral ischemic injury: correlation with clinical course and infarct size. Stroke 28:2425–2428
Takai Y, Hossayamada Y, Kato T (1991) Effect of water soluble and water insoluble fractions of Spirulina over serum lipids and glucose resistance of rats. J Jpn Soc Nutr Food Sci 44:273–277
Thaakur SR, Jyothi B (2007) Effect of Spirulina maxima on the haloperidol induced tardive dyskinesia and oxidative stress in rats. J Neural Transm 114:1217–1225
Thaakur SR, Pushpa B (2008) Effect of Spirulina on Phynotoin induced selected behavioural abnormalities and regional brain lipid peroxidation in rats. Int J Neuro Degeneration Regeneration 4:263–272
Thiyagarajan M, Sharma SS (2004) Neuroprotective effect of Curcumin in middle cerebral artery occlusion induced focal cerebral ischemia in rats. Life Sci 74:969–985
Upasani CD, Khera A, Balaraman R (2001) Effect of lead with vitamin E, C or Spirulina on malondialdehyde, conjugated dienes and hydroperoxides in rats. Indian J Exp Biol 39:70–74
Viglino P, Scara M, Rotilio G, Rigo A (1988) A kinectic study of the reactions between H2O2 and Cu, Zn superoxide dismutase, evidence for an electrostatic control of the reaction rate. Biochem Biophys Acta 952:77–82
Wang Y, Chang CF, Chou J, Chen HL, Deng X, Harvey BK, Cadet JL, Bickford PC (2005) Dietary supplementation with blue berries, spinach or spirulina reduces ischemic brain damage. Exp Neurol 193:75–84
Yang GY, Pang L, Ge HL, Tan M, Ye W, Liu XH, Huang FP, Wu DC, Che XM, Song Y, Wen R, Sun Y (2001) Attenuation of ischemia-induced mouse brain injury by SAG, a redox-inducible antioxidant protein. J Cereb Blood Flow Metab 21:722–733
Yanpallewar SU, Rai S, Kumar M, Acharya SB (2004) Evaluation of antioxidant and neuroprotective effect of Ocimum sanctum on transient cerebral ischemia and long term cerebral hypoperfusion. Pharmacol Biochem Behav 79:155–164
Yao JK, Reddy R, Elhinny LG, Vankammen DP (1998) Effects of haloperidol on antioxidant defense system enzymes in schizophrenia. J Psychiatr Res 32:385–391
Zang C, Yang J (2006) Protective effects of isoliquiritigenin in transient middle cerebral artery occlusion induced focal cerebral ischemia in rats. Pharmacol Res 55:303–309
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thaakur, S., Sravanthi, R. Neuroprotective effect of Spirulina in cerebral ischemia–reperfusion injury in rats. J Neural Transm 117, 1083–1091 (2010). https://doi.org/10.1007/s00702-010-0440-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-010-0440-5