Abstract
Neuregulin-1 (NRG1) gene is implicated in the etiology or neuropathology of schizophrenia, although its biological contribution to this illness is not fully understood. We have established an enzyme-linked immunosorbent assay (ELISA), which recognizes the NRG1β1 immunoglobulin-like (Ig) domain, and measured soluble Ig-NRG1 immunoreactivity in the sera of chronic schizophrenia patients (n = 40) and healthy volunteers (n = 59). ELISA detected remarkably high concentrations of Ig-NRG1 immunoreactivity in human serum (mean 5.97 ± 0.40 ng/mL, ~213 ± 14 pM). Gender and diagnosis exhibited significant effects on serum Ig-NRG1 immunoreactivity. Mean Ig-NRG1 immunoreactivity in the schizophrenia group was 63.2% of that measured in the control group. Ig-NRG1 immunoreactivity in women was 147.1% of that seen in men. We also attempted to correlate six SNPs of NRG1 genome with serum Ig-NRG1 immunoreactivity. Analysis of covariance with compensation for gender identified a significant interaction between diagnosis and SNP8NRG243177 allele. The T allele of this SNP significantly contributed to the disease-associated decrease in Ig-NRG1 immunoreactivity. Although we hypothesized a chronic influence of antipsychotic medications, there was no significant effect of chronic haloperidol treatment on serum Ig-NRG1 immunoreactivity in monkeys. These findings suggest that serum NRG1 levels are decreased in patients with chronic schizophrenia and influenced by their SNP8NRG243177 alleles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many studies have indicated a genetic linkage between the human chromosome locus 8p21-p12 and schizophrenia (Blouin et al. 1998; Kendler et al. 1996; Pulver et al. 1995). Stefansson et al. (2002) first reported that the neuregulin-1 (NRG1) gene, which resides in this genomic locus, is associated with the vulnerability to schizophrenia. Subsequent studies have confirmed the genetic association with NRG1 throughout different countries and populations (Addington et al. 2007; Corvin et al. 2004; Fukui et al. 2006; Gardner et al. 2006; Harrison and Weinberger 2005; Li et al. 2004; Tang et al. 2004; Thomson et al. 2007; Williams et al. 2003; Yang et al. 2003), although there are several contradictory reports (Ikeda et al. 2008; Iwata et al. 2004; Munafò et al. 2006; Thiselton et al. 2004). Postmortem studies support a genetic contribution of NRG1 to schizophrenia (Hashimoto et al. 2004; Law et al. 2006). The expression of mRNAs encoding NRG1 precursors is altered in schizophrenia patients; in particular, the type-I splice variants of NRG1 mRNA are upregulated in the prefrontal cortex and hippocampus of affected patients (Hashimoto et al. 2004; Law et al. 2006). Although studies have attempted to correlate abnormalities in NRG1 mRNA expression with patient single nucleotide polymorphism (SNP) haplotype (Law et al. 2006), controversy surrounds the use of postmortem samples, especially for mRNA analysis (Chagnon et al. 2008; Harrison et al. 1995; Morrison-Bogorad et al. 1995; Tomita et al. 2004). Terminal conditions, such as coma and hypoxia, might influence NRG1 gene expression and RNA quality in the brain (Chagnon et al. 2008; Harrison et al. 1995; Morrison-Bogorad et al. 1995; Tomita et al. 2004).
mRNA and protein measurements in blood are often used to diagnose schizophrenia or investigate the pathological contribution of individual molecules (Chagnon et al. 2008; Petryshen et al. 2005; Zhang et al. 2008). Of the molecules examined, growth factors and cytokines displayed marked abnormalities in both central nervous system and peripheral blood (Bellon 2007; Futamura et al. 2002; Sei et al. 2007; Takahashi et al. 2000; Toyooka et al. 2002, 2003). mRNA expression levels for NRG1 precursors are determined by evaluation of peripheral lymphocytes from schizophrenia patients (Chagnon et al. 2008; Petryshen et al. 2005; Zhang et al. 2008). However, NRG1 peptide levels in peripheral blood have not yet been studied. The production and release of mature NRG1 peptides require proteolytic processing of precursors (i.e., ectodomain shedding) (Marchionni et al. 1993; Shirakabe et al. 2001; Yokozeki et al. 2007). This process liberates mature NRG1 peptides from the cell-anchored precursor proteins, allowing it to activate ErbB receptors. Thus, the measurement of free mature NRG1 peptides is necessary to estimate the biological activity.
Here, we established an enzyme-linked immunosorbent assay (ELISA) for NRG1 and measured trace NRG1-like immunoreactivities (LI) in the cell-free soluble fraction of human serum. ELISA allowed us to estimate the effects of gender, age and schizophrenia diagnosis, disease duration and antipsychotic treatment on human serum NRG1 peptide levels. We also attempted to estimate the contribution of SNPs in NRG1 genome to serum NRG1-LI levels.
Materials and methods
Subjects
This clinical study was approved by the Ethics Committee on Genetics of the Niigata University School of Medicine. Written informed consent was obtained from all participants. Patients meeting the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) criteria for schizophrenia were recruited from two hospitals. The diagnosis of schizophrenia was based on all available sources of information, including unstructured interviews, clinical observations and medical records. Control subjects were recruited primarily from the staff of participating hospitals and associated laboratories. We matched the ages and genders of the control healthy volunteers to those of the patients examined. Although these subjects were not assessed by a structured psychiatric interview, all of them demonstrated good social and occupational skills and did not report any history of psychiatric disorders.
Three young adult male cynomolgus monkeys (Macaca fascicularis), 4 years of age, weighing 3.12–3.98 kg, were used in this study. Monkeys, reared at the animal house of Shinn Nippon Biomedical Lab. Inc. (Kagoshima, Japan), were housed individually in stainless steel cages of 50 cm (W) × 86 cm (D) × 82 cm (H) under temperature-controlled conditions, at 26 ± 2°C and a humidity of 40–60% under a 12/12-h light/dark cycle. Animals were fed 108 g commercial monkey chow daily. Filtered water was delivered by an automatic supplier ad libitum. Experiments were subjected to review by the Ethical Committee of Shinn Nippon Biomedical Lab. Inc., and were performed in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals and the Guidelines of the Central Research Laboratory.
Blood sampling
Blood was collected in vacuum collection tubes (Neotube, NP-PS0507, Nipro, Osaka, Japan) between 9 and 12 a.m. in the morning. Within 1 h of collection, blood was coagulated at 37°C for 60 min. Serum was separated by centrifugation at 4°C for 15 min and stored at −80°C until use for analysis.
NRG enzyme immunoassay
We produced anti-NRG1β1 polyclonal antibodies by immunizing rabbits and guinea pigs with a mouse cerebellar isoform of NRG1β1 peptide (Ozaki et al. 2000). This type-I isoform, with a molecular weight of 25,500 Da, consists of an immunoglobulin-like (Ig) domain and an epidermal growth factor (EGF)-like domain alone. Rabbit and guinea pig antisera were subjected to antigen-affinity chromatography (Affi-Gel 10, Bio-Rad, Hercules, CA, USA). We established ELISA using the affinity-purified antibodies according to the previous procedures (Nawa et al. 1995).
In brief, ELISA titer plates were coated with 100 ng rabbit anti-NRG1β1 antibody per well in 0.1 M Tris buffer (pH 9.0) for 12–18 h and then blocked with ELISA buffer [50 mM Tris, 0.5 M NaCl, 0.1% NaN3, 0.2% Triton X-100, 1% gelatin (pH 6.8)] at 4°C for more than 12 h. Serum (100 μL; quadruplicate) or standard NRG1β3 (10–1,000 pg; quadruplicate) was loaded into wells. After five washes, each well was incubated with 30 ng of guinea pig anti-NRG1β1 antibody followed by rabbit anti-guinea pig Ig biotinylated antibody (1:5,000; Open Biosystems, Huntsville, AL, USA). Biotinylated antibodies bound to wells were incubated with 100 μL avidin-β-galactosidase (1:10,000; Rockland Immunochemicals Inc., Gilbertsville, PA, USA) followed by the fluorogenic substrate, 200 μM 4-methylumbelliferyl-β-d-galactoside (MUG; Sigma Chemicals, St. Louis, MO, USA). The fluorescent product was quantitated using an MTP-601F microplate reader (Corona, Ibaraki, Japan) with excitation and emission at 365 and 450 nm, respectively. To evaluate the cross-reactivity of ELISA, we obtained the following human recombinant factors that exhibit structural homology or similarity to NRG1: betacellulin, EGF, heparin-binding EGF-like growth factor (HB-EGF), epiregulin and transforming growth factor-α (TGFα) (Peprotech, Rocky Hill, NJ, USA, or Sigma Chemicals, St. Louis, MO, USA). The mature human NRG1β3 peptides, which correspond to the respective extracellular domains of NRG1 precursors (Genbank; NM_013956.3 for type I, NM_013962.2 for type II, and NM_013959.3 for type III), were synthesized by an in vitro translation/transcription system (TNT Quick Coupled transcription/Translation Systems; Promega, Addison, WI, USA) using synthetic cDNAs encoding the corresponding domains (GenScript, Piscataway, NJ, USA). The amount of each type of NRG1β3 peptides was determined by immunoblotting for their histidine tag (R. Wang, unpublished data).
Genotyping
DNA was extracted from blood clots using Puregene core kit A (Qiagen, Germantown, MD, USA). We selected six SNPs from the human NRG1 genome, which have been intensively characterized previously, to investigate rs35753505 (SNP8NRG221533), SNP8NRG241930, rs6994992 (SNP8NRG243177), rs1081062, rs3924999 and rs2954041 (Ikeda et al. 2008). These six SNPs were genotyped using the TaqMan assay as described previously (Fukui et al. 2006).
Chronic treatment of cynomolgus monkeys with haloperidol
Young adult monkeys (all male) were given oral haloperidol for 2 months. Haloperidol (Wako Chemical Ltd, Tokyo, Japan), suspended in 0.5% methylcellulose solution, was administered at concentrations of 0.125–0.25 mg/mL to monkeys daily with the aid of a gastric tube for 8 weeks. The initial dose of 0.25 mg/kg for 2 weeks was increased to 0.5 mg/kg for the following 6 weeks. We confirmed normal food consumption daily and monitored body weight gain weekly to avoid adverse effects of haloperidol treatment. Whole blood (5 mL/animal) was collected from the femoral vein before treatment and after 4 and 8 weeks of haloperidol treatment. Serum samples were prepared to measure haloperidol concentrations as well as Ig-NRG-LI levels.
Statistical analysis
To analyze multifactorial interactions and ascertain statistical power, we applied parametric analyses to data. Genotype deviation from Hardy–Weinberg equilibrium (HWE) and allele frequency difference between patients and controls were evaluated by using the χ2 test. ELISA data were initially analyzed with the analysis of variance (ANOVA) using gender, disease, DSM-IV type and/or genotype as between-subject factors. The correlations between each potential confounding factor and Ig-NRG1-LI levels were examined by Pearson correlation analysis. The putative confounding factors included age, gender, duration of illness, age at onset and dose of antipsychotic medication. As we identified a significant and strong correlation of ELISA data with gender in the initial analysis, data were all re-analyzed using analysis of covariance (ANCOVA), adopting gender as a covariate. We used Fisher least significant difference (LSD) test for post hoc analysis. A probability level of P < 0.05 was considered to be statistically significant. All data represent the mean ± SE. Statistical analysis was performed using SPSS software (version 11.5).
Results
Establishment of a sandwich ELISA for NRG1
We raised antisera directed against recombinant mouse NRG1β1 (type I, soluble mature form) in rabbits and guinea pigs and established a sandwich ELISA for NRG1 (Nawa et al. 1995). The combination of these antibodies in ELISA generated a linear standard curve for concentrations of 10–1,000 pg/well for a soluble form of human NRG1β3 (Fig. 1). As NRG1 structurally belongs to the EGF family, we tested the cross-reactivity of this ELISA system to other members in this family, including EGF, HB-EGF, TGFα, betacellulin and epiregulin (Table 1). The cross-reactivity to these factors was <0.1% of the signal for type-I human NRG1β3. We also tested the reactivity of ELISA to the EGF domain of human NRG1 as well as other splice variants of human NRG1. Reactivity to the EGFβ1 domain was negligible (<0.01%), and that to the type-II and type-III variants was 61.0 and 7.2% of the levels seen for type-I NRG1 used as a standard, respectively. These results suggest that this ELISA primarily recognizes not the common EGF domain, but the Ig-like domain of NRG1, which is present in type-I and type-III NRG1, but not type-II NRG1. Using this novel ELISA, we measured Ig-like domain-containing NRG1-like immunoreactivity (Ig-NRG-LI) in human serum.
A standard curve of ELISA with control type-1 NRG-1 peptide. Various amounts of human recombinant NRG1β3 (10–1,000 pg/well/100 μL) were applied to ELISA as a standard. Total immunoreactivity for NRG1 trapped by the primary antibody was determined using the guinea pig secondary anti-NRG1β1 antibody followed by detection with a β-galactosidase-conjugated antibody against guinea pig immunoglobulin. Enzymatic activity in each well was measured using the fluorogenic substrate MUG. The enzymatic product derived from MUG exhibits fluorescence at 450 nm with 365 nm excitation
NRG1 immunoreactivity in sera of schizophrenia patients and control subjects
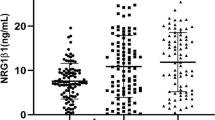
We obtained sera from schizophrenia patients (n = 40) and age- and gender-matched healthy volunteers (n = 59) (Table 2). The time of blood sampling, type of sampling tube and coagulation procedures were consistent between patients to minimize the influence of differences in sampling conditions (see “Materials and methods”). Human serum samples from healthy volunteers contained Ig-NRG-LI with concentrations ranging from 0.4 to 14.0 ng/mL (Fig. 2). The concentrations in healthy women samples (mean 8.02 ± 1.33 ng/mL) were significantly higher than those in men (mean 5.61 ± 0.91 ng/mL, P = 0.048). Two-way ANOVA using subject factors of diagnosis (patient vs. control) and gender (men vs. women) revealed main effects for both diagnosis (F (1,95) = 11.50; P = 0.001) and gender (F (1,95) = 9.23; P = 0.003) without an interaction between these two factors (F (1,95) = 0.24; P = 0.627). Mean Ig-NRG1 immunoreactivity in the schizophrenia group was 63.2% of the mean level observed in the control group. Mean Ig-NRG1 immunoreactivity in the women’s group was 147.1% of that detected in the men’s group.
Serum Ig-NRG1-LI levels in patients with schizophrenia and age-matched controls. Serum samples were collected from 40 patients with schizophrenia (n = 20 for men and n = 20 women) and 59 healthy volunteers (n = 28 for men and n = 31 women) and subjected to ELISA to detect Ig-NRG-LI. Mean levels are marked with thick horizontal lines, while SE levels are denoted by horizontal broken lines. Post hoc analysis detected a significant difference between groups; **P < 0.01 and *P < 0.05
To estimate the influence of patient clinical features on Ig-NRG-LI levels, we performed Pearson correlation analysis with compensation for gender effect (Table 3). There were modest correlations of both age (R = 0.340; P = 0.032) and disease onset (R = 0.333; P = 0.036) with Ig-NRG-LI. Neither disease duration nor medication length, however, correlated significantly with Ig-NRG-LI. As the prescribed antipsychotics differed between patients, we calculated chlorpromazine-equivalent doses based on an antipsychotic potency table of commercial drugs (Inada 2006) (Table 2). We did not find a significant correlation between chlorpromazine-equivalent dose and Ig-NRG-LI level (R = −0.235; P = 0.144). Although we also attempted to assess an interaction between DSM-IV category and Ig-NRG-LI, one-way ANOVA failed to detect a significant difference in Ig-NRG-LI levels among DSM-IV types (F (2,39) = 0.15; P = 0.865).
Association between NRG1 immunoreactivity and SNP types
We examined the six SNP sites within the NRG1 gene that are reported to be associated with schizophrenia risk: SNP8NRG221533, SNP8NRG241930, SNP8NRG243177, rs1081062, rs3924999 and rs2954041 (Bakker et al. 2004; Fukui et al. 2006; Hall et al. 2006; Ikeda et al. 2008; Iwata et al. 2004; Law et al. 2006; Li et al. 2004, 2006; McIntosh et al. 2008; Munafò et al. 2006; Nicodemus et al. 2009; Stefansson et al. 2002; Williams et al. 2003; Yang et al. 2003). The genotype distributions of the SNPs examined did not differ significantly from HME in either group (patient: P = 0.203–0.747, control: P = 0.096–0.678), with the exception of SNP8NRG221533 (patient: P = 0.016, control: P = 0.052; χ2 test). With the given sample sizes, we failed to detect any significant difference in genotype distributions at all SNP loci between patient and control groups (P = 0.073–0.90; χ2 test). As there were significant gender differences in Ig-NRG-LI, we adopted ANCOVA with a covariate of gender to estimate SNP effects.
At the site of SNP8NRG243177, two-way ANCOVA revealed a main effect of diagnosis (F (1,93) = 4.78; P = 0.031), but not that of genotype (F (2,93) = 2.00; P = 0.141), with an significant interaction between the two subject factors (F (2,93) = 4.43; P = 0.015), suggesting differential contribution of genotype to the disease-associated decreases in Ig-NRG1-LI levels (Fig. 3). Post hoc analysis revealed that patients with the SNP T allele (T/T or C/T) exhibited lower levels of Ig-NRG-LI than schizophrenia patients bearing the homozygous C allele (P = 0.038 for T/T vs. C/C, P = 0.016 for T/C vs. C/C) or controls with the same allele (P = 0.025 for T/T, P = 0.0003 for T/C). The allelic difference in serum Ig-NRG1-LI levels was not observed in the control group. Thus, these results suggest that the T allele at SNP8NRG243177 is one of the biological contributors to the disease-associated decreases in serum Ig-NRG-LI levels. We also analyzed the influences of the other SNP genotypes on serum Ig-NRG-LI. However, there were no significant differences detected at these SNP loci (F (2,93) = 0.43–2.28; P = 0.108–0.645) without interaction (F (2,93) = 0.28–4.70; P = 0.098–0.753).
Association between SNP8NRG243177 and serum Ig-NRG1-LI. Serum Ig-NRG-LI levels detected in individuals with each SNP genotype (SNP8NRG243177; T/T, C/T, C/C) were plotted for the control and patient groups. Mean levels are marked with thick horizontal lines, while SE levels are denoted by horizontal broken lines. *P < 0.05, compared between genotypes of patient group, and ### P < 0.001 and # P < 0.05, compared between patient and control groups of the same genotype
Influence of chronic haloperidol administration to monkeys
To estimate the potential influence of antipsychotic medications given to patients with schizophrenia, we administered haloperidol (0.25–0.50 mg/kg) to three cynomolgus monkeys for 2 months and monitored Ig-NRG1-LI levels in serum before and during treatment. Serum concentrations of haloperidol were 4.00 ± 0.62 ng/mL after 4 weeks of treatment and 4.03 ± 0.52 ng/mL after 8 weeks of treatment. Mean levels of IG-NRG1-LI were 7.0 ± 2.1, 7.5 ± 2.6 and 8.4 ± 2.9 ng/mL before and 4 and 8 weeks after beginning treatment, respectively. One-way ANOVA failed to detect any significant difference during drug treatment (F (2,5) = 4.07; P = 0.865).
Discussion
To evaluate the pathological influences of schizophrenia on NRG1 protein, we established ELISA for NRG1 and measured NRG1 immunoreactivity in serum. We found that the human sera contains high concentrations of Ig-NRG1-LI and that the concentrations are significantly lower in men as well as in patients with schizophrenia. In addition, the schizophrenia-associated decrease in serum Ig-NRG1-LI is apparent only in patients carrying the schizophrenia risk allele at the SNP8NRG243177 site.
Neuregulin-1 variants possess a common EGF-like core domain that is responsible for its biological activity, thus sharing significant structural similarity with other EGF-like peptides. It was thus important to evaluate the cross-reactivity of the ELISA system with other EGF-like peptides. Despite careful examination of six distinct peptides in the EGF family, none exhibited significant cross-reactivity. As multiple gene promoters and alternative splicing produce distinct structural variants of NRG1 precursors (Falls 2003; Harrison and Law 2006), it was essential to determine which NRG1 subtypes were recognized by this ELISA system. The antigen used to raise antibodies was a soluble mature form of type-1 NRG1β1 derived from mouse cerebellum, which contains only an Ig-like domain and EGF-like domain (Ozaki et al. 2004). Resulting antibodies derived from rabbits and guinea pigs failed to react with the EGF-like domain, leading to the assumption that these antibodies primarily recognize the Ig-like domain of NRG1. In support of this assumption, ELISA detected type-II NRG1, which contains the Ig domain with high efficiency. Reactivity to type-III NRG1, which lacks the Ig-like domain, was <10% of that to type-I NRG1.
Several clinical features of schizophrenia patients exhibited modest, but significant, correlations with Ig-NRG-LI levels using our novel ELISA. Serum Ig-NRG-LI levels weakly correlated with age (R = 0.340) and disease onset (R = 0.333) following gender compensation. To confirm the statistical results of ANOVA, we also performed ANCOVA, adopting each of these factors individually or together as covariate(s), and yet obtained the same statistical conclusion (data not shown). It is noteworthy that our statistical analysis did not detect a significant correlation between antipsychotic medication and serum Ig-NRG-LI levels, although calculating chlorpromazine equivalents of second-generation antipsychotics is controversial among researchers (Woods 2003; Inada 2006). This statistical result of antipsychotic effects appeared to be supported by the present monkey experiment: the chronic treatment of monkeys with haloperidol had no significant effect on serum Ig-NRG1-LI. Pharmaceutical effectiveness of haloperidol in monkeys was controlled by measuring its blood concentrations. Concentrations of haloperidol in monkey serum corresponded to the human therapeutic range of the antipsychotic medication (3–17 ng/mL) (Guthrie et al. 1987). As the given species of antipsychotics to monkeys and patients were not fully identical, however, the interpretation of the present monkey results is limited and needs to be re-evaluated in drug-naïve schizophrenia patients.
In the present assay, we found that human serum contains markedly high concentrations of Ig-NRG1-LI (mean 5.97 ± 0.40 ng/mL, ~213 ± 14 pM) in comparison to the concentrations of other growth factors and cytokines. Concentrations of EGF, HB-EGF and TGFα are 100–400 pg/mL in human serum (Futamura et al. 2002), and those of inflammatory cytokines such as interleukin-1, interleukin-6 and tumor necrosis factor alpha are also below 1 ng/mL (Akiyama 1999; Schmitt et al. 2005). Our preliminary study indicates that the concentrations of Ig-NRG1-LI in human plasma were as high as in serum (M. Shibuya, unpublished data). As NRG-1 concentrations in human blood are above the reported dissociation constant of the NRG-1 receptor (k d; ~60 pM for ErbB3) (Carraway et al. 1994), human NRG-1 peptides in circulation presumably exert a biological activity or influences on tissue targets.
There was a significant gender difference in serum Ig-NRG-LI levels between men and women. Ig-NRG-LI quantities in females were significantly higher than those observed in males, irrespective of the presence of schizophrenia. This trend contrasts the fact that another neurotrophic factor, nerve growth factor, is enriched in the serum of human males (Martocchia et al. 2002). LaCroix-Fralish et al. (2006, 2008) report that protein and mRNA expression levels of NRG1 are higher in female rats. As there were no significant gender differences in NRG1 mRNA levels observed in postmortem brains (Hashimoto et al. 2004), this gender difference may be limited to NRG1 expression in the peripheral blood.
Although there are several studies examining the expression of NRG1 mRNA in postmortem brains of patients with schizophrenia, most have reported an increase in mRNA levels. The hippocampus and prefrontal cortex of schizophrenia patients contain higher levels of type-I mRNA than samples from control subjects (Hashimoto et al. 2004; Law et al. 2006). Petryshen et al. (2005) report an increase in mRNA encoding type-III NRG1 precursor (SMDF), while Zhang et al. (2008) detect decreases in type-I and type-II mRNAs encoding NRG1 precursors (HRG-β3 and GGF2) in patient lymphocytes. The latter report is consistent with our present findings on NRG1 expression in blood, assuming that our novel ELISA primarily detected the type-I and type-II NRG1 peptides.
The schizophrenia-associated SNP sites within the NRG1 gene are located in the 5′-flanking region of the gene promoter (Harrison and Law 2006; Lawrie et al. 2008; Mei and Xiong 2008). Of the six SNPs examined, patients with the T allele at the SNP8NRG243177 site displayed a reduction in serum Ig-NRG-LI levels. Brain imaging studies reveal that the T allele is associated with the reduction of white matter density in the anterior limb of the internal capsule (McIntosh et al. 2008). This finding is in agreement with the fact that NRG1 is a positive regulator for myelination and oligodendrocyte survival (Fernandez et al. 2000; Corfas et al. 2004). In this context, it is possible that the serum reduction in Ig-NRG1-LI might contribute to the white matter deficits of schizophrenia patients.
This SNP site is located at the 5′ region of transcription initiation sites of type-II and type-IV mRNAs, which both encode Ig-NRG1 (Li et al. 2006). The T allele-specific reduction in Ig-NRG-LI was only found in the patient group and is therefore consistent with the finding that this allele is associated with schizophrenia susceptibility (Law et al. 2006; Steinthorsdottir et al. 2004). It is unknown, however, whether the allele-dependent Ig-NRG-LI reduction is ascribed to the difference in basal or regulated transcription rates of NRG1 gene (Tan et al. 2007). A postmortem brain study combining RT-PCR for NRG mRNAs and SNP typing comes to a conclusion in conflict with our findings. The hippocampus of human subjects bearing the risk allele expresses higher levels of type-IV variants of NRG1 mRNA, irrespective of the diagnosis (Law et al. 2006). Although it remains to be determined whether NRG1 gene transcription is under the same regulation in both periphery and brain, the answer to this discrepancy awaits future experiments with a larger number and the combination of peripheral and brain samples. We hope that the ELISA system for NRG1 peptide will help to explore its biological role in schizophrenia pathogenesis or diagnosis.
Abbreviations
- NRG:
-
Neuregulin
- SNP:
-
Single-nucleotide polymorphism
- ELISA:
-
Enzyme-linked immunosorbent assay
- LI:
-
Like immunoreactivity
- ANOVA:
-
Analysis of variance
- ANCOVA:
-
Analysis of covariance
References
Addington AM, Gornick MC, Shaw P, Seal J, Gogtay N, Greenstein D et al (2007) Neuregulin 1 (8p12) and childhood-onset schizophrenia: susceptibility haplotypes for diagnosis and brain developmental trajectories. Mol Psychiatry 12:195–205
Akiyama K (1999) Serum levels of soluble IL-2 receptor alpha, IL-6 and IL-1 receptor antagonist in schizophrenia before and during neuroleptic administration. Schizophr Res 37:97–106
Bakker SC, Hoogendoorn ML, Selton JP, Verduijn W, Pearson PL, Sinke RJ et al (2004) Neuregulin 1: genetic support for schizophrenia subtypes. Mol Psychiatry 9:1061–1063
Bellon A (2007) New genes associated with schizophrenia in neurite formation: a review of cell culture experiments. Mol Psychiatry 12:620–629
Blouin JL, Dombroski BA, Nath SK, Lasseter VK, Wolyniec PS, Nestadt G et al (1998) Schizophrenia susceptibility loci on chromosomes 13q32 and 8p21. Nat Genet 20:70–73
Carraway KL 3rd, Sliwkowski MX, Akita R, Platko JV, Guy PM, Nuijens A et al (1994) The erbB3 gene product is a receptor for heregulin. J Biol Chem 269:14303–14306
Chagnon YC, Roy MA, Bureau A, Mérette C, Maziade M (2008) Differential RNA expression between schizophrenic patients and controls of the dystrobrevin binding protein 1 and neuregulin 1 genes in immortalized lymphocytes. Schizophr Res 100:281–290
Corfas G, Roy K, Buxbaum JD (2004) Neuregulin 1-erbB signaling and the molecular/cellular basis of schizophrenia. Nat Neurosci 7:575–580
Corvin AP, Morris DW, McGhee K, Schwaiger S, Scully P, Quinn J et al (2004) Confirmation and refinement of an ‘at-risk’ haplotype for schizophrenia suggests the EST cluster, Hs.97362, as a potential susceptibility gene at the Neuregulin-1 locus. Mol Psychiatry 9:208–212
Falls DL (2003) Neuregulins: functions, forms, and signaling strategies. Exp Cell Res 284:14–30
Fernandez PA, Tang DG, Cheng L, Prochiantz A, Mudge AW, Raff MC (2000) Evidence that axon-derived neuregulin promotes oligodendrocyte survival in the developing rat optic nerve. Neuron 28:81–90
Fukui N, Muratake T, Kaneko N, Amagane H, Someya T (2006) Supportive evidence for neuregulin 1 as a susceptibility gene for schizophrenia in a Japanese population. Neurosci Lett 396:117–120
Futamura T, Toyooka K, Iritani S, Niizato K, Nakamura R, Tsuchiya K et al (2002) Abnormal expression of epidermal growth factor and its receptor in the forebrain and serum of schizophrenic patients. Mol Psychiatry 7:673–682
Gardner M, González-Neira A, Lao O, Calafell F, Bertranpetit J, Comas D (2006) Extreme population differences across Neuregulin 1 gene, with implications for association studies. Mol Psychiatry 11:66–75
Guthrie S, Lane EA, Linnoila M (1987) Monitoring of plasma drug concentrations in clinical psychopharmacology. In: Meltzer HY (ed) Psychopharmacology: the third generation of progress. Raven Press, New York, pp 1323–1338
Hall J, Whalley HC, Job DE, Baig BJ, Mclntosh AM, Evans KL et al (2006) A neuregulin 1 variant associated with abnormal cortical function and psychotic symptoms. Nat Neurosci 9:1477–1478
Harrison PJ, Law AJ (2006) Neuregulin 1 and schizophrenia: genetics, gene expression, and neurobiology. Biol Psychiatry 60:132–140
Harrison PJ, Weinberger DR (2005) Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry 10:40–68
Harrison PJ, Heath PR, Eastwood SL, Burnet PWJ, McDonald B, Pearson RCA (1995) The relative importance of premortem acidosis and postmortem interval for human brain gene expression studies: selective mRNA vulnerability and comparison with their encoded proteins. Neurosci Lett 200:151–154
Hashimoto R, Straub RE, Weickert CS, Hyde TM, Kleinman JE, Weinberger DR (2004) Expression analysis of neuregulin-1 in the dorsolateral prefrontal cortex in schizophrenia. Mol Psychiatry 9:299–307
Ikeda M, Takahashi N, Saito S, Aleksic B, Watanabe Y, Nunokawa A et al (2008) Failure to replicate the association between NRG1 and schizophrenia using Japanese large sample. Schizophr Res 101:1–8
Inada T (2006) Pharmacologic treatment of schizophrenia: Summarized in various guidelines and algorithms. Aruta Shuppan Co, Tokyo
Iwata N, Suzuki T, Ikeda M, Kitajima T, Yamanouchi Y, Inada T et al (2004) No association with the neuregulin 1 haplotype to Japanese schizophrenia. Mol Psychiatry 9:126–127
Kendler KS, MacLean CJ, O’Neill FA, Burke J, Murphy B, Duke F et al (1996) Evidence for a schizophrenia vulnerability locus on chromosome 8p in the Irish Study of High-Density Schizophrenia Families. Am J Psychiatry 153:1534–1540
LaCroix-Fralish ML, Tawfik VL, Spratt KF, DeLeo JA (2006) Sex differences in lumbar spinal cord gene expression following experimental lumbar radiculopathy. J Mol Neurosci 30:283–295
LaCroix-Fralish ML, Tawfik VL, Nutile-McMenemy N, Deleo JA (2008) Neuregulin 1 is a pronociceptive cytokine that is regulated by progesterone in the spinal cord: implications for sex specific pain modulation. Eur J Pain 12:94–103
Law AJ, Lipska BK, Weickert CS, Hyde TM, Straub RE, Hashimoto R et al (2006) Neuregulin 1 transcripts are differentially expressed in schizophrenia and regulated by 5′ SNPs associated with the disease. Proc Natl Acad Sci USA 103:6747–6752
Lawrie SM, Hall J, McIntosh AM, Cunningham-Owens DG, Johnstone EC (2008) Neuroimaging and molecular genetics of schizophrenia: pathophysiological advances and therapeutic potential. Br J Pharmacol 153:S120–S124
Li T, Stefansson H, Gudfinnsson E, Cai G, Liu X, Murray RM et al (2004) Identification of a novel neuregulin 1 at-risk haplotype in Han schizophrenia Chinese patients, but no association with the Icelandic/Scottish risk haplotype. Mol Psychiatry 9:698–704
Li D, Collier DA, He L (2006) Meta-analysis shows strong positive association of the neuregulin 1 (NRG1) gene with schizophrenia. Hum Mol Genet 15:1995–2002
Marchionni MA, Goodearl AD, Chen MS, Bermingham-McDonogh O, Kirk C, Hendricks M et al (1993) Glial growth factors are alternatively spliced erbB2 ligands expressed in the nervous system. Nature 362:312–318
Martocchia A, Sigala S, Proietti A, D’Urso R, Spano PF, Missale C et al (2002) Sex-related variations in serum nerve growth factor concentration in humans. Neuropeptides 36:391–395
McIntosh AM, Moorhead TWJ, Job D, Lymer GKS, Munoz Maniega S, McKirdy J et al (2008) The effects of a neuregulin 1 variant on white matter density and integrity. Mol Psychiatry 13:1054–1059
Mei L, Xiong WC (2008) Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci 9:437–452
Morrison-Bogorad M, Zimmerman AL, Pardue S (1995) Heat-shock 70 messenger RNA levels in human brain: correlation with agonal fever. J Neurochem 64:235–246
Munafò MR, Thiselton DL, Clark TG, Flint J (2006) Association of the NRG1 gene and schizophrenia: a meta-analysis. Mol Psychiatry 11:539–546
Nawa H, Carnahan J, Gall C (1995) BDNF protein measured by a novel enzyme immunoassay in normal brain and after seizure: partial disagreement with mRNA levels. Eur J Neurosci 7:1527–1535
Nicodemus KK, Law AJ, Luna A, Vakkalanka R, Straub RE, Kleinman JE et al (2009) A5′ promoter region SNP in NRG1 is associated with schizophrenia risk and type III isoform expression. Mol Psychiatry 14:741–743
Ozaki M, Tohyama K, Kishida H, Buonanno A, Yano R, Hashikawa T (2000) Roles of neuregulin in synaptogenesis between mossy fibers and cerebellar granule cells. J Neurosci Res 59:612–623
Ozaki M, Itoh K, Miyakawa Y, Kishida H, Hashikawa T (2004) Protein processing and releases of neuregulin-1 are regulated in an activity-dependent manner. J Neurochem 91:176–188
Petryshen TL, Middleton FA, Kirby A, Aldinger KA, Purcell S, Tahl AR et al (2005) Support for involvement of neuregulin 1 in schizophrenia pathophysiology. Mol Psychiatry 10:366–374
Pulver AE, Lasseter VK, Kasch L, Wolyniec P, Nestadt G, Blouin JL et al (1995) Schizophrenia: a genome scan targets chromosomes 3p and 8p as potential sites of susceptibility genes. Am J Med Genet 60:252–260
Schmitt A, Bertsch T, Tost H, Bergmann A, Henning U, Klimke A et al (2005) Increased serum interleukin-1beta and interleukin-6 in elderly, chronic schizophrenic patients on stable antipsychotic medication. Neuropsychiatr Dis Treat 1:171–177
Sei Y, Ren-Patterson R, Li Z, Tunbridge EM, Egan MF, Kolachana BS et al (2007) Neuregulin1-induced cell migration is impaired in schizophrenia: association with neuregulin1 and catechol-o-methyltransferase gene polymorphisms. Mol Psychiatry 12:946–957
Shirakabe K, Wakatsuki S, Kurisaki T, Fujisawa-Sehara A (2001) Roles of Meltrin beta/ADAM19 in the processing of neuregulin. J Biol Chem 276:9352–9358
Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S et al (2002) Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet 71:877–892
Steinthorsdottir V, Stefansson H, Ghosh S, Birgisdottir B, Bjornsdottir S, Fasquel AC et al (2004) Multiple novel transcription initiation sites for NRG1. Gene 342:97–105
Takahashi M, Shirakawa O, Toyooka K, Kitamura N, Hashimoto T, Maeda K et al (2000) Abnormal expression of brain-derived neurotrophic factor and its receptor in the corticolimbic system of schizophrenic patients. Mol Psychiatry 5:293–300
Tan W, Wang Y, Gold B, Chen J, Dean M, Harrison PJ et al (2007) Molecular cloning of a brain-specific, developmentally regulated neuregulin1 (NRG 1) isoform and identification of a functional promoter variant associated with schizophrenia. J Biol Chem 282:24343–24351
Tang JX, Chen WY, He G, Zhou J, Gu NF, Feng GY et al (2004) Polymorphisms within 5′ end of the neuregulin 1 gene are genetically associated with schizophrenia in the Chinese population. Mol Psychiatry 9:11–12
Thiselton DL, Webb BT, Neale BM, Ribble RC, O’Neill FA, Walsh D et al (2004) No evidence for linkage or association of neuregulin-1 (NRG1) with disease in the Irish study of high-density schizophrenia families (ISHDSF). Mol Psychiatry 9:777–783
Thomson PA, Christoforou A, Morris SW, Adie E, Pickard BS, Porteous DJ et al (2007) Association of Neuregulin 1 with schizophrenia and bipolar disorder in a second cohort from the Scottish population. Mol Psychiatry 12:94–104
Tomita H, Vawter MP, Walsh DM, Evans SJ, Choudary PV, Li J et al (2004) Effect of agonal and postmortem factors on gene expression profile: quality control in microarray analyses of postmortem human brain. Biol Psychiatry 55:346–352
Toyooka K, Asama K, Watanabe Y, Muratake T, Takahashi M, Someya T et al (2002) Decreased levels of brain-derived neurotrophic factor in serum of chronic schizophrenic patients. Psychiatry Res 110:249–257
Toyooka K, Watanabe Y, Iritani S, Shimizu E, Iyo M, Nakamura R et al (2003) A decrease in interleukin-1 receptor antagonist expression in the prefrontal cortex of schizophrenic patients. Neurosci Res 46:299–307
Williams NM, Preece A, Spurlock G, Norton N, Williams HJ, Zammit S et al (2003) Support for genetic variation in neuregulin 1 and susceptibility to schizophrenia. Mol Psychiatry 8:485–487
Woods SW (2003) Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry 64:663–667
Yang JZ, Si TM, Ruan Y, Ling YS, Han YH, Wang WL et al (2003) Association study of neuregulin 1 gene with schizophrenia. Mol Psychiatry 8:706–709
Yokozeki T, Wakatsuki S, Hatsuzawa K, Black RA, Wada I, Sehara-Fujisawa A (2007) Meltrin beta (ADAM19) mediates ectodomain shedding of Neuregulin beta1 in the Golgi apparatus: fluorescence correlation spectroscopic observation of the dynamics of ectodomain shedding in living cells. Genes Cells 12:329–343
Zhang HX, Zhao JP, Lv LX, Li WQ, Xu L, Ouyang X et al (2008) Explorative study on the expression of neuregulin-1 gene in peripheral blood of schizophrenia. Neurosci Lett 438:1–5
Acknowledgments
The authors thank the patients and healthy volunteers for their participation. This work was supported by Health and Labor Sciences Research Grants, a grant from the Promotion of Niigata University Research Projects, Core Research for Evolutional Science and Technology from the JST Corporation, and a grant-in-aid from the Ministry of Health, Labor and Welfare, Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shibuya, M., Komi, E., Wang, R. et al. Measurement and comparison of serum neuregulin 1 immunoreactivity in control subjects and patients with schizophrenia: an influence of its genetic polymorphism. J Neural Transm 117, 887–895 (2010). https://doi.org/10.1007/s00702-010-0418-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-010-0418-3