Abstract
The role of the ATP-gated receptor, P2X7, has been evaluated in the unilateral 6-OHDA rat model of Parkinson’s disease using the P2X7 competitive antagonist A-438079. Nigral P2X7 immunoreactivity was mainly located in microglia but also in astroglia. A-438079 partially but significantly prevented the 6-OHDA-induced depletion of striatal DA stores. However, this was not associated with a reduction of DA cell loss. Blockade of P2X7 receptors may represent a novel protective strategy for striatal DA terminals in Parkinson’s disease and warrants further future investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
P2X7 receptors are ATP-gated ion channels that are functional in homomeric forms and are only activated by high concentrations of ATP (>100 μM). In the CNS P2X7 receptors are localized to microglia and astrocytes (Sim et al. 2004); however, their localization to central nerve cells still remains controversial (Anderson and Nedergaard 2006).
Prolonged exposure to P2X7 agonists leads to the formation of large cytolytic pores in the plasma membrane of macrophages (Surprenant et al. 1996). The mechanism for this pore-forming activity has not yet been definitively clarified, but may develop through P2X7 receptor signaling events via non-selective hemi-channels that are built up of the protein Pannexin-1 (Pelegrin and Surprenant 2006). This mechanism is of interest to our hypothesis that P2X7 receptors may play a significant role in the degeneration of nigral DA nerve cells in Parkinson’s disease (PD) via disruption of ion homeostasis and release of interleukin-1β, among other things (Fuxe et al. 2006, 2009). Furthermore, a series of studies indicate that the P2X7 receptor plays a pathophysiological role in inflammatory states (Ferrari et al. 2006). In addition, studies using newly developed potent and selective P2X7 antagonists (Honore et al. 2006; Nelson et al. 2006) as well as studies involving P2X7 receptor knock-out mice (Chessell et al. 2005) have demonstrated a clear role of the P2X7 receptor in certain states of chronic pain.
We have postulated that these receptors may not only exist on nigral microglia and astroglia but possibly also on damaged nigral DA cells since cytolytic pores could be directly formed in their plasma membrane upon P2X7 stimulation and cause DA cell death. However, the neurodegeneration in PD could mainly be mediated by astrocyte-born or microglia-born signals released after activation of P2X7 receptors. In PD, these receptors may be activated by free oxygen radicals (radical oxygen species) and thereby increase DA nerve cell degeneration through its cytolytic pore formation activity in nigral glial and DA nerve cells (Fuxe et al. 2006, 2009). Recently in support of our hypothesis, extracellular ATP has been demonstrated to mediate necrotic cell swelling in SN4741 dopaminergic nerve cells obtained from the substantia nigra of transgenic mouse embryos through the activation of P2X7 receptors (Jun et al. 2007).
In this work we have therefore studied the cellular distribution of P2X7 immunoreactivity in the 6-OHDA-lesioned substantia nigra and tested whether the P2X7 receptor antagonist A-438079 (Nelson et al. 2006) can prevent unilateral 6-OHDA-induced injuries of nigrostriatal DA neurons (Ungerstedt 1968) as evaluated in a biochemical, immunohistochemical and stereological analysis. A-438079 is known to have sufficient bioavailability following intraperitoneal injection to establish the role of P2X7 receptors in disease models (Donnelly-Roberts and Jarvis 2007; Nelson et al. 2006).
Materials and methods
Animals
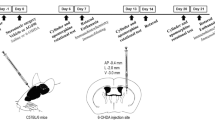
Sprague–Dawley male rats (8- to 12-week old) were used. All animals were housed for a minimum period of 7 days after their arrival to the animal facility before experimental procedures. Unilateral damage to the DA pathway (rat model for Parkinson’s disease; Ungerstedt 1968) was performed at the nigral (DA cell bodies and axons) level. The rats were anaesthetized with isoflurane by inhalation (3–5%) and placed into a stereotaxic frame. A drill with a diameter between 1 and 1.5 mm was used to open the skull and subsequent microinjections (using a Hamilton 702 syringe with a 0.15 mm inner diameter) were made using a low dose of the DA neurotoxin 6-OHDA (0.2 μg/4 μL) to produce a partial lesion of the nigral DA nerve cell group. The experiment was terminated 4 weeks after creating the 6-OHDA lesion.
The selective P2X7 antagonist A-438079 (30 mg/kg dissolved in saline, i.p.) (Tocris, Bristol, United Kingdom) (Nelson et al. 2006) was acutely injected two times, 60 min before and 60 min after microinjection of 6-OHDA in the substantia nigra, representing a dose range with demonstrated anti-inflammatory effects (Donnelly-Roberts and Jarvis 2007; Nelson et al. 2006). A group of lesioned animals was injected with only vehicle. Sham-lesioned rats were, in this manner, injected with either the P2X7 receptor antagonist or saline.
Determination of DA, DOPAC and HVA in brain tissue
The analysis of the damaged nigrostriatal DA neurons in these experiments involved determination of DA, DOPAC and HVA levels in the extracts of dissected tissue samples from dorsal striata by high-performance liquid chromatography (HPLC) with electrochemical detection as described elsewhere (Kehr and Yoshitake 2006) and the DA turnover was calculated as the ratio (DOPAC + HVA)/DA [for details, see (Aguirre et al. 2005)].
The HPLC system consisted of a LC-27A Degasser (ALS, Tokyo, Japan), an EP-300 pump (Eicom, Kyoto, Japan), a CMA/200 refrigerated microsampler (CMA Microdialysis, Stockholm, Sweden) equipped with 20 μL loop and operating at +4°C, and an ECD-300 chromatograph with an electrochemical detector (Eicom, Kyoto, Japan). Typically, 10 μL of the supernatant or the standard was injected into the HPLC column Eicompak SC-5ODS, 150 × 3.0 ID mm (Eicom, Kyoto, Japan). The separation column was protected with a guard column OPTI-GUARD C18 (Optimize Technologies, USA). The mobile phase was a mixture of methanol and 0.1 M acetic acid–citric acid buffer (pH 3.5) (16:84 v/v) containing 200 mg/L octanesulfonic acid sodium salt and 5 mg/L EDTA-2Na. The flow rate was 0.34 mL/min. The limit of detection (defined as signal-to-noise ratio >2) was 10 fmol/10 μL for DA.
Chemical anatomical analysis of the location of P2X7 immunoreactivity in nigral microglial, astroglial and DA nerve cells
In naïve, sham- and 6-OHDA-operated rats, with or without P2X7 antagonist treatment, immunohistochemical experiments on P2X7 immunoreactivity (IR) were performed in the substantia nigra after transcardial perfusion of animals with 4% paraformaldhyde under sodium pentobarbital (60 mg/kg, i.p.) anesthesia according to Rivera et al. (2006). The whole brain was removed and postfixed in the same fixative for 2 h at 4°C and subsequently cryoprotected in 30% sucrose in PBS for 48 h before being frozen on dry ice. Coronal sections (30 µm thick) were obtained using a freezing microtome (CM 1325; Leica, Wetzlar, Germany) and free-floating sections were processed for single or double immunohistochemical techniques.
Single labeling immunohistochemistry
Sections were pretreated for 15 min with 3% H2O2 and incubated overnight at RT with the rabbit polyclonal anti-P2X7 (Alomone Labs Ltd., Jerusalem, Israel) (1:500) or mouse anti-TH (Diasorin, Saluggia, Italy) (1:5,000). Primary antibodies were dissolved in 0.1 M PBS containing 0.2% Triton X-100 (PBS–TX) and 0.1% sodium azide. Following primary antibody incubation, sections were sequentially incubated for 1 h each with the biotinylated goat anti-rabbit IgG or goat anti-mouse IgG (1:500 in PBS–TX; Vector Laboratories, Burlingame, CA, USA) and peroxidase-conjugated streptavidin (1:2,000 in PBS; Sigma, St. Louis, MO, USA). Specificity of the P2X7 antibody used in the present work has been tested by Western blot, in the striatum and substantia nigra, where a band of the molecular weight of rat modified form of the P2X7 protein was observed that was abolished upon preadsorption of the antibody with the antigen peptide (Sanchez-Nogueiro et al. 2005); (data not shown). In brain sections, nigral P2X7 immunolabeling was completely abolished prior to incubation of the antibody with the antigen peptide (Alomone Labs Ltd., Jerusalem, Israel, data not shown).
Immunoreaction was visualized using 0.05% 3,3′-diaminobenzidine (DAB, Sigma) and 0.02% H2O2, intensified with 0.8% nickel ammonium sulfate. Sections were mounted on gelatin-coated slides, air-dried, dehydrated with ethanol, cleared in xylene, and coverslipped with DPX mounting medium. The total number of immunopositive cell bodies for TH in the substantia nigra (between 4.8 mm and 6.04 mm posterior to Bregma according to the atlas of Paxinos and Watson) was quantified with stereology according to the optical fractionator’s methods (Aguirre et al. 2005). Digital images were acquired with the Nikon E400 microscope (Nikon) (100× objective) interfaced with a computer and a digital camera (Nikon DS-Fi1). Systemic random sampling with an unbiased counting frame (108 × 108 µm) from every sixth section (with a distance of 180 µm) through the substantia nigra was undertaken. All cells (nucleated only) that came into focus within the frame were counted and four sites were sampled per section. This unbiased method is not affected by either the volume of the substantia nigra or the size of the counted neurons.
Double labeling immunohistochemistry
Double immunolabeling for P2X7 and TH through use of green and red fluorescence labeling was performed with the aim of determining the localization of P2X7 IR on injured DA cell bodies. Similarly double immunolabeling for P2X7 and GFAP (Chemicon/Millipore) was used for determining the localization on astroglia. Furthermore, incubation with biotinylated lectin purified from lycopersicon esculentum (tomato lectin; Sigma, St. Louis) was performed to selectively demonstrate P2X7 IR in microglia (Acarin et al. 1994; Mariotti and Bentivoglio 2000). Confocal microscopy was used in the immunohistochemical analysis.
Sections were incubated 24 h with antibodies against P2X7/TH, P2X7/GFAP or P2X7 alone. Sections were washed and incubated for 1 h at RT with biotinylated goat anti-rabbit IgG (P2X7/TH), biotinylated goat anti-chicken IgG (P2X7/GFAP) or with tomato lectin at 37°C (P2X7/TL). The sections were washed and incubated in either goat anti-mouse conjugated with Alexa 594 and streptavidin conjugated to Alexa 488 for P2X7/TH visualization or goat anti-rabbit conjugated to Alexa 594 and streptavidin conjugated to Alexa 488 for P2X7/GFAP and P2X7/tomato lectin visualization. Finally, the sections were rinsed in PBS and mounted on gelatin-coated slides using an antifading-mounting medium (Dako Corporation, Carpinteria, USA).
Statistical analysis
The comparison of the changes in the absolute striatal DA levels after P2X7 or saline treatment and the number of TH IR cells in the susbstantia nigra after such treatments was made with Student’s t test followed by the Bonferoni correction (Table 1). The percent striatal DA depletions on the lesioned side with respect to the unlesioned side with P2X7 antagonist or saline treatment were analyzed with the Mann–Whitney U test followed by Bonferoni correction. This test was also used to analyze the changes in striatal DA turnover found in lesioned and sham-operated rats that received P2X7 antagonist treatment.
Results
Effect of the P2X7 receptor antagonist A-438079 on striatal DA levels and turnover after 6-OHDA induced nigral lesions of the nigrostriatal DA pathway
DA levels
As shown in Table 1, a marked reduction of dorsal striatal DA levels was found 4 weeks after the lesion in saline-treated 6-OHDA-lesioned animals. This action was found to be partly and significantly counteracted by A-438079 in two acute i.p. doses of 30 mg/kg given 60 min before and after the 6-OHDA-induced lesion (Table 1). This counteraction became significant (p = 0.016) also when comparing the % DA depletion on the lesioned with respect to the unlesioned side with or without P2X7 antagonist treatment. In the saline group the % median value on the operated side was 23.4% and the interquartile range 21.6 (n = 11), while the % median value in the P2X7 antagonist treated group was 31.3 with an interquartile range of 29.0.
As shown in Table 1, the lesion produced the expected and significant rise of the DOPAC + HVA/DA ratio in the dorsal striatum of the lesioned side with respect to the unlesioned side (p < 0.01). This rise of DA turnover on the lesioned side was reduced by A-438079, but not significantly.
Number of nigral DA nerve cells
A significant reduction (p < 0.05) in the number of TH IR nerve cells was found 4 weeks after partial lesion in saline-treated 6-OHDA-lesioned animals (means ± SEM, n = 6; lesioned side: 3,728 ± 477; unlesioned side: 5,528 ± 519). A-438079-treated 6-OHDA-lesioned animals showed a similar significant reduction of TH IR nerve cells on the lesioned side (2,720 ± 354) versus the unlesioned side (4,776 ± 453).
Effect of the P2X7 receptor antagonist A-438079 on striatal DA levels and turnover in sham-operated animals
DA levels
As shown in Table 1, the absolute DA levels was unaffected by sham operation and the P2X7 antagonist treatment did not significantly change DA levels on the operated and unoperated side compared with saline treatment.
As shown in Table 1, A-438079 treatment produced a significant reduction (p < 0.01) of DA turnover on the sham-operated side in the dorsal striatum 4 weeks after sham operation, versus saline-treated sham-operated rats. A significant reduction (p < 0.05) of DA turnover on the sham-operated side was also observed after A-438079 treatment when the DA turnover on the sham-operated side was expressed in percent of the unlesioned side. The median value on the sham-operated side in the saline-treated group was 100.7% with an interquartile range of 40.35 (n = 17) and in the A-438079-treated group 79.2% with an interquartile range of 22.9 (n = 8).
Number of DA nerve cells
No differences between the lesioned side and unlesioned side were found in both saline and A-438079-treated sham-operated rats.
Colocalization of P2X7/TL and P2X7/GFAP but not of P2X7/TH immunoreactivities in the substantia nigra
The immunocytochemical analysis shows the existence of nigral P2X7 receptor immunoreactivity (IR) mainly in microglia of naïve rat substantia nigra (Fig. 1). Using tomato lectin as a microglial marker, all nigral microglia profiles appeared to have P2X7 IR based on the colocalization of tomato lectin and P2X7 IR (Fig. 1). However, endothelial cells also labeled with tomato lectin lacked P2X7 IR. Double immunolabeling with antibodies against P2X7 and GFAP as an astroglial marker demonstrates very sparse colocalization of their IRs in astrocytes. Using double immunolabeling with antibodies against TH and P2X7 a complete lack of colocalization of these IRs was observed in nerve cells of both naïve, sham-operated and 6-OHDA-lesioned substantia nigra (Fig. 1). The treatment with A-438079 did not modify the localization pattern of the P2X7 receptor.
Double immunofluorescence labeling of P2X7 receptor and TH (A–D; P2X7: green; TH: red), tomato lectin (TL) (E–H; P2X7: red; TL: green) or GFAP (I–L; P2X7: red; GFAP: green) in the SNc. Tomato lectin also labels endothelial cells which are indicated by an arrow and are green, since they do not express P2X7 receptor IR. Co-immnunolabeled TL/P2X7 (E–H) and GFAP/P2X7 (I–L) display a yellowish color. An arrow indicates the few astroglial GFAP immunoreactive profiles with yellowish fluorescence. Scale bar 100 µm
Discussion
The chemical neuroanatomical analysis within the naïve, sham-operated and 6-OHDA-lesioned substantia nigra indicated that P2X7 IR is primarily located in nigral microglial cells with its existence also in some astroglial processes. However, the analysis failed to show the presence of P2X7 IR in nigral nerve cells including DA nerve cells in naïve, sham-operated and 6-OHDA-lesioned substantia nigra in contrast to what has been previously postulated (Fuxe et al. 2006, 2009). These findings make it likely that P2X7 receptors may mainly enhance neuroinflammatory nigral processes through the activation of microglia and the release of inflammatory agents like interleukin 1β [see (Donnelly-Roberts and Jarvis 2007; Pelegrin and Surprenant 2006)]. In this way, P2X7 receptors may contribute to the neurodegeneration or change the functionality of the nigral DA cells in view of the evidence that anti-inflammatory drugs like COX-2 inhibitors can block MPTP-induced DA nerve cell degeneration in the mouse [see (Aguirre et al. 2008)].
The main finding in this work is the demonstration that the P2X7 receptor antagonist A-438079 in a moderate dose (30 mg/kg given twice; see Nelson et al. 2006) can partly and significantly prevent the 6-OHDA-induced striatal DA depletion in the unilateral nigral 6-OHDA rat model of PD. However, since P2X7 antagonist treatment was unable to prevent DA nerve cell loss, the neuroprotection mechanisms initially postulated in the substantia nigra may not exist, but instead a sparing of striatal DA stores in remaining DA nerve terminals appear to take place that may also involve a reduction of DA turnover.
Treatment with A-438079 did not produce any significant effect on striatal DA levels in sham-operated rats either on the sham-operated or on the unlesioned side. However, the interesting finding made was that the P2X7 receptor antagonist treatment substantially reduced striatal DA turnover on the sham-operated side with a trend for a reduction on the 6-OHDA-lesioned side. Therefore, in the sham-operated substantia nigra there may exist a P2X7 receptor-dependent neuroinflammatory microglial process that maintains the neuronal firing in the nigro-striatal DA pathway. It has been in fact proposed that chemokines like stromal cell-derived factor, SDF-1α, may participate in enhancing compensatory transmission processes in the remaining nigral DA cells in early degenerative events (Fuxe et al. 2008; Guyon and Nahon 2007).
In conclusion, it seems possible that P2X7 receptor antagonists may represent a novel strategy for treatment of PD, which must be validated with a full dose response curve and a complete chemical neuroanatomical analysis of the neurodegenerative processes of nigrostriatal DA neurons and microglia proliferation.
References
Acarin L, Vela JM, Gonzalez B, Castellano B (1994) Demonstration of poly-N-acetyl lactosamine residues in ameboid and ramified microglial cells in rat brain by tomato lectin binding. J Histochem Cytochem 42(8):1033–1041
Aguirre JA, Kehr J, Yoshitake T, Liu FL, Rivera A, Fernandez-Espinola S, Andbjer B, Leo G, Medhurst AD, Agnati LF, Fuxe K (2005) Protection but maintained dysfunction of nigral dopaminergic nerve cell bodies and striatal dopaminergic terminals in MPTP-lesioned mice after acute treatment with the mGluR5 antagonist MPEP. Brain Res 1033(2):216–220
Aguirre JA, Leo G, Cueto R, Andbjer B, Naylor A, Medhurst AD, Agnati LF, Fuxe K (2008) The novel cyclooxygenase-2 inhibitor GW637185X protects against l-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine toxicity. Neuroreport 19(6):657–660
Anderson CM, Nedergaard M (2006) Emerging challenges of assigning P2X7 receptor function and immunoreactivity in neurons. Trends Neurosci 29(5):257–262
Chessell IP, Hatcher JP, Bountra C, Michel AD, Hughes JP, Green P, Egerton J, Murfin M, Richardson J, Peck WL, Grahames CB, Casula MA, Yiangou Y, Birch R, Anand P, Buell GN (2005) Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain 114(3):386–396
Donnelly-Roberts DL, Jarvis MF (2007) Discovery of P2X7 receptor-selective antagonists offers new insights into P2X7 receptor function and indicates a role in chronic pain states. Br J Pharmacol 151(5):571–579
Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, Panther E, Di Virgilio F (2006) The P2X7 receptor: a key player in IL-1 processing and release. J Immunol 176(7):3877–3883
Fuxe K, Manger P, Genedani S, Agnati L (2006) The nigrostriatal DA pathway and Parkinson’s disease. J Neural Transm Suppl (70):71–83
Fuxe KG, Tarakanov AO, Goncharova LB, Agnati LF (2008) A new road to neuroinflammation in Parkinson’s disease? Brain Res Rev 58(2):453–458
Fuxe K, Marcellino D, Antonelli T, Mudó G, Manger P, Genedani S, Ferraro L, Belluardo N, Tanganelli S, Agnati LF (2009) The nigro-striatal DA neurons and mechanisms of their degeneration in Parkinson’s disease. In: Ribak CE, Arámburo de la Hoz C, Jones EG, Larriva Sahd JA, Swanson LW (eds) From development to degeneration and regeneration of the nervous system. Oxford University Press, New York, pp 121–143
Guyon A, Nahon JL (2007) Multiple actions of the chemokine stromal cell-derived factor-1alpha on neuronal activity. J Mol Endocrinol 38(3):365–376
Honore P, Donnelly-Roberts D, Namovic MT, Hsieh G, Zhu CZ, Mikusa JP, Hernandez G, Zhong C, Gauvin DM, Chandran P, Harris R, Medrano AP, Carroll W, Marsh K, Sullivan JP, Faltynek CR, Jarvis MF (2006) A-740003 [N-(1-{[(cyanoimino)(5-quinolinylamino) methyl]amino}-2, 2-dimethylpropyl)-2-(3, 4-dimethoxyphenyl)acetamide], a novel and selective P2X7 receptor antagonist, dose-dependently reduces neuropathic pain in the rat. J Pharmacol Exp Ther 319(3):1376–1385
Jun DJ, Kim J, Jung SY, Song R, Noh JH, Park YS, Ryu SH, Kim JH, Kong YY, Chung JM, Kim KT (2007) Extracellular ATP mediates necrotic cell swelling in SN4741 dopaminergic neurons through P2X7 receptors. J Biol Chem 282(52):37350–37358
Kehr J, Yoshitake T (2006) Monitoring brain chemical signals by microdialysis. In: Grimes CA, Dickey EC, Pishko MV (eds) Encyclopedia of sensors. American Scientific Publishers, USA, pp 287–312
Mariotti R, Bentivoglio M (2000) Activation and response to axotomy of microglia in the facial motor nuclei of G93A superoxide dismutase transgenic mice. Neurosci Lett 285(2):87–90
Nelson DW, Gregg RJ, Kort ME, Perez-Medrano A, Voight EA, Wang Y, Grayson G, Namovic MT, Donnelly-Roberts DL, Niforatos W, Honore P, Jarvis MF, Faltynek CR, Carroll WA (2006) Structure-activity relationship studies on a series of novel, substituted 1-benzyl-5-phenyltetrazole P2X7 antagonists. J Med Chem 49(12):3659–3666
Pelegrin P, Surprenant A (2006) Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J 25(21):5071–5082
Rivera A, Agnati LF, Horvath TL, Valderrama JJ, de La Calle A, Fuxe K (2006) Uncoupling protein 2/3 immunoreactivity and the ascending dopaminergic and noradrenergic neuronal systems: relevance for volume transmission. Neuroscience 137(4):1447–1461
Sanchez-Nogueiro J, Marin-Garcia P, Miras-Portugal MT (2005) Characterization of a functional P2X(7)-like receptor in cerebellar granule neurons from P2X(7) knockout mice. FEBS Lett 579(17):3783–3788
Sim JA, Young MT, Sung HY, North RA, Surprenant A (2004) Reanalysis of P2X7 receptor expression in rodent brain. J Neurosci 24(28):6307–6314
Surprenant A, Rassendren F, Kawashima E, North RA, Buell G (1996) The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science 272(5262):735–738
Ungerstedt U (1968) 6-Hydroxy-dopamine induced degeneration of central monoamine neurons. Eur J Pharmacol 5(1):107–110
Acknowledgments
This work has been supported by a grant from Parkinsonfonden (Swedish Parkinson’s Foundation) to KF and Spanish Research Council grant BFU2008-02030 to AR.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marcellino, D., Suárez-Boomgaard, D., Sánchez-Reina, M.D. et al. On the role of P2X7 receptors in dopamine nerve cell degeneration in a rat model of Parkinson’s disease: studies with the P2X7 receptor antagonist A-438079. J Neural Transm 117, 681–687 (2010). https://doi.org/10.1007/s00702-010-0400-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-010-0400-0