Abstract
Recent studies have indicated that gamma band oscillations participate in the temporal binding needed for the synchronization of cortical networks involved in short-term memory and attentional processes. To date, no study has explored the temporal dynamics of gamma band in the early stages of dementia. At baseline, gamma band analysis was performed in 29 cases with mild cognitive impairment (MCI) during the n-back task. Based on phase diagrams, multiple linear regression models were built to explore the relationship between the cognitive status and gamma oscillation changes over time. Individual measures of phase diagram complexity were made using fractal dimension values. After 1 year, all cases were assessed neuropsychologically using the same battery. A total of 16 MCI patients showed progressive cognitive decline (PMCI) and 13 remained stable (SMCI). When adjusted for gamma values at lag −2, and −3 ms, PMCI cases displayed significantly lower average changes in gamma values than SMCI cases both in detection and 2-back tasks. Gamma fractal dimension of PMCI cases displayed significantly higher gamma fractal dimension values compared to SMCI cases. This variable explained 11.8% of the cognitive variability in this series. Our data indicate that the progression of cognitive decline in MCI is associated with early deficits in temporal binding that occur during the activation of selective attention processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Electroencephalography (EEG) is an easily accessible, high temporal resolution procedure that may be particularly efficient for the identification of subtle functional changes before the occurrence of significant brain damage in Alzheimer disease (AD). Among the different EEG markers that have been studied in this context, frequency analysis is thought to provide a good indication of the integrity of cortical connectivity. Classically, AD is associated with a power increase in the lower delta and theta band frequencies and decrease in the alpha and beta frequencies (Huang et al. 2000; Jelic et al. 1996; Jeong 2004). In mild cognitive impairment (MCI), these EEG parameters showed intermediate values between those of controls and AD patients with substantial inter-individual variability (Wolf et al. 2003). Earlier and more recent contributions reported significant changes in power and event-related synchronization of the very high gamma band frequency (30–100 Hz) that occur in both MCI and AD cases (Driver et al. 2007; Jiang et al. 2008; Koenig et al. 2005; Ribary et al. 1991; Rossini et al. 2006; Stam et al. 2002, 2003; Teipel et al. 2008; van Deursen et al. 2008). The exact significance of this phenomenon remains a matter of debate (Basar-Eroglu et al. 2007; Haig et al. 2000; Naatanen and Alho 1995; Ruusuvirta and Huotilainen 2005; Tallon-Baudry and Bertrand 1999; Tallon-Baudry et al. 1998). Overall, high oscillatory activities are thought to reflect the selective assembly of neurons into functional groups needed for the temporal coding supporting fast selection and multiregional cortical binding (Bressler et al. 1993; Engel et al. 2001; Fries et al. 2007; Gruber et al. 2004; Jensen and Colgin 2007; Llinás and Ribary 1992; Salinas and Sejnowski 2001; Tallon-Baudry and Bertrand 1999; Uhlhaas et al. 2008; Varela et al. 2001). It has been proposed that gamma band oscillations play a key role in the synchronization of the cortical networks involved in short-term cognitive processes (Babiloni et al. 2004; Daskalakis et al. 2008; Kaiser and Lutzenberger 2003; Rodriguez et al. 1999; Schneider et al. 2008; Tao and Tian 2005). Appropriate synchronization depends on the ability of neural networks to self-organize and form stable EEG patterns at the initial stages of cognitive activation (Stam 2005). A well-organized network displays a temporal memory in that its current state may be predicted on the basis of an earlier one. According to this recent conceptualization, it is crucial to investigate not only gamma power or event-related synchronization, but also the temporal evolution of the gamma band in early stages of cognitive decline. Using an n-back working memory activation paradigm, we report here that changes in gamma band temporal dynamics at baseline may predict further cognitive decline after 1 year with MCI.
Methods
Inclusion procedure and follow-up
Mild cognitive impairment participants were recruited in a large acute and intermediate care geriatric hospital. Subjects with MMSE scores between 25 and 28 underwent an additional clinical evaluation, which included the Hospital Anxiety and Depression Scale (HAD, Zigmond and Snaith 1983) and Lawton’s Instrumental Activities of Daily Living (IADL, Barberger-Gateau et al. 1992). Depressive co-morbidity was excluded on the basis of a HAD score consistently <8. Extensive neuropsychological testing was performed including attention (Wechsler 1981), orientation (MMSE items), short-term memory [Mattis Dementia rating scale items (DRS)] (Gardner et al. 1981), digit span forward (Wechsler 1981), Corsi block-tapping test (Milner 1971), episodic memory (Buschke double memory test, Buschke et al. 1997; Shapes test, Baddeley et al. 1994), executive functions (verbal fluency test, Butters et al. 1987), trail making test (Reitan 1958), language (Boston naming test, Kaplan et al. 1983), ideomotor (Schnider et al. 1997), reflexive (Poeck 1985) and constructional praxis (Welsh et al. 1994), and visual gnosis (Ghent overlapping figures) (Ghent 1956). Global cognitive function was assessed by the clinical dementia rating scale (CDR, Hughes et al. 1982) and the Mattis DRS. Subjects having a test score of more than 1.5 SD below the age-appropriate mean in any of the above tests and a CDR score of 0.5, but no dementia, were diagnosed with possible MCI (Petersen et al. 2001). Among these cases, 8 had single domain amnestic MCI (decreased performance in the Buschke double memory test) and 21 had multiple domain amnestic MCI (impaired performance in the Buschke double memory test and either the trail making test or the Corsi block-tapping test (Petersen and Morris 2005). These cases were reviewed independently by two highly experienced clinicians blinded to each other’s findings and included in the MCI group only if both clinicians concurred on this diagnosis. The final sample included 29 MCI cases (mean age 82.5 years, age range 77–91) who underwent a detailed neuropsychological follow-up evaluation 1 year after inclusion using the same neuropsychological battery. On follow-up, subjects were considered to have progressed and were included in the PMCI group if: (1) they exhibited a significant deterioration (defined as ≥0.5 SD compared to inclusion values) in the Buschke double memory test and at least ≥1.0 SD among the other neuropsychological tests, and (2) they were considered to have clinically deteriorated based on the review of all the neuropsychological tests by an independent physician highly experienced in cognitive disorders, who was blinded to all other data. Subjects were considered stable and included in the SMCI group if (1) they exhibited no or marginal changes in their neuropsychological performances on follow-up (test result improved or decreased to <0.5 SD compared to inclusion values), and (2) they were considered to be clinically stable by the independent physician described above.
All participants had normal or corrected-to-normal visual acuity, and none reported a history of sustained head injury, or other neurological or psychiatric disorders. All participants with regular use of neuroleptics, antidepressants, anti-epileptic medications, stimulants, opioids and β-blockers were excluded from the present study. In contrast, regular use of low doses of hypnotics was tolerated. After formal approval of the local ethics committee, informed written consent was obtained from all participants included in this study.
Design
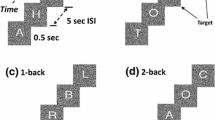
Subjects watched a computer-controlled display screen at a distance of 57 cm. They viewed pseudo-random sequences of consonants and vowels common to the French alphabet and pressed a computer-controlled button with their right index finger as soon as a target appeared (response trials). For non-target stimuli, no motor response was required (no-response trials). Targets were defined either according to the oddball (rare event) or to the n-back design.
Stimuli consisted of white letters, arial font (2° × 2.5° visual angle), with 10% gray noise, embedded in a 50% random noise gray rectangular background patch (6° × 6.7° visual angle). They were presented for a duration of 0.5 s, separated by 5-s intervals (onset to onset) during which a dot helped subjects maintain fixation. In addition, subjects were instructed to remain quiet and to only move their right index finger in accordance with the nature of the task to minimize any muscle artifacts during the EEG recording.
Two different tasks were used: in a simple detection task (control), sequential letters or background patches without letters were presented. Subjects responded as fast as possible when background patches without letters appeared; in the 2-back task, the target was any letter that was identical to the one presented two trials back. Thus, working memory load increased from control (memory free-condition) to 2-back task (highly demanding).
Each task was tested in three stimulus sequences of 30 images each (7 targets), adding up to 90 trials (epochs) per task (21 targets). Before each sequence block, subjects were informed about the nature of the task, and several warm-up trials were performed for MCI patients. A control task block was followed by three blocks of the 2-back condition, and two blocks of the control task. Reaction time (RT) and performance were systematically recorded, but no feedback on performance was provided. The absence of difference in RT and performance between blocks of the same task indicated that learning, habituation and fatigue effects were minimal during the recording session. Before and after the experimental paradigm, an open and close eye EEG session without task was realized during a period of 3 min. Electrophysiological and neuropsychological assessments were all performed in the morning.
Electrophysiological recordings
Continuous EEG (Micromed, Brain Quick system 98, Treviso, Italy) was recorded, using 20 surface electrodes placed over the scalp according to the 10–20 international electrode placement system (Homan et al. 1987), with linked earlobes as reference. Skin impedance was kept below 5 kΩ. Electrophysiological signals were software filtered and digitized (sampling rate 1,024 Hz), with a lower cutoff of 0.33 Hz and an upper cutoff of 120 Hz (DC amplifiers Micromed). The electrooculogram (EOG) was recorded using two pairs of bipolar electrodes in both vertical and horizontal directions. Single pulses (TTL) synchronized with stimulus onset (letter or background patches) were recorded and used off-line to segment the continuous EEG data into epochs time-locked to stimulus onset.
Data processing
Electroencephalography data were analyzed using the NeuroScan software (NeuroScan Inc., Herndon, VA, USA). EEG signals were corrected for ocular artifacts using an off-line threshold reduction algorithm (NeuroScan Inc.). This method operates by subtracting EOG signals from EEG channels using linear derivation approach with a spatial filter transform. The EEG signals were automatically cleared of movement artifacts in which voltage exceeded 100 μV criteria and the remaining trials were inspected visually to control for minor artifacts. EEG data were detached into epochs of 1,050 ms, starting 525 ms before the stimulus onset. To eliminate effects from manual responses and exclude the confounding effect of motor processing, correct answers without motor response were analyzed according to the task condition (detection, 2-back). Then, the EEG data were analyzed using two distinct types of electrophysiological analysis: (i) spectral power analysis and (ii) event-related oscillations (EROs).
Spectral power analysis
Artifact-free close and open eyes EEG recordings at rest were converted into the frequency domain using an FFT function computed on overlapping 2-s windows (10% Hanning filter). For resting EEG data, gamma frequency band power was measured at the frontal (F3, Fz, F4), central (C3, Cz, C4) and parietal (P3, Pz, P4) electrode sites as the average power between 35 and 45 Hz, respectively.
Event-related oscillations
Event-related oscillations were averaged over a window of 1050 ms with a 525-ms pre-stimulus onset and band-pass filtered at 35–45 Hz (−48 dB/octave). They were also averaged for artifact-free open eye EEG recordings at rest. Analyses of the segment-evoked oscillations included the frontal (F3, Fz, F4), central (C3, Cz, C4) and parietal (P3, Pz, P4) electrodes. Additionally, to study the possible group and task effects on visual sensory processing, we performed an analysis restricted to the occipital (O1, Oz, O2) electrodes. Over a maximum of 90 epochs, on average for SMCI subjects, the percentage of artifact-free trials was 65 ± 14, and 61 ± 16 in control and 2-back tasks, respectively. Similar percentages were obtained for PMCI cases with 61 ± 10 and 61 ± 13, respectively.
Statistical analysis
Demographic, clinical characteristics at inclusion, differences in n-back performance, as well as neuropsychological values at baseline and on follow-up were compared between the PMCI and SMCI groups using the Mann–Whitney non-parametric U test. Cognitive changes over time were assessed using the Wilcoxon matched-pairs signed-ranks test. RT differences were assessed using two-way repeated measures ANOVA.
To normalize the variance of the EEG data (i.e., magnitude of gamma power at rest), a logarithmic transformation was used to perform parametric two-way repeated comparisons. The normality of data distribution was verified with skewness and kurtosis tests. Statistical analysis was performed to compare EEG measures between groups, using a two-way repeated measures ANOVA, with task condition (detection, 2-back) and electrode site (frontal, central, parietal, occipital) as within-subject factors. The significance values were determined using the Greenhouse–Geisser correction. Post hoc analysis was performed using Fisher’s least square difference test (Milliken and Johnson 1984).
As the time series were not stationary (as assessed with run tests), we explored the association between the temporal evolution of gamma oscillations and cognitive decline using the following procedures. First, phase diagrams were used to explore the best time lag to discriminate between PMCI and SMCI. Phase space represents all the possible states of a dynamic system. A phase plot corresponds to a graph of gamma oscillation values at time t minus a lag (t − lag) on the y axis versus gamma values at time t on the x axis. Plots were drawn for each electrode, task and subject for lag time periods ranging from 1 to 20 ms. Then, linear regression models (Dwyer and Feinleib 1992), taking into account the clustering of measures within each subject, were built with gamma values as the dependent variable and group, task, electrode and gamma values at a given time lag (gammalag) as the independent variables. Geometrically, the regression coefficient associated with the gammalag parameter corresponds to the slope of the local regression line of the ellipse in the phase diagrams. The present analysis of phase space representation was performed for each electrode site and is thus quite different from that of the degree of linear relatedness between two channels (i.e., the functional coupling of cerebral areas explored by the non-linear coherence function).
Fractal dilation was used to estimate the fractal dimension (a measure of complexity represented by a unique value) of the phase diagrams using the Fractal analysis software (Fractalyse, CNRS, Franche-Comté and Bourgogne University, Version: 2.4; downloadable from Web site: http://www.fractalyse.org). Then, fractal dimensions were compared between the two MCI groups for each task and electrode sites using unpaired Student’s t test. In addition, univariate and multiple logistic regression models were built to assess the relationship between cognitive decline in MCI (binary dependent variable) and the fractal dimension of the gamma oscillation controlling for task and electrode site effects (independent variables). This method can also evaluate the amount of variability of the dependent variable (cognitive decline) that can be explained by the model and thus provide an estimate of the strength of their relationship. Statistical threshold was set at p < 0.05. Statistical analyses were performed using the Stata software package (Statcorp, College Station, TX, USA, 2007), release 10.1.
Results
Clinical data
One year after baseline assessment (range 12.0 ± 0.4 months), 16 (55%) of the original MCI cases demonstrated significant cognitive decline and constituted the progressive MCI group (PMCI). Among these cases, only three converted into AD. The remaining 13 cases (45%) showed no change in cognitive function, except an executive motor slowing indicated by the trail making test A (78.6 ± 32.0 vs. 96.1 ± 33.4 s; p < 0.01). This difference was not clinically significant with respect to normative thresholds and is consistent with the low test–retest reliability of Part A in older adults (Mitrushina et al. 1991). These participants were therefore included in the stable MCI group (SMCI). PMCI cases showed a significant deterioration of their performances in the MMSE test (25.5 ± 1.8 vs. 23.3 ± 3.7; p < 0.01), verbal fluency (14.6 ± 4.8 vs. 11.1 ± 5.4; p < 0.01), Boston naming test (18.6 ± 1.5 vs. 17.4 ± 2.0; p < 0.05) and Buschke double memory test (3 blocks of 16 items) (total free recall: 16.9 ± 3.7 vs. 13.6 ± 3.4; p < 0.01).
Demographic and clinical characteristics at inclusion did not differ between SMCI and PMCI cases, for instance the mean age was 82.4 ± 9.6 and 83.1 ± 4.9 years (p = 0.819), respectively. Moreover, there was no significant difference in baseline scores for any of the neuropsychological tests between SMCI and PMCI. The HAD scale scores for anxiety and depression as well as gender distribution were comparable between groups.
Reaction times and performances in n-back tasks
All subjects performed well in the 2-back (SMCI 87.3% ± 3.9 and PMCI 82.8% ± 6.0) and detection tasks (SMCI 98.8% ± 2.2 and PMCI 97.9% ± 2.2). No significant differences were found in both performances and RT between SMCI and PMCI cases. In all groups, RTs significantly increased for the 2-back task (F(1, 27) = 54.48, p < 0.001).
Electrophysiological data
Spectral power analysis
The magnitude of gamma power open eyes at rest was independent of group (F(1, 27) = 0.40, p = 0.53), electrode (F(2, 54) = 2.73, p = 0.08) or interaction between group and electrode location (F(2, 54) = 0.06, p = 0.94) effects (Fig. 1a). Similarly, power gamma close eyes was independent of group-, electrode- or task-related effects (respectively, F(1, 27) = 0.46, p = 0.57; F(2, 54) = 1.32, p = 0.28; and (F(2, 54) = 1.20, p = 0.31) (Fig. 1b).
Repeated measure linear regression of event-related oscillations
The visual stimuli elicited a series of gamma oscillations in the time range 0–525 ms for the three electrode locations (Fig. 2). These oscillations did not depend on groups (p = 0.877), tasks (p = 0.178) or electrode location (central vs. frontal p = 0.624; parietal vs. frontal p = 0.391).
The assessment of regression coefficients showed that group differences in gamma dynamics concerned only two lag times (Table 1). When adjusted for gamma value at lag −2, and −3 ms in regression models, the gamma oscillations of PMCI were significantly different from SMCI (t = −2.18 and t = −2.13) during the interval 0–525 ms post-stimulus. The negative regression coefficients mean that the PMCI group (coded as 1) had lower average changes in gamma values (delta of gamma values between time and lag −2 or −3) than the SMCI group (coded as 0). As an example, Fig. 2 displays the phase diagram plots that correspond to the average frontal gamma oscillations at time t on the x axis and at lag time t − 2 on the y axis. It illustrates the elliptical shapes of the cyclic patterns and the lower variability of gamma dynamics in the PMCI compared to SMCI groups.
a Average frontal gamma based on group during the 2-back task as a function of time (a, d), and as a function of average frontal gamma at lag time −2 (phase diagrams BC and EF). See text for details. The colored section represents the traces obtained during three limited time periods: blue (0 [ms] < t < 24 [ms]), red (275 [ms] < t < 301 [ms]) and cyan (468 [ms] < t < 496 [ms]). They correspond to different loops of the ellipse in the SMCI and the PMCI group. For example, the red section corresponds to the envelope (external layer) of the ellipse in the SMCI group, but to an inner loop in the PMCI group
Fractal dimension comparisons between PMCI and SMCI
The distribution of fractal dimensions in PMCI and SMCI cases is illustrated in Fig. 3. For instance, in the 2-back task, the fractal dimension of PMCI cases (mean ± SD 0.88 ± 0.61) was significantly higher (N = 29; t = −2.087, degree of freedom 27, p < 0.05) compared to that of SMCI (mean ± SD 0.45 ± 0.49) cases. When considering the distinction between SMCI and PMCI as a binary dependent variable, univariate logistic regression models showed that the fractal dimension of gamma oscillation was significantly related to the cognitive outcome and explained 11.8% of its variability (95% confidence interval 0.34–2.77, p < 0.01). This association persisted when controlling for task and electrode site (see Fig. 3).
In terms of MCI classification, the best combination of sensitivity and specificity (after controlling for task and electrode sites) was obtained for a threshold fractal dimension of gamma at 1.0 (sensitivity: 0.81, specificity: 0.85) with an area under the corresponding ROC curve reaching 82%. This model correctly classified 79.3% of MCI cases.
Discussion
Earlier studies focusing on gamma band parameters in AD and MCI reported discrepant data due to sampling differences and variability in the assessment of gamma band oscillations (Driver et al. 2007; Koenig et al. 2005; Ribary et al. 1991; Stam et al. 2002; van Deursen et al. 2008; Wright et al. 2001). In agreement with the hypothesis of Singer (Singer 2009) who proposed to include internal timing mechanisms in the analysis of high brain oscillations, we observed significant differences in gamma band dynamics, but not in magnitude, between PMCI and SMCI. This observation cannot be due to motor processing or sensory deficits since our analysis was performed on correctly recognized non-target trials. The observed changes in gamma dynamics appear to be strictly related to the cognitive processes common to both detection and 2-back tasks, since they were absent in spectral power analysis at rest and also before stimulus onset. The n-back task used in our study involves not only a pure working memory component that becomes evident in the 2-back condition, but also a major attentional component engaged already in the detection task (Baddeley 1992; LaBar et al. 1999; Krause et al. 2000). Physiologically, previous magneto-encephalographic investigations indicated that the gamma band activity was specific to the retained stimulus during auditory short-term memory maintenance and postulated that stimulus-specific gamma band activity was related to neural activations preceding test stimuli rather than short-term memory load (Kaiser et al. 2003; Lutzenberger et al. 2002). Supporting further this hypothesis, a recent study based on delayed auditory matching-to-sample task revealed stimulus-specific gamma activity (>70 Hz) in anticipation of test stimuli that predicted short-term memory performance (Kaiser et al. 2009). In line with the involvement of gamma oscillations in attention processes, a cross-frequency coupling between theta (4–8 Hz) and gamma neuronal oscillations was recently reported (Demiralp et al. 2007; Jensen and Colgin 2007; Lisman and Buzsaki 2008). This coupling is necessary for the interaction between wide groups of neurons located within distant brain regions during cognitive processing. Recent studies demonstrated that attention processes need theta–gamma frequency coupling to facilitate the transient coordination of cortical areas required for this function (Canolty et al. 2006; Fan et al. 2007, 2005). Interestingly, our time–frequency analysis showed that the frontal-induced theta activity at baseline was significantly reduced in PMCI compared to SMCI in all n-back tasks that were similar in terms of directed attention requirements (Deiber et al. 2009). In conjunction with these observations, the present data suggest that theta–gamma frequency uncoupling during the activation of directed attention may be an early electrophysiological event that predicts worst cognitive evolution among MCI cases.
In our study, the lag time that makes it possible to distinguish SMCI from PMCI coincides strictly with the thalamo-cortical conduction time (Albe-Fessard et al. 1986; Allison et al. 1991; Pantev et al. 1991; Ribary et al. 1991). Cortical and deep brain thalamic implanted electrodes reported stimulus-evoked responses that point to the millisecond conduction time among thalamo-cortical axons in both humans and animals (Allison et al. 1991; Klostermann et al. 2002; Simons et al. 2007). Most importantly, previous contributions using magneto-encephalography and magnetic field tomography revealed waveform deterioration and intensity reduction of gamma oscillations at 2–3 ms lag times in AD (Ribary et al. 1991, 1990, 1989). These findings may be explained by the loss of long distance cortico-cortical connections that characterize AD (Koenig et al. 2005; Stam et al. 2006, 2003). In this context, our results are compatible with a disruption of long distance connections that would be already present in PMCI. One should note, however, that the increased gamma band power during task performance observed in normal aging persists even in certain AD patients (Fitzgibbon et al. 2004; van Deursen et al. 2008) suggesting that the local networks may be at least partly preserved in the course of neurodegeneration. Moreover, a significant increase of gamma band power was reported during cognitive activation in AD cases compared to healthy controls and MCI cases, implying the presence of compensatory mechanisms for a possible decrease in long distance connectivity (van Deursen et al. 2008). In line with our previous studies focusing on the research of EEG markers predictive of cognitive decline in MCI (Missonnier et al. 2007, 2006), the observed deficits in gamma band activation in PMCI were present despite the successful performance of the n-back task further supporting the presence of probable compensatory activation of additional cerebral areas. Accordingly, recent data suggest that high working memory performance can be maintained through the recruitment of alternative cortical networks even in presence of early brain activation deficits in MCI (Yetkin et al. 2006). The changes in the temporal evolution of gamma band value reported here were also identified at an individual level using the fractal dimension analysis that revealed only a limited overlap of individual scores between SMCI and PMCI cases. The average observed value of 0.88 for the PMCI group can be interpreted as being close to a straight line (dimension 1.0) and thus more complex, than the value of 0.45 for the SMCI group that lies between a dot (dimension 0.0) and a straight line (dimension 1.0). This single EEG parameter predicts almost 12% of the cognitive variability in our MCI group. Although this percentage may appear modest, it is significantly higher than that reported for theta event-related synchronization in previous studies (Missonnier et al. 2007, 2006), suggesting that the investigation of early changes in high, rather than low, frequency brain oscillations may be useful to predict rapid cognitive decline in MCI. Importantly, our fractal dimension findings disagree with those reported recently by Gomez et al. (2009), who found a decrease of this parameter in AD cases. Several methodological differences may explain this discrepancy. First, magneto-electroencephalographic signal in this latter study was filtered between 0.5 and 40 Hz, and 72% of the MEG epochs that retained the majority of the signal energy were below 10.6 Hz. In contrast, EEG signal was band-pass filtered at 35–45 Hz in the present study. Second, our observations concern gamma reactivity during the successful performance of a working memory task, while Gomez et al. recorded background activity that was not influenced by functional compensation phenomena (van Deursen et al. 2008). Finally, fractal dilations were calculated in Pointcarré maps using the method of Minkowski and Bouligand in our study, whereas Gomez et al. used Higuchi’s fractal dimension in EEG signals.
Several limitations should be considered when interpreting the present data. First, no distinction was made between different MCI subgroups (i.e., single versus multiple domain). Second, group differences for other lag times may be overlooked because of the small sample size. Most importantly, the analysis of gamma band oscillations in experimental paradigms exploring cognition showed that oscillations in the gamma band included three distinct components typically recorded within 500 ms after stimulus presentation: a gamma band-evoked response directly related to the stimulus, a 40-Hz steady-state response related to primary sensory processing and an induced component associated with meaningful information processing (Fell et al. 2003; Herrmann and Demiralp 2005; Klimesch 1999; Tallon-Baudry and Bertrand 1999). Given the limited number of MCI cases, no separate analysis of each of these components was made in this study. Although one can argue that p values may be significant by chance only with an alpha value set at 0.05 in our regression models, this is a highly unlikely scenario since the two significant p values are consecutive in a cyclic pattern, associated with the time lag instead of occurring randomly, and it holds after adjusting for age.
In conclusion, the present data reveal lower variability, but higher complexity, of the gamma signal over time in PMCI compared to SMCI cases that is already present before their rapid cognitive decline. Further longitudinal studies including larger cohorts of MCI subgroups including time–frequency analysis to differentiate induced from evoked functional processes, and use of additional paradigms addressing other cognitive modalities (i.e., directed attention) are needed to explore the possible relevance of gamma band abnormalities related to attentional and pre-attentional levels of cognitive processing in the prediction of dementia.
Abbreviations
- EEG:
-
Electroencephalography
- ERO:
-
Event-related oscillations
- MCI:
-
Mild cognitive impairment
- SMCI or PMCI:
-
Stable or progressive mild cognitive impairment
- MMSE:
-
Mini-mental state examination
- HAD:
-
Hospital anxiety and depression scale
- IADL:
-
Lawton’s instrumental activities of daily living
References
Albe-Fessard D, Tasker R, Yamashiro K, Chodakiewitz J, Dostrovsky J (1986) Comparison in man of short latency averaged evoked potentials recorded in thalamic and scalp hand zones of representation. Electroencephalogr Clin Neurophysiol 65:405–415
Allison T, McCarthy G, Wood CC, Jones SJ (1991) Potentials evoked in human and monkey cerebral cortex by stimulation of the median nerve. A review of scalp and intracranial recordings. Brain 114(Pt 6):2465–2503
Babiloni C, Babiloni F, Carducci F, Cincotti F, Vecchio F, Cola B, Rossi S, Miniussi C, Rossini PM (2004) Functional frontoparietal connectivity during short-term memory as revealed by high-resolution EEG coherence analysis. Behav Neurosci 118:687–697
Baddeley A (1992) Working memory. Science 255:556–559
Baddeley A, Emslie H, Nimmo-Smith L (1994) The Doors and People Test: a test of visual and verbal recall and recognition. Thames Valley Test Company, Bury St Edmonds, England
Barberger-Gateau P, Chaslerie A, Dartigues JF, Commenges D, Gagnon M, Salamon R (1992) Health measures correlates in a French elderly community population: the PAQUID study. J Gerontol 47:S88–S95
Basar-Eroglu C, Brand A, Hildebrandt H, Karolina Kedzior K, Mathes B, Schmiedt C (2007) Working memory related gamma oscillations in schizophrenia patients. Int J Psychophysiol 64:39–45
Bressler SL, Coppola R, Nakamura R (1993) Episodic multiregional cortical coherence at multiple frequencies during visual task performance. Nature 366:153–156
Buschke H, Sliwinski MJ, Kuslansky G, Lipton RB (1997) Diagnosis of early dementia by the Double Memory Test: encoding specificity improves diagnostic sensitivity and specificity. Neurology 48:989–997
Butters N, Granholm E, Salmon DP, Grant I, Wolfe J (1987) Episodic and semantic memory: a comparison of amnesic and demented patients. J Clin Exp Neuropsychol 9:479–497
Canolty RT, Edwards E, Dalal SS, Soltani M, Nagarajan SS, Kirsch HE, Berger MS, Barbaro NM, Knight RT (2006) High gamma power is phase-locked to theta oscillations in human neocortex. Science 313:1626–1628
Daskalakis ZJ, Farzan F, Barr MS, Rusjan PM, Favalli G, Levinson AJ, Fitzgerald PB (2008) Evaluating the relationship between long interval cortical inhibition, working memory and gamma band activity in the dorsolateral prefrontal cortex. Clin EEG Neurosci 39:150–155
Deiber MP, Ibanez V, Missonnier P, Herrmann F, Fazio-Costa L, Gold G, Giannakopoulos P (2009) Abnormal-induced theta activity supports early directed-attention network deficits in progressive MCI. Neurobiol Aging 30:1444–1452
Demiralp T, Bayraktaroglu Z, Lenz D, Junge S, Busch NA, Maess B, Ergen M, Herrmann CS (2007) Gamma amplitudes are coupled to theta phase in human EEG during visual perception. Int J Psychophysiol 64:24–30
Driver JE, Racca C, Cunningham MO, Towers SK, Davies CH, Whittington MA, LeBeau FE (2007) Impairment of hippocampal gamma-frequency oscillations in vitro in mice overexpressing human amyloid precursor protein (APP). Eur J Neurosci 26:1280–1288
Dwyer J, Feinleib M (1992) Introduction to statistical models for longitudinal observation. In: Dwyer JH, Feinleib M, Lippert P et al (eds) Statistical models for longitudinal studies of health. Oxford University Press, New York, pp 3–47
Engel AK, Fries P, Singer W (2001) Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci 2:704–716
Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI (2005) The activation of attentional networks. Neuroimage 26:471–479
Fan J, Byrne J, Worden MS, Guise KG, McCandliss BD, Fossella J, Posner MI (2007) The relation of brain oscillations to attentional networks. J Neurosci 27:6197–6206
Fell J, Fernandez G, Klaver P, Elger CE, Fries P (2003) Is synchronized neuronal gamma activity relevant for selective attention? Brain Res Brain Res Rev 42:265–272
Fitzgibbon SP, Pope KJ, Mackenzie L, Clark CR, Willoughby JO (2004) Cognitive tasks augment gamma EEG power. Clin Neurophysiol 115:1802–1809
Fries P, Nikolic D, Singer W (2007) The gamma cycle. Trends Neurosci 30:309–316
Gardner R Jr, Oliver-Munoz S, Fisher L, Empting L (1981) Mattis Dementia Rating Scale: internal reliability study using a diffusely impaired population. J Clin Neuropsychol 3:271–275
Ghent L (1956) Perception of overlapping and embedded figures by children of different ages. Am J Psychol 69:575–587
Gomez C, Hornero R, Abasolo D, Fernandez A, Poza J (2009) Study of the MEG background activity in Alzheimer’s disease patients with scaling analysis methods. Conf Proc IEEE Eng Med Biol Soc 1:3485–3488
Gruber T, Malinowski P, Muller MM (2004) Modulation of oscillatory brain activity and evoked potentials in a repetition priming task in the human EEG. Eur J Neurosci 19:1073–1082
Haig AR, Gordon E, Wright JJ, Meares RA, Bahramali H (2000) Synchronous cortical gamma-band activity in task-relevant cognition. Neuroreport 11:669–675
Herrmann CS, Demiralp T (2005) Human EEG gamma oscillations in neuropsychiatric disorders. Clin Neurophysiol 116:2719–2733
Homan RW, Herman J, Purdy P (1987) Cerebral location of international 10–20 system electrode placement. Electroencephalogr Clin Neurophysiol 66:376–382
Huang C, Wahlund L, Dierks T, Julin P, Winblad B, Jelic V (2000) Discrimination of Alzheimer’s disease and mild cognitive impairment by equivalent EEG sources: a cross-sectional and longitudinal study. Clin Neurophysiol 111:1961–1967
Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL (1982) A new clinical scale for the staging of dementia. Br J Psychiatry 140:566–572
Jelic V, Shigeta M, Julin P, Almkvist O, Winblad B, Wahlund LO (1996) Quantitative electroencephalography power and coherence in Alzheimer’s disease and mild cognitive impairment. Dementia 7:314–323
Jensen O, Colgin LL (2007) Cross-frequency coupling between neuronal oscillations. Trends Cogn Sci 11:267–269
Jeong J (2004) EEG dynamics in patients with Alzheimer’s disease. Clin Neurophysiol 115:1490–1505
Jiang ZY, Zheng LL, Yu EY (2008) EEG coherence characteristics at rest and during a three-level working memory task in normal aging and mild cognitive impairment. Med Sci Monit 14:CR515–CR523
Kaiser J, Lutzenberger W (2003) Induced gamma-band activity and human brain function. Neuroscientist 9:475–484
Kaiser J, Ripper B, Birbaumer N, Lutzenberger W (2003) Dynamics of gamma-band activity in human magnetoencephalogram during auditory pattern working memory. Neuroimage 20:816–827
Kaiser J, Rahm B, Lutzenberger W (2009) Temporal dynamics of stimulus-specific gamma-band activity components during auditory short-term memory. Neuroimage 44:257–264
Kaplan EF, Goodglass H, Weintraub S (1983) The Boston Naming Test. Lea and Febiger, Philadelphia
Klimesch W (1999) EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Brain Res Rev 29:169–195
Klostermann F, Gobbele R, Buchner H, Curio G (2002) Dissociation of human thalamic and cortical SEP gating as revealed by intrathalamic recordings under muscle relaxation. Brain Res 958:146–151
Koenig T, Prichep L, Dierks T, Hubl D, Wahlund LO, John ER, Jelic V (2005) Decreased EEG synchronization in Alzheimer’s disease and mild cognitive impairment. Neurobiol Aging 26:165–171
Krause CM, Sillanmaki L, Koivisto M, Saarela C, Haggqvist A, Laine M, Hamalainen H (2000) The effects of memory load on event-related EEG desynchronization and synchronization. Clin Neurophysiol 111:2071–2078
LaBar KS, Gitelman DR, Parrish TB, Mesulam M (1999) Neuroanatomic overlap of working memory and spatial attention networks: a functional MRI comparison within subjects. Neuroimage 10:695–704
Lisman J, Buzsaki G (2008) A neural coding scheme formed by the combined function of gamma and theta oscillations. Schizophr Bull 34:974–980
Llinás R, Ribary U (1992) Rostrocaudal scan in human brain: a global characteristic of the 40-Hz response during sensory input. In: Bassar E, Bullock T (eds) Induced rhythms in the brain. Birkhäuser, Boston, pp 147–154
Lutzenberger W, Ripper B, Busse L, Birbaumer N, Kaiser J (2002) Dynamics of gamma-band activity during an audiospatial working memory task in humans. J Neurosci 22:5630–5638
Milliken GA, Johnson DE (1984) Designed experiments. Van Nostrand Reinhold, New York
Milner B (1971) Interhemispheric differences in the localization of psychological processes in man. Br Med Bull 27:272–277
Missonnier P, Gold G, Herrmann FR, Fazio-Costa L, Michel JP, Deiber MP, Michon A, Giannakopoulos P (2006) Decreased theta event-related synchronization during working memory activation is associated with progressive mild cognitive impairment. Dement Geriatr Cogn Disord 22:250–259
Missonnier P, Deiber MP, Gold G, Herrmann FR, Millet P, Michon A, Fazio-Costa L, Ibanez V, Giannakopoulos P (2007) Working memory load-related electroencephalographic parameters can differentiate progressive from stable mild cognitive impairment. Neuroscience 150:346–356
Mitrushina M, Satz P, Chervinsky A, D’Elia L (1991) Performance of four age groups of normal elderly on the Rey Auditory–Verbal Learning Test. J Clin Psychol 47:351–357
Naatanen R, Alho K (1995) Mismatch negativity—a unique measure of sensory processing in audition. Int J Neurosci 80:317–337
Pantev C, Makeig S, Hoke M, Galambos R, Hampson S, Gallen C (1991) Human auditory evoked gamma-band magnetic fields. Proc Natl Acad Sci USA 88:8996–9000
Petersen RC, Morris JC (2005) Mild cognitive impairment as a clinical entity and treatment target. Arch Neurol 62:1160–1163 (discussion 1167)
Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor M, Thal L, Winblad B (2001) Current concepts in mild cognitive impairment. Arch Neurol 58:1985–1992
Poeck K (1985) Clues to the nature of disruption to limb praxis. In: Roy EA (ed) In neuropsychological studies of apraxia and related disorders. Elsevier, North-Holland, Amsterdam, pp 99–109
Reitan RM (1958) Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills 8:271–276
Ribary U, Llinas R, Kluger A, Suk J, Ferris SH (1989) Neuropathological dynamics of magnetic, auditory, steady-state responses in Alzheimer’s disease. In: Williamson SJ, Hoke M, Stroink G et al (eds) Advances in biomagnetism. Plenum Press, New York, pp 311–314
Ribary U, Llinas R, Kluger A, Cappell J, Suk J, Ferris SH (1990) Dynamics of magnetic, auditory evoked steady-state responses in Alzheimer patients. Electroencephalogr Clin Neurophysiol 75:S126
Ribary U, Ioannides AA, Singh KD, Hasson R, Bolton JP, Lado F, Mogilner A, Llinas R (1991) Magnetic field tomography of coherent thalamocortical 40-Hz oscillations in humans. Proc Natl Acad Sci USA 88:11037–11041
Rodriguez E, George N, Lachaux JP, Martinerie J, Renault B, Varela FJ (1999) Perception’s shadow: long-distance synchronization of human brain activity. Nature 397:430–433
Rossini PM, Del Percio C, Pasqualetti P, Cassetta E, Binetti G, Dal Forno G, Ferreri F, Frisoni G, Chiovenda P, Miniussi C, Parisi L, Tombini M, Vecchio F, Babiloni C (2006) Conversion from mild cognitive impairment to Alzheimer’s disease is predicted by sources and coherence of brain electroencephalography rhythms. Neuroscience 143:793–803
Ruusuvirta T, Huotilainen M (2005) The fast detection of rare auditory feature conjunctions in the human brain as revealed by cortical gamma-band electroencephalogram. Neuroscience 130:91–94
Salinas E, Sejnowski TJ (2001) Correlated neuronal activity and the flow of neural information. Nat Rev Neurosci 2:539–550
Schneider TR, Debener S, Oostenveld R, Engel AK (2008) Enhanced EEG gamma-band activity reflects multisensory semantic matching in visual-to-auditory object priming. Neuroimage 42:1244–1254
Schnider A, Hanlon RE, Alexander DN, Benson DF (1997) Ideomotor apraxia: behavioral dimensions and neuroanatomical basis. Brain Lang 58:125–136
Simons DJ, Carvell GE, Kyriazi HT, Bruno RM (2007) Thalamocortical conduction times and stimulus-evoked responses in the rat whisker-to-barrel system. J Neurophysiol 98:2842–2847
Singer W (2009) Distributed processing and temporal codes in neuronal networks. Cogn Neurodyn 3:189–196
Stam CJ (2005) Nonlinear dynamical analysis of EEG and MEG: review of an emerging field. Clin Neurophysiol 116:2266–2301
Stam CJ, van Walsum AM, Pijnenburg YA, Berendse HW, de Munck JC, Scheltens P, van Dijk BW (2002) Generalized synchronization of MEG recordings in Alzheimer’s disease: evidence for involvement of the gamma band. J Clin Neurophysiol 19:562–574
Stam CJ, van der Made Y, Pijnenburg YA, Scheltens P (2003) EEG synchronization in mild cognitive impairment and Alzheimer’s disease. Acta Neurol Scand 108:90–96
Stam CJ, Jones BF, Manshanden I, van Walsum AM, Montez T, Verbunt JP, de Munck JC, van Dijk BW, Berendse HW, Scheltens P (2006) Magnetoencephalographic evaluation of resting-state functional connectivity in Alzheimer’s disease. Neuroimage 32:1335–1344
Tallon-Baudry C, Bertrand O (1999) Oscillatory gamma activity in humans and its role in object representation. Trends Cogn Sci 3:151–162
Tallon-Baudry C, Bertrand O, Peronnet F, Pernier J (1998) Induced gamma-band activity during the delay of a visual short-term memory task in humans. J Neurosci 18:4244–4254
Tao HY, Tian X (2005) Coherence Characteristics of Gamma-band EEG during rest and cognitive task in MCI and AD. Conf Proc IEEE Eng Med Biol Soc 3:2747–2750
Teipel SJ, Pogarell O, Meindl T, Dietrich O, Sydykova D, Hunklinger U, Georgii B, Mulert C, Reiser MF, Möller HJ, Hampel H (2008) Regional networks underlying interhemispheric connectivity: an EEG and DTI study in healthy ageing and amnestic mild cognitive impairment. Hum Brain Mapp 30:2098–2119
Uhlhaas PJ, Haenschel C, Nikolic D, Singer W (2008) The role of oscillations and synchrony in cortical networks and their putative relevance for the pathophysiology of schizophrenia. Schizophr Bull 34:927–943
van Deursen JA, Vuurman EF, Verhey FR, van Kranen-Mastenbroek VH, Riedel WJ (2008) Increased EEG gamma band activity in Alzheimer’s disease and mild cognitive impairment. J Neural Transm 115:1301–1311
Varela F, Lachaux JP, Rodriguez E, Martinerie J (2001) The brainweb: phase synchronization and large-scale integration. Nat Rev Neurosci 2:229–239
Wechsler D (1981) Adult Intelligence Scale, Revised (WAIS-R). Psychological Corporation, San Antonio
Welsh KA, Butters N, Mohs RC, Beekly D, Edland S, Fillenbaum G, Heyman A (1994) The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part V. A normative study of the neuropsychological battery. Neurology 44:609–614
Wolf H, Jelic V, Gertz HJ, Nordberg A, Julin P, Wahlund LO (2003) A critical discussion of the role of neuroimaging in mild cognitive impairment. Acta Neurol Scand Suppl 179:52–76
Wright JJ, Robinson PA, Rennie CJ, Gordon E, Bourke PD, Chapman CL, Hawthorn N, Lees GJ, Alexander D (2001) Toward an integrated continuum model of cerebral dynamics: the cerebral rhythms, synchronous oscillation and cortical stability. Biosystems 63:71–88
Yetkin FZ, Rosenberg RN, Weiner MF, Purdy PD, Cullum CM (2006) FMRI of working memory in patients with mild cognitive impairment and probable Alzheimer’s disease. Eur Radiol 16:193–206
Zigmond AS, Snaith RP (1983) The hospital anxiety and depression scale. Acta Psychiatr Scand 67:361–370
Conflict of interest statement
This study has not been submitted elsewhere for publication, in whole or in part, and all the authors listed have approved the manuscript. The authors declare that there are no actual or potential conflicts of interest. All have read and have abided by the statement of ethical standards for manuscripts submitted to this journal.
Author information
Authors and Affiliations
Corresponding author
Additional information
P. Missonnier and F. R. Herrmann have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Missonnier, P., Herrmann, F.R., Michon, A. et al. Early disturbances of gamma band dynamics in mild cognitive impairment. J Neural Transm 117, 489–498 (2010). https://doi.org/10.1007/s00702-010-0384-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-010-0384-9