Abstract
The study objective was to assess the efficacy, safety and feasibility of switching from levodopa/benserazide (LB) or levodopa/carbidopa (LC) to levodopa/carbidopa/entacapone (LCE) in Parkinson’s disease (PD) patients with wearing-off. This was a multicenter, open-label, 6-week study; the primary outcome was success rate based on the patient-assessed Clinical Global Impression of Change (P-CGI-C). Secondary outcomes included investigator-assessed CGI-C (I-CGI-C), change from baseline in Unified Parkinson’s Disease Rating Scale (UPDRS), motor/non-motor wearing-off symptoms and quality of life–visual analog scale (QoL-VAS). After switching to LCE, 77% of patients reported an ‘improvement’ (p < 0.0001 vs. patients reporting ‘no change or worsening’). Significant improvements were seen in I-CGI-C, UPDRS and QoL-VAS, regardless of prior therapy. Oral levodopa dosing was increased in 28% of patients; the primary outcome remained significant when these patients were excluded. The data suggest that switching from LB/LC to LCE provided a significant benefit in PD patients with wearing-off.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is a consensus that levodopa, in combination with a dopa-decarboxylase inhibitor (DDCI), provides the most effective symptomatic treatment for Parkinson’s disease (PD) (Olanow et al. 2004). However, long-term therapy is often associated with the development of motor complications. These are thought to be a result of both disease progression and the short half-life of levodopa, which results in deep troughs of plasma levodopa levels and subsequent fluctuations of clinical response (Stocchi 2006; Muller et al. 2006a, b; Kuoppamäki et al. 2009).
Levodopa/carbidopa/entacapone (LCE) is an enhanced levodopa formulation that provides dual inhibition of dopa-decarboxylase (DDC) and catechol-O-methyltransferase (COMT), the two main enzymes involved in levodopa metabolism. When compared with levodopa/DDCI formulations, LCE increases the bioavailability of levodopa by 25–50% and extends the plasma half-life of levodopa by 25–75% (Gordin et al. 2004; Kuoppamäki et al. 2009). The clinical benefit of levodopa/DDCI and entacapone therapy in PD patients with established wearing-off has been demonstrated in double-blind, placebo-controlled, long-term studies. At baseline, these patients had a mean of 5–6 h of motor OFF time and received up to ten daily doses of levodopa/DDCI (Brooks and Sagar 2003; Poewe et al. 2002; Rinne et al. 1998; Parkinson Study Group 1997). However, there are fairly limited data available on the benefits of LCE in clinical practice. In addition, although the above-mentioned studies included patients on both levodopa/carbidopa (LC) and levodopa/benserazide (LB), there are limited prospective data investigating the clinical efficacy and safety of the direct switch from levodopa/DDCI formulations to LCE (Koller et al. 2005; Brooks et al. 2005; Linazasoro et al. 2008). Over the last 10 years, awareness of the incidence of non-motor symptoms during periods of wearing-off has increased. The 9-item Wearing-off Questionnaire (WOQ-9) has been developed to assess the presence of these non-motor features and to help in identifying patients with PD who present with both motor and non-motor wearing-off symptoms (Stacy et al. 2006). However, as yet, there have been no studies using the WOQ-9 as a screening tool.
The aim of this open-label study was to investigate the efficacy, safety and feasibility of the direct switch from either LB or LC to LCE in a study resembling routine clinical practice in PD patients with wearing-off. The WOQ-9 was used to identify the presence of motor and/or non-motor wearing-off symptoms.
Materials and methods
Study design
The SENSE study was a multinational, multicenter, open-label, single-arm Phase IV study, conducted in 25 centers in Germany, Sweden and the UK. The study consisted of a 2-week screening period, followed by a 6-week treatment period. A screening visit was made during the 2 weeks prior to the baseline visit. Following the baseline visit, subsequent visits were made at weeks 1, 2, 4 and 6, and a telephone call was made on either day 3 or 4. Study treatment was initiated the day after the baseline visit and subjects acted as their own controls. This Good Clinical Practice (GCP) study was approved by the responsible ethical committee.
Study population
The study population included male or female patients with PD (≥35 years old). All study subjects fulfilled the following inclusion criteria: wearing-off, defined as the presence of at least one positive symptom in the WOQ-9 questionnaire; treatment with standard release levodopa/DDCI at a dosing frequency of three or four daily doses (maximum total daily levodopa dose of 600 mg); and a Hoehn and Yahr (H&Y) stage of 1–3 assessed during the ON state. Other anti-parkinsonian medications [amantadine, monoamine oxidase (MAO)-B inhibitors, anticholinergics and dopamine agonists] were allowed and the doses of these drugs and the patient’s levodopa/DDCI therapy remained unchanged for at least 6 weeks preceding baseline. An evening dose of controlled-release levodopa/DDCI and one dose of soluble levodopa/DDCI as the first dose of the morning were also allowed.
Exclusion criteria included intake of a COMT inhibitor during the 6 weeks prior to baseline; concomitant treatment with a non-selective MAO inhibitor, or simultaneous use of higher than recommended doses of MAO-A and MAO-B inhibitors; and use of apomorphine, drugs with anti-dopaminergic action, such as reserpine, antipsychotic drugs (1 evening dose of an atypical antipsychotic drug was allowed) and D2-receptor-blocking antiemetics, except domperidone. Other exclusion criteria were patients with unpredictable OFF periods, dyskinesia, clinically significant concomitant diseases, or abnormal laboratory values or electrocardiogram (ECG). Females who were of childbearing potential, pregnant or lactating were also excluded.
Study treatment
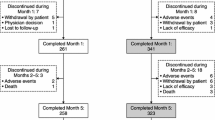
The study medication included three strengths of LCE: 50 levodopa/12.5 carbidopa/200 mg entacapone; 100/25/200 mg and 150/37.5/200 mg (Stalevo® 50, Stalevo® 100 and Stalevo® 150, respectively, manufactured by Orion Pharma, Espoo, Finland). Patients were to be stratified 50:50 according to their previous therapy (LB or LC; Fig. 1) and switched to LCE at the equivalent levodopa dose and dosing frequency (three or four times daily) of the previous standard levodopa preparation. The oral levodopa dosing could be adjusted by altering the strength of LCE during weeks 1 and 2, depending on the clinical response and tolerability of the patient. After this adjustment period, the dose was to remain as constant as possible. The number of doses was to remain unchanged during the 6-week study period.
Outcome variables
The primary outcome variable was success rate based on the patient-assessed clinical global impression of change (P-CGI-C) at the last visit (week 6) or at premature discontinuation. Patients were assessed on any change in their condition using a 7-point scale: ‘very much improved’, ‘much improved’, ‘a little improved’, ‘no change’, ‘a little worse’, ‘much worse’ or ‘very much worse’. Secondary outcome variables included investigator-assessed CGI-C (I-CGI-C), change from baseline in Unified Parkinson’s Disease Rating Scale (UPDRS) Parts II and III scores (determined 2–3 h after the study drug intake), health-related quality of life (QoL) [assessed with a 100 mm visual analog scale (VAS)] and response to LCE on the wearing-off symptoms (assessed by the patients using a seven-point scale similar to CGI-C), which were identified by the WOQ-9 at screening. The oral levodopa dosing was also analyzed as an additional variable.
Safety
A standard 12-lead ECG, laboratory tests (hematology and clinical chemistry) and a physical examination that included vital signs (systolic and diastolic blood pressure and heart rate) and body weight were performed at screening. All adverse events (AEs), serious AEs (SAEs) and vital signs were recorded at each study visit. Adverse events and SAEs were classified according to the Medical Dictionary for Regulatory Activities (MedDRA). All examinations performed at baseline, except the ECG, were repeated at week 6.
Statistical analysis
The intention-to-treat (ITT) population consisted of all patients who provided post-baseline efficacy data, and all patients who received at least one dose of study medication were included in the safety analysis. For the analyses, a two-sided p value of <0.05 was considered statistically significant. The results of the P-CGI-C at week 6 were grouped as an improvement (‘very much improved’, ‘much improved’ and ‘a little improved’) versus no difference or a worsening (‘a little worse’, ‘much worse’ and ‘very much worse’) of disease. The success rate was defined as the percentage of patients with an outcome of improvement and the treatment success rate was analyzed by calculating a p value comparing the success rate to a threshold of 50% using binomial distribution. UPDRS Parts II and III scores at the study end were compared with baseline scores with analysis of covariance for repeated measures. Any missing values in the UPDRS scores and levodopa doses were replaced using the last observation carried forward (LOCF) imputation. Frequency tabulations were used to assess the changes from baseline to week 6 in all the wearing-off symptoms identified at baseline. Furthermore, a sub-analysis for the primary variable was performed excluding patients whose oral levodopa dosing had increased.
A post hoc, exploratory analysis was performed to determine the factors that appeared to be associated with the observed changes (improvement/no improvement) in the non-motor symptoms of wearing-off. Based on a stepwise logistic regression analysis, H&Y staging classified as earlier disease stage (H&Y ≤2) or more advanced disease stage (H&Y ≥2.5) was identified as the most predictive factor. Therefore, the proportion of patients with an ‘improved’ outcome in the non-motor symptoms was compared between earlier disease stage and more advanced disease stage patients using the Cochran–Mantel–Haenszel test.
Results
Demographics and other baseline characteristics
A total of 130 patients were screened and, of these, 115 patients (71 males and 44 females) were enrolled into the study. Out of the 115 enrolled patients, 113 were included in the ITT population (2 patients were excluded due to lack of post-baseline efficacy measurements) and 107 completed the study. At screening, all patients had at least one motor symptom and 82% had at least one non-motor symptom of wearing-off. At baseline, 68 patients (59%) were receiving LB and 47 patients (41%) were receiving LC (Fig. 1). No significant differences were seen between the patients receiving either LB or LC, and the levodopa dose (mean ± SD) was comparable between the LB (325 ± 97 mg) and LC (342 ± 84 mg) groups (Table 1). Detailed baseline demographics and other baseline characteristics are described in Table 1, showing data for the LB and LC populations, as well as those with early stage (H&Y ≤2) and advanced stage (H&Y ≥2.5) disease.
Primary efficacy variable
Regardless of prior therapy (LB or LC), switching to LCE was associated with improved P-CGI-C, with significantly more patients reporting an improvement compared with no change or worsening (p < 0.0001). In detail, 2.7% of patients reported an improvement, as assessed the by P-CGI-C, as ‘very much improved’, 25.7% of patients reported an improvement as ‘much improved’, and 48.7% of patients as ‘a little improved’. Overall, 15.0% of patients found no difference, 5.3% of patients estimated their P-CGI-C scores as ‘a little worse’, 1.8% of patients as ‘much worse’ and 0.9% of patients as ‘very much worse’. The estimated success rate (defined as the percentage of patients with at least a little improvement) was 77.0% (95% confidence interval [CI]: 69.2, 84.8) (Fig. 2a).
Success rate (% of patients with at least a little improvement) according to CGI-C assessed by patient (a) and investigator (b); success rate was analyzed by calculating a p value comparing the success rate to a threshold of 50% using binomial distribution (ITT population) LB levodopa/benserazide, LC levodopa/carbidopa, CGI-C Clinical Global Impression of Change, ITT intention-to-treat
Secondary efficacy variables
Investigator-assessed CGI-C scores demonstrated a significant benefit when patients were switched to LCE. In detail, 1.8% of investigators reported improvements, as assessed by the I-CGI-C, as ‘very much improved’, 23.9% of investigators as ‘much improved’ and 58.4% of investigators as ‘a little improved’. A total of 15.0% of investigators found no difference and 0.9% of investigators rated the I-CGI-C score as ‘much worse’. The estimated success rate was 84.1% (95% CI: 77.3, 90.8; p < 0.0001). The benefit was significant regardless of whether patients received LB or LC at baseline (Fig. 2b). There was a high correlation between the patient-assessed and the investigator-assessed CGI-C scores (Spearman’s correlation coefficient = 0.66, p < 0.001). Patients’ and investigators’ assessments matched in 68% of the cases; in 19% of the cases the investigators’ assessments were more positive, and in 13% of the cases the patients’ assessments were more positive.
Significant improvements were also seen in the mean change from baseline for UPDRS Part II [activities of daily living (ADL); p < 0.0001] and Part III (motor; p < 0.0001) scores (Table 2). For UPDRS Part II, the change (improvement) from baseline was significantly greater in the LB than in the LC group (p = 0.021), while for UPDRS Part III, the change from baseline in both the LB and LC groups was comparable (p = 0.341). When analyzed by sub-scores, significant improvements were observed in UPDRS III for tremor (Questions 20, 21; p < 0.0001), rigidity (Question 22; p < 0.0001) and akinesia (Questions 23–26, 31; p < 0.0001), accordingly (Table 2). There was no correlation between these sub-scores and mood changes that were detected by WOQ-9 at screening.
Quality of life-VAS scores also showed a significant improvement after the switch to LCE regardless of prior therapy. At week 6, the estimated improvement from baseline in QoL-VAS scores was 4.7 mm (95% CI: 1.6, 7.8 mm) for the total population (p = 0.003).
The most commonly reported motor wearing-off symptoms at screening were ‘any slowness of movement’ (91% of patients) and ‘reduced dexterity’ (90%), while the most common non-motor wearing-off symptoms were ‘cloudy mind/slowness of thinking’ (54%) and ‘mood changes’ (43%; Table 3). Overall, positive responses to LCE were reported in all five motor wearing-off symptoms (at least ‘little improvement’ reported by 53–74% of patients) and in all four non-motor wearing-off symptoms (30–52%) included in the WOQ-9. After switching to LCE, the highest frequencies of improvement were observed in ‘tremor’ (74% of patients) and ‘any stiffness’ (62%) among the motor symptoms, and in ‘mood changes’ (52%) and ‘cloudy mind/slowness of thinking’ (34%) among the non-motor symptoms.
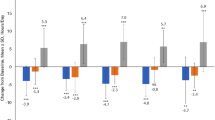
In the post hoc analysis, H&Y staging classified as earlier disease stage (H&Y ≤2) or more advanced disease stage (H&Y ≥2.5) was identified as the most predictive factor of change in non-motor wearing-off symptoms. The proportion of patients with any non-motor symptom was similar at baseline between earlier and more advanced disease stage. Switching to LCE improved mood in significantly more patients with earlier compared with more advanced disease stage (75 vs. 29%, respectively; p = 0.002) (Fig. 3). There was an observed trend towards more patients improving in the earlier compared with more advanced disease stage in anxiety (46 vs. 10%; p = 0.068) and pain/aching (37 vs. 26%; p = 0.388), while no difference was seen in cloudy mind/slowness of thinking (33 vs. 36%; p = 0.861). Overall, 96% of patients with improvement in mood, 89% in pain/aching, 91% in cloudy mind/slowness of thinking and 100% in anxiety also reported treatment success by improvement in P-CGI-C score.
Levodopa dose
An additional variable was the analysis of adjustments in levodopa dose that occurred after the switch to LCE. The levodopa dose was maintained at baseline level in 63% of patients, increased in 28% and decreased in 9%. Of the patients whose dose increased [mean dose increase was 119 mg (range 50–200 mg)] 17 (15%) increased their dose during week 1, 11 (10%) during week 2, and 4 (3%) after 2 weeks. Slightly more patients on LB (n = 20, 29%) compared with LC (n = 12, 26%) needed levodopa dose escalation after the switch, the increase being comparable between the LB (123 mg) and LC (113 mg) groups. Of those patients whose dose decreased, mean dose decrease was 80 mg (range 50–200 mg), six (5%) decreased the dose during week 1, and one (1%) during weeks 2 and 3 (3%) after 2 weeks. The lack of efficacy was the most common reason for a dose increase, and an AE was the most common reason for a dose decrease. Adverse events leading to a dose decrease included three cases of dyskinesia, three of insomnia and one each of restlessness, dystonia, edema and vertigo. At week 6, the dosing frequency was maintained at baseline level in 94% of patients, and in 6% of patients the dosing frequency had increased from three to four times daily. The primary variable, improvement in P-CGI-C score following the switch to LCE at week 6, remained significant (p < 0.001) in a sub-analysis that excluded patients whose oral levodopa dosing had increased, the estimated success rate being 71.6% (95% CI: 61.8, 81.4).
Safety and tolerability
A total of 68.7% of patients (61.8 and 78.7% in patients receiving LB and LC at baseline, respectively) developed an AE during the course of the study. The most common AEs (>3%) included chromaturia (20%), nausea (11%), diarrhea (5%), dyskinesia (5%), vertigo (5%), insomnia (4%), dizziness (3%), fatigue (3%) and nasopharyngitis (3%). Most of the AEs were either mild (72.3%) or moderate (25.9%) in severity. Only three of the AEs were rated as severe (1.8%); one case each of vomiting, tremor and freezing. All of the severe AEs were reported in patients who had switched from LC. In general, there were no clear differences in AEs reported by patients receiving either LB or LC at baseline (Table 4). Four SAEs were experienced during the study by three patients (2.6%). These were skin infection and pacemaker complication experienced by one patient and upper respiratory tract infection and hallucination each experienced by one patient, respectively. The only SAE assessed to be related to the study treatment was hallucination in a patient receiving additional pramipexole who subsequently discontinued the study. There were no deaths during the study.
Altogether, eight patients (7.0%) discontinued treatment: two patients (3%) on LB and six patients (13%) on LC. The main reasons for the discontinuations were AE/SAEs in five patients, two of them on LB (1 hallucination, 1 dizziness) and three on LC (1 dyskinesia, 1 nausea, 1 vomiting). The other three discontinuations (all in the LC group) were due to the lack of efficacy in one patient, withdrawal of consent in one patient and ‘other reason’ (abnormal laboratory values that were present at screening) in one patient.
Discussion
The SENSE study aimed to investigate the efficacy, safety and feasibility of the direct switch from either LB or LC to LCE in PD patients with wearing-off. The predefined primary endpoint of this open-label study reports clinical benefits in the context of a direct switch to LCE. These benefits have been previously demonstrated in controlled, randomized studies (Brooks and Sagar 2003; Parkinson Study Group 1997; Poewe et al. 2002; Rinne et al. 1998) and our present findings show that these benefits are also observed in an open-label study design which more closely resembles that of routine clinical practice. Although the non-controlled design of our study may limit evidence-based conclusions, the impact of placebo effects in controlled clinical trials should not be underestimated. Placebo-induced clinical benefits in PD trials have been associated with reward-related pathways that involve dopamine release in the striatum (de la Fuente-Fernandez and Stoessl 2004; de la Fuente-Fernandez et al. 2004). The results of a meta-analysis of Goetz et al. showed an overall placebo response rate of 16% (range 0–55%), with the highest rate occurring in trials of patients with advanced PD who required symptomatic intervention because of motor fluctuations (Goetz et al. 2008).
The primary efficacy variable (success rate based on a patient’s CGI-C score) was positive in this study showing improvement in 77.0% of patients. The success rate was based on the patients reporting any improvement in P-CGI-C score and summarized the three P-CGI-C items ‘very much improved’, ‘much improved’ and ‘little improved’. This last item, ‘little improved’, was reported by 48.7% of all patients in the study, thus representing almost two-thirds of the 77% of patients who reported any type of improvement. Therefore, the clinical impact of the observed primary outcome (a sum of any type of improvement in the patient’s CGI-C score) should be considered with caution. The I-CGI-C, one of the secondary efficacy variables, gave a similar result with ‘little improvement’ in 58.4% of all patients, and ‘any improvement’ in 84.1% of patients. The high correlation observed between the physician and the patient rating of CGI-C is further evidence for the reliability of these results.
Overall, it was found that patients previously treated with LB responded at least as well as those previously treated with LC. This observation suggests that changing two substances in the direct switch from LB to LCE is feasible, safe and associated with mild benefits in efficacy.
There were clinically relevant improvements in UPDRS scores following the switch to LCE. Significant improvements were seen in the patient-derived questionnaire on activities of daily living (UPDRS-ADL, Part II) and the physician-derived testing of motor function (UPDRS motor, Part III) scores. Interestingly, significant improvements were also seen in tremor, rigidity and akinesia sub-scores of the UPDRS scale. As these parameters were assessed 2–3 h after the intake of the LCE preparation, their improvement most likely reflects a reduction in the time or severity of the wearing-off phenomenon. These improvements in UPDRS outcomes may perhaps have more clinical relevance than the primary endpoint in the assessment of PD patients.
Significant improvement was also seen in QoL-VAS scores after switching to LCE. Quality of life-VAS scores were also seen to improve after switching to LCE in another study by Brooks et al. and, interestingly, it was also found that patients randomized to switch from levodopa/DDCI to LCE, as a single tablet, had significantly better improvement in QoL-VAS scores compared with patients who switched to separately administered LC and entacapone tablets (Brooks et al. 2005). The exact reasons for this difference are not fully understood, but better compliance due to a more convenient intake of LCE may explain this finding.
After the switch to LCE, the levodopa dose was maintained at baseline level in 63% of patients, increased in 28% (mostly due to lack of adequate efficacy) and decreased in 9% (mostly due to AEs). Slightly more patients on LB compared with those on LC needed levodopa dose escalation after the switch, the amount of increase being comparable between the LB versus LC patients (i.e. 123 vs. 113 mg, respectively). However, the primary efficacy variable remained significant in a sub-analysis excluding patients whose oral levodopa dosing was increased during the follow-up. In addition, the dosing frequency of levodopa was increased in 6% of patients from three to four times daily. These data reflect experience in clinical practice. Therefore, physicians should be aware that after a direct switch from LB or LC to an equivalent dose and dosing frequency of LCE in PD patients with wearing-off, levodopa may have to be increased to achieve optimal motor control in more than a quarter of patients. On the other hand, appearance or exacerbation of dyskinesia is a possible side effect of entacapone and for those patients taking high doses of levodopa. Thus, comprehensive patient education of the efficacy and possible side effects, and a close patient surveillance during the first 2–3 weeks after the switch to LCE could stabilize therapy outcomes.
The WOQ-9 was developed to enable standardized and easily implemented detection of wearing-off symptoms in PD patients (Stacy et al. 2006). This questionnaire was used as a screening tool in the SENSE study to more easily identify patients with motor and non-motor symptoms of wearing-off. All patients had at least one motor wearing-off symptom, and 82% of patients had at least one additional non-motor wearing-off symptom. This indicates the fact that non-motor symptoms are frequently present during wearing-off. The wearing-off tool was not used as a rating scale; however, when patients were questioned about the change in their symptoms detected at screening, the motor and non-motor wearing-off symptoms were reported to be improved in 62 and 38% of patients, respectively, following the switch to LCE. When interpreting these results, it should be taken into consideration that the WOQ-9 has not been validated as a tool to assess treatment effects.
Hoehn and Yahr staging was identified as the most important factor in determining the responsiveness of non-motor symptoms when switching to LCE. Earlier disease stage, as defined by H&Y ≤2, was associated significantly more frequently with improvement in mood changes when compared with more advanced disease stage, defined as H&Y ≥2.5. A similar trend was seen for anxiety and pain and aching. The association of relief in non-motor symptoms with lower H&Y stages may be an indication that entacapone helps to improve non-motor symptoms in the early stages of the disease. However, there were no correlations between changes in mood and the UPDRS sub-scores of tremor, rigidity and akinesia symptoms. A compound with additional properties, over and above a dopaminergic mode of action, may be required to optimally control non-motor symptoms in the advanced stages of the disease, during which non-motor symptoms become a source of increasing disability for the patient. Nevertheless, the observation that over 90% of these patients reported an improvement, as assessed by P-CGI-C, may be considered as support in favor of switching to LCE for the treatment of the non-motor symptoms of wearing-off. Based on these results, the WOQ-9 appears to be a useful tool to detect patients with wearing-off who will benefit from a switch to LCE.
The direct switch to LCE was generally well tolerated without any clinically significant differences occurring between patients who were receiving LB or LC at baseline. The incidence and frequency of AEs were similar to those reported in previous studies (Linazasoro et al. 2008; Brooks et al. 2005; Koller et al. 2005). With respect to dyskinesia, previous studies with levodopa/DDCI and entacapone in PD patients with wearing-off symptoms have reported the frequency of dyskinesia to be around 20–30% (Brooks and Sagar 2003; Poewe et al. 2002; Rinne et al. 1998; Parkinson Study Group 1997). During this study, only 6 out of 115 (5%) patients reported dyskinesia as an AE after switching to LCE; the difference in the incidence of dyskinesia between previously reported studies and our findings may reflect differences in study duration or stage of disease.
In conclusion, the study conducted in PD patients with wearing-off showed that the direct switch from LB or LC to LCE was feasible and safe in a setting resembling routine clinical practice, and that the WOQ-9 appears to be a valuable tool to help in identifying patients with wearing-off who are likely to benefit from switching to LCE.
References
Brooks DJ, Sagar H (2003) Entacapone is beneficial in both fluctuating and non-fluctuating patients with Parkinson’s disease: a randomised, placebo controlled, double blind, six month study. J Neurol Neurosurg Psychiatry 74:1071–1079
Brooks DJ, Agid Y, Eggert K, Widner H, Ostergaard K, Holopainen A, The TC-INIT Study Group (2005) Treatment of end-of-dose wearing-off in Parkinson’s disease: Stalevo® (Levodopa/Carbidopa/Entacapone) and levodopa/DDCI given in combination with Comtess®/Comtan® (Entacapone) provide equivalent improvements in symptom control superior to that of traditional levodopa/DDCI treatment. Eur Neurol 53:197–202
de la Fuente-Fernandez R, Stoessl AJ (2004) The biochemical bases of the placebo effect. Sci Eng Ethics 10:143–150
de la Fuente-Fernandez R, Schulzer M, Stoessl AJ (2004) Placebo mechanisms and reward circuitry: clues from Parkinson’s disease. Biol Psychiatry 56:67–71
Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stern MB, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, LeWitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, van Hilten JJ, LaPelle N (2008) Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 23:2129–2170
Gordin A, Kaakkola S, Teravainen H (2004) Clinical advantages of COMT inhibition with entacapone––a review. J Neural Transm 111:1343–1363
Koller W, Guarnieri M, Hubble J, Rabinowicz AL, Silver D (2005) An open-label evaluation of the tolerability and safety of Stalevo® (carbidopa, levodopa and entacapone) in Parkinson’s disease patients experiencing wearing-off. J Neural Transm 112:221–230
Kuoppamäki M, Korpela K, Marttila R, Kaasinen V, Hartikainen P, Lyytinen J, Kaakkola S, Hänninen J, Löyttyniemi E, Kailajärvi M, Ruokoniemi P, Ellmén J (2009) Comparison of pharmacokinetic profile of levodopa throughout the day between levodopa/carbidopa/entacapone and levodopa/carbidopa when administered four- or five-times daily. Eur J Clin Pharmacol 65:443–455
Linazasoro G, Kulisevsky J, Hernandez B (2008) Should levodopa dose be reduced when switched to stalevo? Eur J Neurol 15:257–261
Muller T, Erdmann C, Muhlack S, Bremen D, Przuntek H, Goetze O, Woitalla D (2006a) Pharmacokinetic behaviour of levodopa and 3-O-methyldopa after repeat administration of levodopa/carbidopa with and without entacapone in patients with Parkinson’s disease. J Neural Transm 113:1441–1448
Muller T, Erdmann C, Muhlack S, Bremen D, Przuntek H, Woitalla D (2006b) Inhibition of catechol-O-methyltransferase contributes to more stable levodopa plasma levels. Mov Disord 21:332–336
Olanow CW, Agid Y, Mizuno Y, Albanese A, Bonuccelli U, Damier P, De Yebenes J, Gershanik O, Guttman M, Grandas F, Hallett M, Hornykiewicz O, Jenner P, Katzenschlager R, Langston WJ, LeWitt P, Melamed E, Mena MA, Michel PP, Mytilineou C, Obeso JA, Poewe W, Quinn N, Raisman-Vozari R, Rajput AH, Rascol O, Sampaio C, Stocchi F (2004) Levodopa in the treatment of Parkinson’s disease: current controversies. Mov Disord 19:997–1005
Parkinson Study Group (1997) Entacapone improves motor fluctuations in levodopa-treated Parkinson’s disease patients. Ann Neurol 42:747–755
Poewe WH, Deuschl G, Gordin A, Kultalahti ER, Leinonen M (2002) Efficacy and safety of entacapone in Parkinson’s disease patients with suboptimal levodopa response: a 6-month randomized placebo-controlled double-blind study in Germany and Austria (Celomen study). Acta Neurologica Scand 105:245–255
Rinne UK, Larsen JP, Siden A, Worm-Petersen J, The Nomecomt Study Group (1998) Entacapone enhances the response to levodopa in parkinsonian patients with motor fluctuations. Neurology 51:1309–1314
Stacy M, Hauser R, Oertel W, Schapira A, Sethi K, Stocchi F, Tolosa E (2006) End-of-dose wearing off in Parkinson disease: a 9-question survey assessment. Clin Neuropharmacol 29:312–321
Stocchi F (2006) The levodopa wearing-off phenomenon in Parkinson’s disease: pharmacokinetic considerations. Expert Opin Pharmacother 7:1399–1407
Acknowledgments
This study was funded by Orion Corporation Orion Pharma. The authors would like to thank Diya Lahiri, Ph.D. for her editorial assistance.
Conflict of interest statement
Karla Eggert has worked as a consultant for Orion Pharma, Schwarz Pharma Neuroscience (UCB), Solvay Pharmaceuticals, Valeant Pharmaceuticals International, Desitin, Orphane Europe, Boehringer Ingelheim, GlaxoSmithKline. She receives scientific grants from the German Ministry of Education and Health, from the Pitzer Foundation and from the Rhön Foundation; Helena Nissinen, Mikko Kuoppamäki and Liisa Luotonen are employees of Orion Pharma; Örjan Skogar and Khaled Amar have acted as consultants for Orion Pharma; statistical analysis was carried out my Mika Leinonen who has served as a consultant for Orion Pharma; W. H. Oertel has worked as a consultant for Boehringer Ingelheim, EISAI Medical Research, GE Health, GlaxoSmithKline, Novartis, Orion Pharma, Schwarz Pharma Neuroscience (UCB), Solvay Pharmaceuticals, Teva Neuroscience, Valeant Pharmaceuticals International. Prof. Dr. W. H. Oertel and his collaborators have received grants from Boehringer Ingelheim, Hoffmann LaRoche, Schwarz Pharma Neuroscience for basic science research and educational support. He receives scientific grants from the German Ministry of Education and Health, from the German Research Foundation, from the Pitzer Foundation and from the Rhön Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
On behalf of the SENSE study group.
Appendix
Appendix
The members of the SENSE Study Group are: Khaled Amar, The Royal Bournemouth Hospital, Bournemouth, UK; Ralf Bodenschatz, Pharmakologisches Studienzentrum Chemnitz, Chemnitz, Germany; Carsten Buhmann, Universitätskrankenhaus Eppendorf, Hamburg, Germany; Sven-Erik Bysell, Visby lasarett, Visby, Sweden; Ilona Csoti, Parkinson-Zentrum Gertrudis-Klinik, Leun-Biskirchen, Germany; Paul Davies, Northampton General Hospital, Northampton, UK; Karla Eggert, Klinik für Neurologie, Philipps-Universität Marburg, Germany; Christer Ewaldsson, Neurologmottagningen, Västra Frölunda, Sweden; Duncan Forsyth, Addenbrookes Hospital, Cambridge, UK; Thomas Gasser, Eberhard-Karls-Universität, Tübingen, Germany; Jan Kassubek, Neurologische Klinik der Universität Ulm, Ulm, Germany; Andreas Kupsch, Humboldt Universität Charité Neurologische Klinik, Berlin, Germany; Nelson Lo, Leicester General Hospital, Leicester, UK; Wolfhard Lünser, Facharzt für Neurologie und Neurochirurgie, Hamm, Germany; Thomas Müller, St. Josef-Hospital Klinikum der Ruhr-Universität-Bochum, Bochum, Germany; Alexander Nass, Nervenarztpraxis, Köln, Germany; Udo Polzer, Asklepios Fachklinikum Stadtroda, Stadtroda, Germany; Gerd Reifschneider, Gemeinschaftspraxis für Neurologie & Psychiatrie, Erbach, Germany; Stuart Rochow, Woodend Hospital, Aberdeen, UK; Anirban Saha, Royal Sussex County Hospital, Brighton, UK; Christine Schuster, Neurologische Praxis, Giessen, Germany; Örjan Skogar, Länssjukhuset Ryhov, Jönköping, Sweden; Alexander Storch, Technische Universität Dresden, Dresden, Germany; Claudia Trenkwalder, Paracelsus-Elena Klinik, Kassel, Germany; Wolfgang Oertel, Klinik für Neurologie, Philipps-Universität Marburg, Germany; Hans-Jürgen von Giesen, Alexianer-Krankenhaus, Krefeld, Germany; Görel Wachtmeister, Nyköpings Lasarett, Nyköping, Sweden; Richard Walker, North Tyneside General Hospital, North Shields, UK; Ulf Wigow, Höglandssjukhuset i Nässjö, Nässjö, Sweden.
Rights and permissions
About this article
Cite this article
Eggert, K., Skogar, Ö., Amar, K. et al. Direct switch from levodopa/benserazide or levodopa/carbidopa to levodopa/carbidopa/entacapone in Parkinson’s disease patients with wearing-off: efficacy, safety and feasibility—an open-label, 6-week study. J Neural Transm 117, 333–342 (2010). https://doi.org/10.1007/s00702-009-0344-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-009-0344-4