Abstract
Dopamine replacement therapy in Parkinson’s disease (PD) using l-dopa is invariably associated with a loss of drug efficacy (“wearing off”) and the onset of dyskinesia. The use of dopamine receptor partial agonists might improve therapeutic benefit without increased dyskinesia expression but may antagonise the effects of l-dopa. We now examine the effects of the novel high affinity, dopamine D2 receptor partial agonist, aplindore alone and in combination with l-dopa in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated common marmoset. In non-dyskinetic MPTP treated animals, aplindore (0.05–1.0 mg/kg p.o.) produced a dose-dependent reversal of motor disability and an increase in locomotor activity that was maximal at doses of 0.2 mg/kg and above. In animals previously exposed to l-dopa to induce dyskinesia, escalating and repeated dosing of aplindore (0.05–0.5 mg/kg p.o.) produced a sustained, dose-related improvement in motor disability and an increase in locomotor activity. The effects were maximal at a dose of 0.1 mg/kg and above and not different from those produced by l-dopa (12.5 mg/kg plus carbidopa 12.5 mg/kg p.o.). Aplindore administration also led to dose-dependent expression of dyskinesia but at 0.1 mg/kg, this was significantly less intense than that produced by l-dopa. Administration of aplindore (1.0 mg/kg p.o.) in combination with l-dopa (2.5 mg/kg plus carbidopa 12.5 mg/kg p.o.) did not inhibit the reversal of motor deficits but improved motor disability and increased both locomotor activity and dyskinesia expression equivalent to that produced by l-dopa (12.5 mg/kg plus carbidopa 12.5 mg/kg p.o.). These data suggest that dopamine receptor partial agonists would be effective in the treatment of Parkinson’s disease and would not inhibit the beneficial actions of l-dopa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dopamine replacement therapy in the form of l-dopa or dopamine agonists continues to dominate the treatment of the motor symptoms of Parkinson’s disease (PD). While highly effective in the control of the early stages of PD, motor complications appear on chronic treatment and with disease progression that are a major problem in the management of mid- to late-stage illness. In particular, there is a decrease in the duration of drug effect (‘wearing off’), unpredictable drug response (‘on–off’) and the onset of dyskinesia (dystonia, chorea and athetosis) (Obeso et al. 2000; Ahlskog and Muenter 2001; Rascol et al. 2003). When used as monotherapy, dopamine agonists, such as pramipexole and ropinirole, lack the efficacy of l-dopa but prevent the early onset of dyskinesia although they do not protect against motor complications developing with time (Rascol et al. 2000; Holloway et al. 2004). When “wearing off’ to l-dopa appears, dopamine agonists are commonly added to therapy but the increased dopaminergic load leads to increased intensity of established dyskinesia. As a consequence, there is a need for novel dopaminergic approaches to the treatment of PD that can provide increased efficacy but avoid the expression of dyskinesia.
Currently used dopamine agonists are full agonists at dopamine (DA) D2/D3 receptors and an alternative approach might be to use dopamine receptor partial agonists (Lieberman et al. 1987; Baronti et al. 1992; Kehne et al. 2008). Partial agonists would have the advantage of being able to fully occupy normosensitive dopamine receptors in striatal and extra-striatal structures without producing a maximal or supramaximal response because of their low intrinsic activity. Partial agonists could be fully efficacious in producing an antiparkinsonian response by stimulating supersensitive dopamine receptors in the denervated striatum in PD without overstimulation leading to dyskinesia expression. However, there is also the possibility that they might inhibit the actions of l-dopa, acting as a functional antagonist by inhibiting dopamine’s access to post-synaptic receptors. This approach has been tried previously in the treatment of PD but with mixed findings. For example, the dopamine partial agonists, terguride and roxindole (EMD 49980) both avoided and induced dyskinesia when used as monotherapy, both increased and decreased established l-dopa induced dyskinesia, and either had no effect on the activity of l-dopa or inhibited it (Ruggieri et al. 1991; Baronti et al. 1992; Bravi et al. 1993; Pacchetti et al. 1993; Martignoni et al. 1995). Differences in intrinsic activity between compounds could be one factor that contributes to the variable results reported for different D2 partial agonists (Kehne et al. 2008).
Aplindore is a high affinity dopamine D2 receptor partial agonist (pKi = 9.1) (pKi = 8.5, 7.2, 7.6 and 4.9 for D3, D4, 5-HT1A and 5-HT2A receptors, respectively) that exhibits low intrinsic activity relative to the full agonist quinpirole (Heinrich et al. 2006). Depending upon the sensitivity of the functional assay and the signal transduction pathway evoked, aplindore produced 20–76% maximal stimulation in these assays (Heinrich et al. 2006). By definition, a partial agonist should be able to stimulate receptors in the absence of a more efficacious agonist but provide some degree of blockade in the presence of a more efficacious agonist (Stephenson 1956; Carlsson 1983; Kenakin 2002). Aplindore’s partial agonist activity is shown in vivo by its ability to block apomorphine-induced climbing and amphetamine-induced hyperlocomotor activity in normal rats and mice (Andree et al. 1999). In contrast, aplindore’s potential for the treatment of PD is shown in vivo in the 6-hydroxydopamine (6-OHDA) lesioned rat model where it is highly potent in causing contralateral rotation through stimulation of dopamine receptors in the denervated striatum (Heinrich et al. 2006). However, the effects of aplindore in the most highly predictive model of clinical activity, namely the MPTP-treated non-human primate, have not so far been investigated.
In this report, three studies were undertaken to evaluate the actions of aplindore in the MPTP-treated common marmoset. Initially, aplindore was evaluated in a range of doses for its ability to reverse motor disability and to increase locomotor activity in comparison to l-dopa and to the dopamine D2/D3 full agonist, ropinirole in non-dyskinetic animals. Aplindore was subsequently examined alone and in combination with l-dopa in MPTP-treated marmosets previously treated with l-dopa to exhibit dyskinesia in response to acute drug challenge. The objective was to establish whether a maximally effective dose of aplindore produced less dyskinesia than l-dopa. Finally, aplindore in high doses was combined with l-dopa to determine whether this partial agonist did, or did not inhibit the ability of l-dopa to reverse motor disability.
Methods
Animals
Eight adult common marmosets (Callithrix jacchus 6 males and 2 females, weight 340–400 g, King’s College, London, UK) were housed either singly or in pairs, in a room maintained at constant temperature of 24–27°C, 50% relative humidity and with a 12-h light/dark cycle. All animals had ad libitum access to Mazuri food pellets (Mazuri Primate Diet, Special Diet Services Ltd, UK) and water and were fed fresh fruit once-daily.
MPTP administration
To induce locomotor and behavioural deficits 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine hydrochloride (MPTP, Sigma Chemical Co., UK) was dissolved in 0.9% sterile saline solution and administered at 2.0 mg/kg daily for five consecutive days (Jackson et al. 2007). For the following 6–8 weeks, animals were hand fed on a high protein/carbohydrate liquid diet (Marmoset jelly, Special Diet Services Ltd, UK) and Complan (Complan Foods Ltd, UK) until they had recovered from the acute effects of MPTP treatment and were able to self-feed and maintain a stable bodyweight. The animals were then assigned to one of two groups (n = 4/group); one group remained drug naïve until the start of the study and one group was primed to express dyskinesia. At the start of the study, all animals had stable locomotor and behavioural deficits. All experimental work was carried out in accordance with the Animals (Scientific Procedures) Act 1986, under project licence PPL 70/4986.
l-dopa priming
Following recovery from the acute effects of MPTP treatment, four animals (2 males, 2 females) were primed to express dyskinesia. Each animal was administered a daily dose of l-dopa (10–12.5 mg/kg, p.o.) plus carbidopa (12.5 mg/kg, p.o.) for 22–29 days at which time they had reached a stable dyskinetic state expressing intense choreiform and dystonic movements inhibiting normal behaviour (Pearce et al. 1995).
Drug preparation and administration
Aplindore (0.05–2.0 mg/kg, p.o.), ropinirole (0.5 mg/kg) and l-dopa (12.5 mg/kg, p.o.) were all prepared by dissolving in a 10% sucrose solution and were administered at a volume of 2.0 mL/kg. Carbidopa (12.5 mg/kg, p.o.) was prepared as a suspension in a 10% sucrose solution and was administered at a volume of 2.0 mL/kg, 45–60 min prior to l-dopa treatment to prevent peripheral dopa decarboxylation.
Drug naïve animals were treated in the following order with l-dopa (12.5 mg/kg, p.o.), ropinirole (0.5 mg/kg) and aplindore (0.05, 0.1, 0.2, 0.5 and 1.0 mg/kg, p.o.) on separate occasions once weekly, providing a 6-day drug-free washout period between treatments (see flow chart below).

Those animals previously treated chronically with l-dopa (10–12.5 mg/kg, p.o.) to induce dyskinesia received vehicle treatment on day 1 and l-dopa (12.5 mg/kg, p.o.) on day 2. Thereafter, aplindore was administered in escalating doses (see flow chart below), each treatment being given for four consecutive days, 0.05 mg/kg (days 3 to 6), 0.1 mg/kg (days 7 to 10), 0.25 mg/kg (days 11 to 14) and 0.5 mg/kg (days 15 to 18). A further series of experiments was performed with l-dopa (2.5 and 12.5 mg/kg, p.o.) being administered alone and combined with aplindore (1.0 and 2.0 mg/kg, p.o.) on one occasion as follows: l-dopa (12.5 mg/kg, p.o.) plus aplindore (1.0 mg/kg, p.o.) on day 23; l-dopa (2.5 mg/kg, p.o.) plus aplindore (1.0 mg/kg, p.o.) on day 25; l-dopa (12.5 mg/kg, p.o.) plus aplindore (2.0 mg/kg, p.o.) on day 26; l-dopa (12.5 mg/kg, p.o.) on day 30 and l-dopa (2.5 mg/kg, p.o.) on day 32 (see flow chart below).

Behavioural assessments
For each experiment, animals were weighed and placed into locomotor activity test units and allowed 45–60 min to acclimatise to the new environment before the administration of test drugs. Each unit was fitted with eight infrared beams arranged so as to detect floor, perch and climbing activity. Interruption of an infrared beam, by a moving marmoset, was automatically recorded as a single locomotor count. Locomotor counts were accumulated over 30 min periods for up to 8 h.
Rating of motor disability
Motor disability was assessed simultaneously with assessment of locomotor activity through a one-way mirror by experienced observers blinded to treatment. Basal disability was assessed during the 45–60 min acclimatisation period and once every 30 min after drug administration for up to 8 h using an established motor disability rating scale (Smith et al. 2003), alertness (normal 0, reduced 1, sleepy 2), checking movements (present 0, reduced 1, absent 2), posture (normal 0, abnormal trunk +1, abnormal limbs +1, abnormal tail +1, or grossly abnormal 4), balance/co-ordination (normal 0, impaired 1, unstable 2, spontaneous falls 3), reaction (normal 0, reduced 1, slow 2, absent 3), and vocalization (normal 0, reduced 1, absent 2), and motility (normal 0, bradykinesia/hyperkinesia 1, akinesia/hyperkinesia 2). A total motor disability score of 0/10 min indicates normal behaviour and a score of 18/10 min indicates a high degree of behavioural deficits.
Rating of dyskinesia
The rating of dyskinesia was performed only for experiments with the group of animals primed to express dyskinesia.
Dyskinesia was assessed simultaneously with motor disability using an established dyskinesia rating scale (Pearce et al. 1995). Chorea rapid random flicking movements of the fore and hind limbs, Athetosis sinuous writhing limb movements, Dystonia sustained abnormal posturing. Dyskinesia was scored as follows: 0 = absent; 1 = mild, fleeting and rare dyskinetic postures and movements; 2 = moderate, more prominent abnormal movements, but not significantly affecting normal behaviour; 3 = marked, frequent and at times continuous dyskinesia affecting the normal pattern of activity; 4 = severe, virtually continuous dyskinetic activity, disabling to the animal and replacing normal behaviour.
Data analysis
Total locomotor activity, motor disability and dyskinesia scores are presented as the mean of the individual values for each treatment and were analysed using a Kruskal–Wallis one-way analysis of variance (ANOVA) for non-parametric data followed where appropriate by a post hoc Mann–Whitney U test. Significance was set at P < 0.05 for all analyses. Time course data are presented as the mean values per 30 min.
Results
Drug naïve group
The administration of vehicle (10% sucrose solution) did not increase locomotor activity or reverse motor disability (Figs. 1, 2). All animals exhibited behavioural deficits including akinesia, bradykinesia, reduced alertness and head checking movements and impaired balance and coordination.
The effect of aplindore on locomotor activity in MPTP-treated, drug naïve, common marmosets. a Locomotor activity time course; drug administered at t = 0 minutes, data presented as the mean (n = 6) counts/30 min. b Total locomotor activity counts over 3 h. *Significantly different to vehicle, #significantly different to l-dopa, △significantly different to ropinirole (P = 0.0014 Kruskal–Wallis; P < 0.05 Mann–Whitney)
The effect of aplindore on motor disability in MPTP-treated, drug naïve, common marmosets. a Motor disability time course; drug administered at t = 0 min, data presented as the mean (n = 6) score/30 min. b Total motor disability score over 3 h. *Significantly different to vehicle, #significantly different to l-dopa, △significantly different to ropinirole (P = 0.0005 Kruskal–Wallis; P < 0.05 Mann–Whitney)
Treatment with l-dopa (12.5 mg/kg, p.o.) significantly increased locomotor activity compared to vehicle for approximately 4 h with a peak at 90 min (Fig. 1). There was also a significant reversal of motor disability which lasted between 4 and 5 h before returning to baseline levels, with maximum reversal at 60 min after treatment (Fig. 2). Ropinirole treatment also reversed motor deficits, with locomotor activity being significantly increased and motor disability scores significantly reduced compared to that produced by vehicle (Figs. 1, 2).
Aplindore (0.05 and 0.1 mg/kg, p.o.) produced a small, transient increase in locomotor activity that was not significantly different to that produced by vehicle and was significantly less than that produced by either l-dopa or ropinirole (Fig. 1). A moderate, but significant reversal of motor disability, corresponding to the increase in locomotor activity was produced by administration of aplindore (0.05 and 0.1 mg/kg, p.o.). However, although the reversal of motor disability produced was significantly different to vehicle it was significantly less than that produced by l-dopa or ropinirole (Fig. 2). Treatment with higher doses of aplindore (0.2, 0.5 and 1.0 mg/kg, p.o.) produced increases in locomotor activity over the first 3 h after dosing which were significantly greater than that produced by vehicle and not different to that produced by l-dopa and ropinirole (Fig. 1). Motor disability was also significantly reversed compared to vehicle, and aplindore (0.5 mg/kg, p.o.) treatment produced a reversal of motor disability that was not different to that produced by both l-dopa and ropinirole.
Although a dyskinesia assessment was not performed for this group of animals, there was no indication of adverse effects such as stereotypy or hyperactivity occurring with any of the treatments.
l-dopa primed dyskinesia group
The administration of vehicle (10% sucrose solution) did not produce increased locomotor activity, reverse motor disability or express significant levels of dyskinesia (Figs. 3, 4, 5). All animals exhibited behavioural deficits including akinesia, bradykinesia, reduced alertness and head checking movements and impaired balance and coordination for the duration of the test.
The effect of aplindore on locomotor activity in MPTP treated, l-dopa primed, common marmosets. a Locomotor activity time course for day 1; drug administered at t = 0 min, data presented as the mean (n = 6) counts/30 min. b Total locomotor activity counts over 3 h. *Significantly different to vehicle, #significantly different to l-dopa (P = 0.0014 Kruskal–Wallis; P < 0.05 Mann–Whitney)
The effect of aplindore on motor disability in MPTP treated, l-dopa primed, common marmosets. a Motor disability time course for day 1; drug administered at t = 0 min, data presented as the mean (n = 6) score/30 min. b Total motor disability score over 3 h. *Significantly different to vehicle, #significantly different to l-dopa (P = 0.0007 Kruskal–Wallis; P < 0.05 Mann–Whitney)
The effect of aplindore on dyskinesia in MPTP treated, l-dopa primed, common marmosets. a Dyskinesia time course for day 1; drug administered at t = 0 min, data presented as the mean (n = 6) score/30 min. b Total dyskinesia score over 3 h. *Significantly different to vehicle, #significantly different to l-dopa (P = 0.0002 Kruskal–Wallis; P < 0.05 Mann–Whitney)
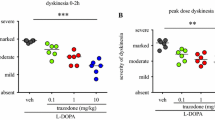
Treatment with l-dopa (12.5 mg/kg, p.o.) significantly increased locomotor activity compared to vehicle for approximately 4 h with a peak at 120 min after administration (Fig. 3). There was also a significant reversal of motor disability for approximately 4 h after treatment, before returning to baseline levels (Fig. 4). l-dopa (12.5 mg/kg, p.o.) also caused the expression of intense dyskinesia that was significantly greater than that produced by vehicle (Fig. 5). Aplindore was then given in escalating doses 0.05, 0.1, 0.25 and 0.5 mg/kg, each treatment being given for four consecutive days. As seen in the previous experiment in the drug-naïve group of animals, aplindore (0.05 mg/kg, p.o.) did not significantly increase locomotor activity compared to vehicle (Fig. 3). However, on the first day of aplindore (0.05 mg/kg, p.o.) administration, there was a moderate, but significant reversal of motor disability compared to vehicle, with only mild and infrequent expression of dyskinesia (Figs. 4, 5). This moderate reversal of motor disability was significantly different than that produced by l-dopa (12.5 mg/kg, p.o.).
Thereafter, treatment with aplindore (0.1, 0.25 and 0.5 mg/kg, p.o.) induced locomotor activity and reversal of motor disability that was not different to that produced by l-dopa (12.5 mg/kg, p.o.) and significantly greater than vehicle (Figs. 3, 4). Aplindore (0.5 mg/kg, p.o.) in particular produced a long duration of increased locomotor activity and reversal of motor disability. The total dyskinesia expressed by aplindore (0.5 mg/kg, p.o.) was similar to that produced by l-dopa (12.5 mg/kg, p.o.), whereas lower doses of aplindore (0.1 and 0.25 mg/kg, p.o.) expressed significantly less dyskinesia than l-dopa (Fig. 5).
For the combined administration of l-dopa plus aplindore experiments, the administration of a low dose of l-dopa (2.5 mg/kg) alone did not increase locomotor activity compared to vehicle treatment. However, when l-dopa (2.5 mg/kg) was co-administered with aplindore (1.0 mg/kg) there was an increase in locomotor activity that was significantly greater compared to that produced by vehicle and l-dopa (2.5 mg/kg) (Fig. 6). Although locomotor activity was not significantly increased by l-dopa (2.5 mg/kg) alone, motor disability was moderately reversed and significantly different compared to vehicle. When co-administered with aplindore (1.0 mg/kg) motor disability was further improved such that it was significantly different to that produced by both vehicle and l-dopa (2.5 mg/kg) alone (Fig. 7). The dyskinesia expressed by l-dopa (2.5 mg/kg) alone was mild to moderate and not significantly different to that produced by vehicle. However, following administration of l-dopa (2.5 mg/kg) plus aplindore (1.0 mg/kg), dyskinesia expression was marked to severe and significantly greater than that expressed by vehicle and l-dopa (2.5 mg/kg) alone (Fig. 8).
The effect of co-administration of l-dopa + aplindore on locomotor activity in MPTP treated, l-dopa primed, common marmosets. a Locomotor activity time course; drug administered at t = 0 min, data presented as the mean (n = 6) counts/30 min. b Total locomotor activity counts over 3 h. *Significantly different to vehicle, #significantly different to l-dopa (2.5 mg/kg) (P = 0.0083 Kruskal–Wallis; P < 0.05 Mann–Whitney)
The effect of co-administration of l-dopa + aplindore on motor disability in MPTP treated, l-dopa primed, common marmosets. a Motor disability time course; drug administered at t = 0 min, data presented as the mean (n = 6) score/30 min. b Total motor disability score over 3 h. *Significantly different to vehicle, #significantly different to l-dopa (2.5 mg/kg) (P = 0.0041 Kruskal–Wallis; P < 0.05 Mann–Whitney)
The effect of co-administration of l-dopa + aplindore on dyskinesia in MPTP treated, l-dopa primed, common marmosets. a Motor disability time course; drug administered at t = 0 min, data presented as the mean (n = 6) score/30 min. b Total motor disability over score 3 h. *Significantly different to vehicle, #significantly different to l-dopa (2.5 mg/kg) (P = 0.0024 Kruskal–Wallis; P < 0.05 Mann–Whitney)
Administration of a higher dose of l-dopa (12.5 mg/kg) alone produced the expected significant increase in locomotor activity, reversal of motor disability and expression of dyskinesia compared to that produced by vehicle (Figs. 6, 7, 8). Combined l-dopa (12.5 mg/kg) plus aplindore (1.0 mg/kg) administration produced similar significant increases in locomotor activity, reversal of motor disability and expression of dyskinesia compared to that produced by l-dopa (12.5 mg/kg) alone (Figs. 6, 7, 8). A combination of l-dopa (12.5 mg/kg) plus a higher dose of aplindore (2.0 mg/kg) produced a similar effect compared to that produced by both l-dopa (12.5 mg/kg) alone and the combination of l-dopa (12.5 mg/kg) plus aplindore (1.0 mg/kg) and was significantly different to vehicle for all assessed parameters (Figs. 6, 7, 8).
Discussion
While dopaminergic therapy currently dominates the treatment of PD, it leads to the onset of motor complications which become increasingly difficult to control (Obeso et al. 2000; Ahlskog and Muenter 2001; Rascol et al. 2003). In particular, dyskinesia induction is difficult to reverse and the expression of established involuntary movements is inevitably worsened by any form of increase in dopaminergic medication. Dyskinesia develops as a result of increasing loss of nigral dopaminergic cells but importantly, as a consequence of the nature of post-synaptic dopamine receptor stimulation produced by drug treatment (Jenner 2008a). Short acting drugs producing pulsatile stimulation are thought to more likely provoke dyskinesia than longer acting drugs producing a more physiological response through continuous dopaminergic stimulation (Jenner 2008b). However, as complete receptor occupation by a partial agonist may not result in functional overstimulation and dyskinesia expression, partial agonists could be a more effective means of treating PD, and the objective of this study was to clarify this issue.
The MPTP-treated primate is thought to be the most predictive model of efficacy of dopaminergic drugs in the control of the motor symptoms of PD in man and the risk of development of dyskinesia. Previously, there has been relatively little investigation of the actions of D2 partial agonists in this model system. In animals primed to exhibit dyskinesia, the findings are confusing with the D2 partial agonists preclamol and terguride reported to produce dyskinesia that was equivalent to that produced by l-dopa or full dopamine agonists while in another study, terguride did not provoke the dyskinesia that was observed with full dopamine agonists (Brucke et al. 1988; Akai et al. 1995). The current studies with aplindore were designed to provide a more comprehensive assessment.
In non-dyskinetic MPTP-treated common marmosets, aplindore produced a robust reversal of motor deficits in a dose-related manner following its oral administration consistent with a dopamine agonist action of the drug in the denervated caudate putamen. This confirms the ability of D2 partial agonists to exert an antiparkinsonian effect when administered as monotherapy. Perhaps important was the fact that the extent of the reversal of motor deficits was not that different from the response produced by the dopamine full agonist ropinirole despite the lower intrinsic activity of aplindore. This would suggest that DA receptors under these experimental conditions have greater “receptor reserve” and that some D2 partial agonists are able to occupy enough of these receptors to produce a fully efficacious response similar to a full agonist. The effects of aplindore were maximal at a dose of 0.2 mg/kg p.o. and there was no significant improvement in its antiparkinsonian activity at higher dose levels. This suggests that maximal receptor activation for reversal of motor deficits was achieved and that no further improvement could be achieved at higher doses. The findings with aplindore are also consistent with the ability of the D2/D3 partial agonists, pardoprunox (SLV 308) and terguride to improve motor function in MPTP-treated primates (Brucke et al. 1988; Lange et al. 1992; Akai et al. 1993; De Vries et al. 2004; Glennon et al. 2006). This is significant since pardoprunox, which also acts as a low potency 5HT1A full agonist is now in phase III clinical evaluation in PD and has shown clear evidence of producing an antiparkinsonian response in man again supporting a role for D2 partial agonists in treatment (Glennon et al. 2006).
In animals primed to express dyskinesia in response to dopaminergic stimulation, aplindore again showed a dose-related ability to reverse motor disability and to increase locomotor activity. Interestingly, the drug appeared more potent in these primed animals than in the non-dyskinetic group and a maximal effect was now observed at 0.1 mg/kg p.o. This may reflect differences in the sensitivity of individual animals making up the different treatment groups since these involve only a small number of common marmosets. On the other hand, we have previously noted that the priming process used to provoke dyskinesia expression does lead to differences in the effects of drugs on locomotor activity and motor disability. This in turn may reflect the priming process but it may also be related to a disruption of behavioural repertoire by the emergence of involuntary movement. We have noted previously that there is a relationship between the expression of dyskinesia and changes in locomotor activity in these primed animals (Kuoppamaki et al. 2007). Certainly in these animals the antiparkinsonian response produced by aplindore was not different from that resulting from l-dopa administration despite its partial agonist characteristics.
Aplindore induced dyskinesia in these primed MPTP-treated common marmosets and at the higher doses, this was not different from that produced by l-dopa treatment. This was somewhat unexpected as one potential scenario was that the partial agonist activity of aplindore would prevent hyperstimulation of dopamine receptors and that a full behavioural response might not be observed. The fact that this did occur suggests that the expression of marked dyskinesia may result from stimulation of supersensitive D2 receptors in which partial agonists are equieffective to full agonists. Importantly, however, at doses of aplindore up to 0.1 mg/kg p.o., which produced a full antiparkinsonian response, the expression of dyskinesia was restricted compared to that produced by l-dopa. Only at doses above those producing a maximal pharmacological response did dyskinesia expression approach that resulting from l-dopa treatment. This means that in clinical use, there is the potential for aplindore to be used in patients exhibiting dyskinesia to produce a full reversal of motor symptoms while avoiding the occurrence of troublesome dyskinesia that becomes treatment limiting.
Another important question that arises from the potential use of partial agonists in the middle to late stages of PD is whether they will functionally inhibit the actions of l-dopa. This could arise from full occupation of the receptor by the partial agonist leading to competition with the actions of dopamine derived from l-dopa. For this reason, we looked at the effects of a high dose of aplindore (e.g. 1.0 mg/kg, a dose that was tenfold greater than the 0.1 mg/kg dose which resulted in a maximal reversal of motor deficits), combined with a threshold (2.5 mg/kg) dose of l-dopa. Administration of aplindore resulted in a marked improvement in motor function and locomotor activity compared to the low dose of l-dopa alone to produce an overall response that was not different from the standard high dose of l-dopa used in the other parts of this study. In summary, there was no indication of any inhibition of the actions of l-dopa by aplindore suggesting that they could be used in combination in treating mid- to late-stage PD in man.
An obvious question is why inhibition of l-dopa efficacy was not observed with a very high dose of aplindore? Indeed, based on the partial agonist concept and the clear evidence from rodent studies that aplindore functions as a partial agonist, inhibition or a lack of an additive effect might almost have been the expected outcome. The most plausible explanation is that the partial agonist activity of aplindore was demonstrated in normal rodents with normosensitive dopamine receptors and the present studies were carried out in primates with a highly denervated caudate putamen that presumably leads to receptor up regulation and adaptive changes in basal ganglia outflow as a consequence (Andree et al. 1999). Under these conditions a partial agonist, such as aplindore, could occupy enough receptors (due to a presumably large receptor reserve) to produce a fully efficacious response and not be capable of demonstrating antagonist actions.
In conclusion, the present studies show that the D2 partial agonist aplindore has potential for the symptomatic treatment of PD both as monotherapy and in conjunction with l-dopa. Aplindore is able to maximally reverse motor deficits at doses that result in reduced dyskinesia potential and so might be an effective therapy for patients with PD with established involuntary movements. Overall the data show that dopamine partial agonists might be a useful addition to dopaminergic treatment strategies in PD.
References
Ahlskog JE, Muenter MD (2001) Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord 16:448–458
Akai T, Yamaguchi M, Mizuta E, Kuno S (1993) Effects of terguride, a partial D2 agonist, on MPTP-lesioned parkinsonian cynomolgus monkeys. Ann Neurol 33:507–511
Akai T, Ozawa M, Yamaguchi M, Mizuta E, Kuno S (1995) Combination treatment of the partial D2 agonist terguride with the D1 agonist SKF 82958 in 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-lesioned parkinsonian cynomolgus monkeys. J Pharmacol Exp Ther 273:309–314
Andree TH, Stack G, Rosenzweig-Lipson S, Coupet J (1999) WAY-135452: a potent novel D2/D3 partial agonist for the treatment of schizophrenia. In: 38th annual meeting of the American College of Neuropsychopharmacology
Baronti F, Mouradian MM, Conant KE, Giuffra M, Brughitta G, Chase TN (1992) Partial dopamine agonist therapy of levodopa-induced dyskinesias. Neurology 42:1241–1243
Bravi D, Davis TL, Mouradian MM, Chase TN (1993) Treatment of Parkinson’s disease with the partial dopamine agonist EMD 49980. Mov Disord 8:195–197
Brucke T, Bankiewicz K, Harvey-White J, Kopin I (1988) The partial dopamine receptor agonist terguride in the MPTP-induced hemiparkinsonian monkey model. Eur J Pharmacol 148:445–448
Carlsson A (1983) Dopamine receptor agonists: intrinsic activity vs. state of receptor. J Neural Transm 57:309–315
De Vries MH, Hesselink MB, Winsemius AA, Jenner P, Johnston L, Salvage S (2004) SLV 308: plasma levels of effective doses in MPTP-treated marmosets in comparison with plasma levels and D2 receptor occupancy (PET) in humans. Clin Pharmacol Ther 75:70
Glennon JC, Van SG, Ronken E, Hesselink MB, Reinders JH, Van Der NM, Long SK, Feenstra RW, McCreary AC (2006) In vitro characterization of SLV308 (7-[4-methyl-1-piperazinyl]-2(3H)-benzoxazolone, monohydrochloride): a novel partial dopamine D2 and D3 receptor agonist and serotonin 5-HT1A receptor agonist. Synapse 60:599–608
Heinrich JN, Brennan J, Lai MH, Sullivan K, Hornby G€piolek M, Jiang LX, Pausch MH, Stack G, Marquis KL, Andree TH (2006) Aplindore (DAB-452), a high affinity selective dopamine D2 receptor partial agonist. Eur J Pharmacol 552:36–45
Holloway RG, Shoulson I, Fahn S, Kieburtz K, Lang A, Marek K, McDermott M, Seibyl J, Weiner W, Musch B, Kamp C, Welsh M, Shinaman A, Pahwa R, Barclay L, Hubble J, LeWitt P, Miyasaki J, Suchowersky O, Stacy M, Russell DS, Ford B, Hammerstad J, Riley D, Standaert D, Wooten F, Factor S, Jankovic J, Atassi F, Kurlan R, Panisset M, Rajput A, Rodnitzky R, Shults C, Petsinger G, Waters C, Pfeiffer R, Biglan K, Borchert L, Montgomery A, Sutherland L, Weeks C, DeAngelis M, Sime E, Wood S, Pantella C, Harrigan M, Fussell B, Dillon S, exander-Brown B, Rainey P, Tennis M, Rost-Ruffner E, Brown D, Evans S, Berry D, Hall J, Shirley T, Dobson J, Fontaine D, Pfeiffer B, Brocht A, Bennett S, Daigneault S, Hodgeman K, O’Connell C, Ross T, Richard K, Watts A (2004) Pramipexole vs levodopa as initial treatment for Parkinson disease: a 4-year randomized controlled trial. Arch Neurol 61:1044–1053
Jackson MJ, Smith LA, Al-Barghouthy G, Rose S, Jenner P (2007) Decreased expression of l-dopa-induced dyskinesia by switching to ropinirole in MPTP-treated common marmosets. Exp Neurol 204:162–170
Jenner P (2008a) Molecular mechanisms of L-DOPA-induced dyskinesia. Nat Rev Neurosci 9:665–677
Jenner P (2008b) Preventing and controlling dyskinesia in Parkinson’s disease—a view of current knowledge and future opportunities. Mov Disord 23(Suppl 3):S585–S598
Kehne JH, Andree TH, Heinrich JN (2008) D2 receptor partial agonists: treatment of CNS disorders of dopamine function. Curr Top Med Chem 8:1068–1088
Kenakin T (2002) Drug efficacy at G protein-coupled receptors. Annu Rev Pharmacol Toxicol 42:349–379
Kuoppamaki M, Al-Barghouthy G, Jackson MJ, Smith LA, Quinn N, Jenner P (2007) L-dopa dose and the duration and severity of dyskinesia in primed MPTP-treated primates. J Neural Transm 114:1147–1153
Lange KW, Loschmann PA, Wachtel H, Horowski R, Jahnig P, Jenner P, Marsden CD (1992) Terguride stimulates locomotor activity at 2 months but not 10 months after 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine treatment of common marmosets. Eur J Pharmacol 212:247–252
Lieberman A, Gopinathan G, Neophytides A, Pasternack P, Goldstein M (1987) Advanced Parkinson’s disease: use of partial dopamine agonist, ciladopa. Neurology 37:863–865
Martignoni E, Pacchetti C, Aufdembrinke B, Godi L, Albani G, Mancini F, Nappi G (1995) Terguride in stable Parkinson’s disease. Funct Neurol 10:143–146
Obeso JA, Olanow CW, Nutt JG (2000) Levodopa motor complications in Parkinson’s disease. Trends Neurosci 23:S2–S7
Pacchetti C, Martignoni E, Bruggi P, Godi L, Aufdembrinke B, Miltenburger C, Voet B, Nappi G (1993) Terguride in fluctuating parkinsonian patients: a double-blind study versus placebo. Mov Disord 8:463–465
Pearce RK, Jackson M, Smith L, Jenner P, Marsden CD (1995) Chronic L-DOPA administration induces dyskinesias in the 1-methyl-4- phenyl-1, 2, 3, 6-tetrahydropyridine-treated common marmoset (Callithrix Jacchus). Mov Disord 10:731–740
Rascol O, Brooks DJ, Korczyn AD, De Deyn PP, Clarke CE, Lang AE (2000) A five-year study of the incidence of dyskinesia in patients with early Parkinson’s disease who were treated with ropinirole or levodopa. 056 Study Group. N Engl J Med 342:1484–1491
Rascol O, Payoux P, Ory F, Ferreira JJ, Brefel-Courbon C, Montastruc JL (2003) Limitations of current Parkinson’s disease therapy. Ann Neurol 53(Suppl 3):S3–S12
Ruggieri S, Stocchi F, Baronti F, Viselli F, Horowski R, Lucarelli C, Agnoli A (1991) Antagonist effect of terguride in Parkinson’s disease. Clin Neuropharmacol 14:450–456
Smith LA, Jackson MJ, Hansard MJ, Maratos E, Jenner P (2003) Effect of pulsatile administration of levodopa on dyskinesia induction in drug-naive MPTP-treated common marmosets: effect of dose, frequency of administration, and brain exposure. Mov Disord 18:487–495
Stephenson RP (1956) A modification of receptor theory. Br J Pharmacol Chemother 11:379–393
Acknowledgments
This study was funded by Wyeth Research, the Neurogen Corporation, the Parkinson’s Disease Society and the National Parkinson Foundation, Miami.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jackson, M.J., Andree, T.H., Hansard, M. et al. The dopamine D2 receptor partial agonist aplindore improves motor deficits in MPTP-treated common marmosets alone and combined with l-dopa. J Neural Transm 117, 55–67 (2010). https://doi.org/10.1007/s00702-009-0323-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-009-0323-9