Abstract
Green tea polyphenol epigallocatechin-3-gallate (EGCG) is reported to have antioxidant abilities and to counteract beneficially mitochondrial impairment and oxidative stress. The present study was designed to investigate neuroprotective effects of EGCG on rotenone-treated dissociated mesencephalic cultures and organotypic striatal cultures. Rotenone is a potent inhibitor of complex I of the respiratory chain, which in vitro causes pathological and neurochemical characteristics of diseases in which mitochondrial impairment is involved, e.g., Parkinson’s disease. Treatment with EGCG (0.1, 1, 10 μM) alone had no significant effects on mesencephalic cultures. In striatal slice cultures, EGCG led to a significant increase of propidium iodide (PI) uptake and 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FM), but not dihydroethidium (DHE) fluorescence intensity. Rotenone (20 nM on the eighth DIV for 48 h) significantly decreased the numbers and the neurite lengths of TH ir neurons by 23 and 34% in dissociated mesencephalic cell cultures compared to untreated controls. Exposure of striatal slices to rotenone (0.5 mM for 48 h) significantly increased PI uptake, and DAF-FM and DHE fluorescence intensities by 41 and 136 and 19%, respectively, compared to controls. Against rotenone, in dissociated mesencephalic cultures, EGCG produced no significant effect on either the number or neurite lengths of THir neurons compared to rotenone-treated cultures, but EGCG significantly decreased PI uptake by 19% and DAF-FM fluorescence intensity by 19 and 58%, respectively, compared to increase in rotenone-exposed striatal slices. On the other hand, EGCG did not affect superoxide (O2 −) formation as detected with DHE. These data indicate that EGCG slightly protects striatal slices by counteracting nitric oxide (NO·) production by rotenone. In conclusion, EGCG partially protects striatal slices but not dissociated cells against rotenone toxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Green tea (GT) made from tea plants (Camellia sinensis) is one of the most popular beverages in the world. GT contains a variety of chemically characterized polyphenol compounds generally known as catechins. The main catechins in GT are epicatechin (EC), epicatechin gallate (ECG), epigallocatechin (EGC) and epigallocatechin gallate (EGCG). EGCG is the major polyphenol (flavan-3-ol) in tea plants (Stewart et al. 2005). It shows a high bioavailability, can be absorbed from the digestive tract to the blood system and can enter the brain (Suganuma et al. 1998). In medical research there is evidence that EGCG may act as an anticarcinogenic and anti-angiogenic compound (Lin et al. 1999; Cao and Cao 1999). Similar to other flavanols, EGCG may act as a radical scavenger by reacting with hydrogen, alkoxyl or peroxyl radicals (Wang et al. 2000). Moreover, EGCG is reported to act as an iron chelator (Grinberg et al. 1997). Notably EGCG’s actions may not only depend on its powerful antioxidant properties by directly scavenging free radicals (Qiong et al. 1996), but as well by indirect increase of endogenous antioxidants (Skrzydlewska et al. 2002). In this context EGCG accumulates in mitochondria and acts locally as a free radical scavenger (Schroeder et al. 2008) and preserves the activity of superoxide dismutase (SOD) and catalase, two major oxygen radical species-metabolizing enzymes. Due to its antioxidant characteristics, EGCG has attracted interest in models related to mitochondrial impairment such as Parkinson’s disease (PD). EGCG prevents cell damage by 6-hydroxydopamine in rat PC12 and human neuroblastoma SH-SY5Y cells (Levites et al. 2002). Using another PD model drug, N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP, a complex I inhibitor of the respiratory chain), Levites et al. (2001) could show that pretreating with EGCG can potently reduce cell degeneration of dopaminergic neurons of mice by this agent.
Rotenone is a lipophilic isoflavonoid that can be found in different plants such as Derris or Lonchocarpus species. This compound is widely used as an organic pesticide that non-competitively inhibits NADH ubiquinone reductase (complex I) of the electron transport chain (Singer and Ramsay 1994). When incorporated in the brain, this consecutive lack of energy metabolites (ATP) leads to formation of free radicals, deregulation of Ca2+ homeostasis, and the initiation of neurodegenerative processes. In similarity to parkinsonian brains the formation of protein aggregates resembling Lewy bodies found can be induced by rotenone (Betarbet et al. 2000). In our study, we prepared dissociated mesencephalic cultures containing dopaminergic neurons and striatal slice cultures representing the target region for these cultures. The aim of this study was to investigate the effects of EGCG on rotenone-induced oxidative damage in culture models.
Materials
Eight-week-old mice (C57/B16) and pregnant mice (OF1/SPF) were obtained from the Institute for Laboratory Zoology and Veterinary Genetics (Himberg, Austria) for preparation of organotypic slice and dissociated mesencephalic cultures, respectively. Animals were cared and handled in accordance with the guidelines of the European Union Council (86/609/EU) for the use of laboratory animals. TPTZ [2,4,6-tri(2-pyridyl)-s-triazine], Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), Dulbecco’s Modified Eagles Medium (DMEM), fetal calf serum (FCS), diaminobenzidine, paraformaldehyde, rotenone, EGCG, propidium iodide (PI) and l-glutamic acid (monosodium salt) were obtained from Sigma (Germany). Penicillin/streptomycin, and DNase I were purchased from Roche Molecular Biochemicals (Germany). Anti-tyrosine hydroxylase antibody (anti-TH antibody) was from Chemicon. Vectastain ABC Elite Kit (Mouse IgG) was purchased from Vector Laboratories (USA). B-27 supplement (without antioxidants), trypsin–EDTA and soybean trypsin inhibitor were from Gibco (Invitrogen, Germany). 4-amino-s-methylamino-2′,7′-difluorescein diacetate (DAF-FM) and dihydroethidium (DHE) were obtained from Molecular Probes (Invitrogen, Germany). Sterile plasticware was bought from Nunc (USA). Millicell-CM-membranes (0.4 μm) were obtained from Millipore (USA).
Methods
Measurement of antioxidative activity of EGCG
Antioxidative activity of EGCG was determined according to Benzie and Strain (1996) and expressed as % of vitamin E derivative Trolox. In brief, after reduction of Fe3+ to Fe2+ by antioxidants, a Fe2+-TPTZ complex is built that can be measured photometrically determining ΔA593 nm using Trolox as a standard.
Preparation of organotypic striatal culture
Organotypic striatal slice cultures were prepared according to Franke et al. (2003). Briefly, animals were quickly decapitated, the brains were isolated under aseptic conditions and the striata were removed. The striata were coronally cut into 400-μm-thick sections on a McIlwain tissue chopper (Mickle Laboratory, Cambridge, UK). Slices were cooled in PBS and immediately transferred onto semi-permeable Millicell-CM-membrane on 6-well culture plates with 1 ml culture medium in each well. The medium was composed of 75% DMEM, 20% HBSS, 25 mM Hepes buffer, 2 mM glutamine, 24 mM glucose, 10 U/ml penicillin and 10 μg/ml streptomycin.
Treatment of striatal slices with rotenone and EGCG
In a previous study, Moldzio et al. (2008) used 0.01–1 mM of rotenone in striatal slice cultures. Authors could show dose-dependent effects of this complex I inhibitor. In this study, we chose a concentration of 0.5 mM of rotenone. Prepared striatal slices were kept for 48 h in culture medium, rotenone, culture medium with EGCG (0.1, 1, 10 μM) and culture medium concomitantly supplemented with EGCG (0.1, 1, 10 μM) and rotenone. Rotenone was prepared from a stock solution of 1 mM in 0.1% of dimethyl sulfoxide (DMSO) and then diluted in DMEM to the final concentrations. Freshly prepared solutions of EGCG in DMEM were used at each treatment.
PI uptake and detection of NO· and O2 − in striatal slice cultures
Following treatment protocols, slices were washed three times, 5 min each, with colorless DMEM. PI and DHE staining were performed according to Radad et al. (2006) with slight modifications. For DAF-FM staining, a modified protocol according to Moldzio et al. (2006) was used. PI (final concentration, 2 μM in colorless DMEM) was added to slices for 10 min at 37°C. DAF-FM acetate (final concentration, 10 μM in colorless DMEM) was added to another set of slices for 90 min at 37°C to detect NO· radical. DAF-FM diacetate enters cells when it is deacetylated by esterases to the non-fluorescent DAF-FM which then reacts with NO· to form fluorescent benzotriazole. DHE (final concentration, 10 μM in PBS) was added to another set of slices for 3 min at 37°C to detect O2 − radical formation. DHE can be oxidized in the presence of O2 − radicals to ethidium that intercalates into DNA and emits an intense red fluorescence at 605 nm. After incubation with each fluorescence dye, cultured slices were rinsed with colorless DMEM and ten photos were taken for each tested parameter by a digital camera at 100× magnification attached to an inverted microscope with epifluorescence equipment using TRITC (G-2A) and FITC (B-2A) filters for DHE and PI, and DAF-FM, respectively (Nikon, Japan). Color intensities of the digital pictures were analyzed by Adobe Photoshop® software. The averaged color intensity was measured with the histogram modus in the regions of interest (1.68 mm2/field). Chosen methods are semi-quantitative. Therefore, data are expressed as % compared to controls.
Preparation of dissociated mesencephalic cultures
Primary mesencephalic cell cultures were prepared from brains of embryonic OF1/SPF mice at GD 14 according to Radad et al. (2009). Briefly, dissociated cells were collected in DMEM supplemented with Hepes buffer (25 mM), glucose (30 mM), glutamine (2 mM), penicillin–streptomycin (10 U/ml and 0.1 mg/ml, respectively) and heat-inactivated FCS (10%). The cells were plated into four-well multidishes at 750,000 cells/well pre-coated with poly-d-lysine (50 μg/ml). Cultures were grown at 37°C in an atmosphere of 5% CO2/95% air and 100% relative humidity. The medium was exchanged on the first day in vitro (DIV) and on the third DIV. On the fifth DIV half of the medium was replaced with serum-free DMEM containing 0.02 ml B-27/ml. Serum-free supplemented DMEM was used for feeding from the sixth DIV and subsequently replaced every second day.
Treatment of dissociated mesencephalic cultures with rotenone and EGCG
To investigate the effect of EGCG on the survival of THir neurons in primary mesencephalic culture, EGCG (0.1, 1 and 10 μM), rotenone or EGCG/rotenone were added on the eighth DIV for 48 h. A concentration of rotenone was chosen according to Radad et al. (2006).
Identification of tyrosine hydroxylase immunoreactive (THir) neurons
Cells were fixed with 4% paraformaldhyde (PFA) for 45 min at 4°C and rinsed three times with PBS (pH 7.2). Cells were permeabilized with 0.4% Triton X-100 for 30 min at room temperature. After washing another three times with PBS, cells were incubated with 5% horse serum (Vectastain ABC Elite kit) for 90 min to block nonspecific binding sites. To stain THir cells, sequentially anti-TH primary antibody was added overnight at 4°C, followed by biotinylated secondary antibody (Vectastain) and avidin–biotin–horseradish peroxidase complex (Vectastain) for 90 min at room temperature and washed with PBS between the steps. The brown reaction product was developed in a solution of diaminobenzidine (1.4 mM) in PBS containing 3.3 mM hydrogen peroxide. Stained cells were counted with a Nikon inverted microscope in ten randomly selected fields (1.13 mm2/field) in each well at 100× magnification. On the tenth DIV, the average number of THir cells in the various experiments was between 1,000 and 1,500 cells/well.
Statistics
Data were expressed as mean ± standard error of mean (SEM). Statistical significance was calculated using the Kruskal–Wallis (H) test followed by χ2 test. For comparison of control and rotenone values, the Mann–Whitney U test was used. Differences within the groups of P < 0.05 were regarded as statistically significant.
Results
Antioxidative activity of EGCG
Using the iron reduction assay, an antioxidative activity of EGCG of about 61.48% ± 20.2 compared to Trolox could be shown (data not shown).
Effects of EGCG on striatal and mesencephalic cultures
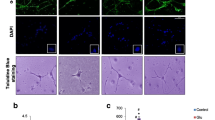
In dissociated cultures, cell numbers were unaffected and the morphology of THir cells within 48 h did not change after treatment with up to 10 μM of EGCG (Figs. 1, 5a, b).
Effect of EGCG on the survival of THir neurons and neurites outgrowth in rotenone-treated mesencephalic cultures. 100% corresponds to the total number of THir neurons and averaged neurite lengths after 10 DIV in untreated controls. Values represent the means ± SEM of five independent experiments with two wells in each treatment. In ten randomly selected cells per well, neurite lengths were measured. EGCG (0.1, 1, 10 μM) alone or in combination with rotenone (20 nM) was added to mesencephalic cultures on DIV 8 for 48 h. Kruskal–Wallis (H) test followed by χ2 test for statistical analysis within groups displayed no significant changes. Statistical significance for the comparison between control and rotenone was calculated using the Mann–Whitney U test (*P < 0.01)
In striatal cultures, radical processes were followed after exposing to EGCG (Fig. 2) for 48 h. The PI uptake was increased significantly by 24, 31, 28% by adding 0.1, 1 or 10 μM of EGCG. DAF fluorescence was increased from 132% (0.1 μM) to significantly increased values of 162% (1 and 10 μM). However, no changes in O2 − formation after 48 h were detectable in striatal slice cultures.
Effects of EGCG on propidium iodide uptake, and DHE and DAF fluorescence in striatal slice cultures. EGCG (0.1, 1, 10 μM) was added to striatal slice cultures on day 0 for 48 h. 100% corresponds to the propidium iodide uptake or the fluorescence intensity of DAF-FM or DHE that formed in the presence of NO· or O2 − radicals, respectively, in control cultures after 2 days in vitro. The overall fluorescence intensity was evaluated using image analysis software from two digital photographs per slice. Data are expressed as means ± SEM of seven (PI) or five (DAF-FM and DHE) experiments with four slices per experiment. Statistical significance was calculated using the Kruskal–Wallis (H) test followed by χ2 test (*P < 0.05)
The number of dopaminergic neurons in the controls varied in the preparations from 184 to 987 cells per well, the number of neurite length between 566 and 1665 μm per dopaminergic neuron.
Effects of rotenone on striatal and mesencephalic cultures
Rotenone was found to be toxic to both striatal slice and dissociated mesencephalic cultures (Fig. 3). Exposure of striatal slices to 0.5 mM of rotenone for 48 h significantly increased PI uptake by 41% compared to controls. Cellular damage by rotenone in striatal slices indicated by the increase of PI uptake could be confirmed by significantly increased NO· detected by DAF-FM (increase 136%) and O2 − production by DHE fluorescent dyes (19%) (Fig. 3).
Effects of rotenone on striatal and mesencephalic cultures. 100% corresponds to the PI uptake (7 experiments), DAF-FM (5 experiments), DHE (5 experiments) fluorescence in control cultures after 2 days in vitro, or to the number of dopaminergic neurons (5 experiments) and neurite growth in control cultures after treatment with rotenone (0.5 mM) for 48 h on the eighth DIV. The overall fluorescence intensity was evaluated using image analysis software taken with low magnification in two digital photographs per slice. Data are expressed as mean ± SEM. Statistical significance was calculated using the Mann–Whitney U test (*P < 0.05)
In dissociated mesencephalic cultures, rotenone (20 nM on the eighth DIV for 48 h) significantly decreased the number of THir neurons by 23% compared to untreated controls (Fig. 3). Morphological degenerations were evident as particularly shortened neurites (significantly reduced by 34% compared to controls) could be observed (Figs. 3, 5c).
Effects of EGCG on rotenone-affected striatal and mesencephalic cultures
Concomitant treatment of striatal slice cultures with rotenone and EGCG on the day 0 for 48 h significantly decreased PI uptake by 19% and DAF-FM fluorescence intensity by 58% (Fig. 4) in the highest concentration of EGCG compared to increase in rotenone-exposed slices. On the other hand, EGCG did not affect O2 − production as detected with DHE (Fig. 4). These data indicated that EGCG protected striatal slices slightly through counteracting NO· production by rotenone.
Effects of EGCG on propidium iodide uptake, and DHE and DAF fluorescence in rotenone-treated striatal slice cultures. EGCG (0.1, 1, 10 μM) and rotenone (0.5 mM) were concomitantly added to striatal slice cultures on day 0 for 48 h. 100% corresponds to the propidium iodide uptake or the fluorescence intensity of DAF-FM or DHE that formed in the presence of NO· or O2 − radicals, respectively, in control cultures after 2 days in vitro. The overall fluorescence intensity was evaluated using image analysis software taken in two digital photographs per slice. Data are expressed as means ± SEM of seven (PI) or five (DAF-FM and DHE) experiments with four slices per experiment. Statistical significance was calculated using the Kruskal–Wallis (H) test followed by χ2 test (*P < 0.05)
In dissociated mesencephalic cultures, addition of EGCG on the eighth DIV for 48 h produced no significant effect on the number of TH ir neurons compared to rotenone-treated cultures (Fig. 1). Also, EGCG did not attenuate morphological changes and shortened neurites produced by rotenone (Fig. 5d).
Discussion
Among medicinal plants, green tea is known to have a wide variety of beneficial actions on the human body. It is reported that green tea polyphenols possess significant antioxidant, anti-carcinogenic, anti-inflammatory and anti-atherosclerotic properties (For review see Crespy and Williamson 2004; Zaveri 2006). The present study was carried out to investigate the protective effect of EGCG against the neurotoxic rotenone on striatal slice and dissociated mesencephalic cultures.
In our study, treatment with EGCG alone produced no significant effects on dissociated mesencephalic cultures (Figs. 1,5b). This is in agreement with the findings of Yin et al. (2008) who reported that EGCG alone did not affect the viability and ROS formation at concentration up to 50 μM in hippocampal neurons. In our culture system, we could show an enhanced production of NO radicals after 48 h and an increased cell degeneration detected by PI uptake in striatal cultures (Fig. 2).
Several studies indicate a selective degeneration of dopaminergic neurons after rotenone exposure (Alam and Schmidt 2002), but also other cell types are affected. Additionally, chronic administration and systemic administration of rotenone over a period of several months lead to destruction of dopaminergic nerve terminals and a retrograde degeneration of substantia nigra neurons (Greenamyre et al. 1999; Sherer et al. 2003). Exposure to rotenone affected both striatal slice and dissociated mesencephalic cultures. We observed a decrement in the number of dopaminergic neurons and an abnormal morphology of these cells (Figs. 3, 5). Similarly, Betarbet et al. (2000) reported that rotenone damaged dopaminergic terminals in striatum of rats as the result of complex I inhibition and subsequent ROS formation. In dissociated mesencephalic cultures, loss of THir neurons and deterioration of neurite lengths of remaining neurons as the result of rotenone exposure coincided with our previous studies (Radad et al. 2006; Radad et al. 2009; Moldzio et al., 2008). In striatal slice cultures, rotenone (0.5 mM for 48 h) caused significant cell death as indicated by increasing PI uptake. Measurements of DAF-FM and DHE intensities in striatal slices after rotenone exposure indicate an increasing production of NO· and O2 − radicals that might point to neurodegenerative processes.
Especially brain regions rich in dopamine such as the striatum are affected by radical damage, because dopamine causes oxidative stress conditions by its metabolism by monoamine oxidase. Therefore, we used EGCG that is reported to have antioxidative capacities. In parallel in vitro studies we showed using an iron reduction test according to Benzie and Strain (1996) that EGCG has an antioxidative capacity of 61.5% compared to TROLOX (data not shown). EGCG has the ability to chelate transition metal ions and thereby preventing the formation of iron-induced free radicals. It has been speculated that this compound could even could act as a putative therapeutic drug in diseases linked to abnormal metal (iron, copper and zinc) metabolism, such as PD (Mandel et al. 2006). Ferrous ions are capable of oxidizing a wide range of substrates and causing radical damage by the Fenton reaction (Lee et al. 2003). Nonetheless in our study, a rescue of dopaminergic neurons or a conservation of their morphology was not detectable in mesencephalic cultures (Figs. 1, 5). Interestingly, it could be shown by Li et al. (2006) that pretreatment with EGCG significantly attenuated MPP+-induced THir cell loss in murine cell cultures. MPP+ is a complex I inhibitor that enters specifically dopaminergic neurons via dopamine transporters. EGCG pretreatment significantly inhibited microglial activation and CD11b expression induced by MPP+. Therefore, next to direct antioxidative mechanisms, EGCG may exert dopaminergic neuroprotective activity by microglial inhibition what may be interesting for the potential use of EGCG in the treatment of neurodegenerative diseases. Also antiapoptotic effects of EGCG are described. EGCG can downregulate BAX and inhibit MPP+-induced increase of Bax. An MPP+-induced decrease of Bcl-2 can be reincreased likewise (Mandel et al. 2004). The neuroprotective findings of the MPP+ studies could not be confirmed by our rotenone experiments, perhaps due to the different ways of entering brain cells.
To assess the involvement of radicals in the cell loss after rotenone and EGCG treatment, we used fluorescent indicator DAF-FM for NO· radicals in striatal cultures. As mentioned above, both, rotenone and EGCG increased the DAF fluorescence (Figs. 2, 3). EGCG was found to provide some counteraction against rotenone toxicity in striatal slice cultures in the form of decreasing cellular damages and production of NO· radical. Co-treatment of substances together reduced DAF fluorescence by 24.4% compared to rotenone treatment to a level that was significantly lower, but still significantly above control levels (Fig. 4).
O2 − radicals were detected by DHE in our study. These radicals may have a damaging role in neurodegeneration. Under normal conditions, mitochondria are the main producers of O2 − and H2O2. Miyazawa (2000) found that EGCG is a direct scavenger of superoxide radicals. This result is not supported by our study. We did not see reductions of O2 − production when EGCG was administered to the cells and additionally no reduction when EGCG was co-treated with rotenone (Figs. 2, 4). In parallel, Schroeder et al. (2008) showed that EGCG did not affect the endogenous activities or expression of SOD in primary cultures of cerebellar granule neurons. Our findings are in accordance to a study of Chung et al. (2007). They found that EGCG decreased cell viability in SH-SY5Y cells. The superoxide concentrations were increased likewise. But the concentrations of EGCG were comparatively high (25–50 μM). Pretreatment augmented rotenone toxicity that might be due to the measured enhanced superoxide radical concentration. Up to a concentration of 10 μM, EGCG showed protective effects in their model.
Besides the detection of superoxide radical formation, NO· formation was determined in our study. Nagai et al. (2002) could show that EGCG can protect against NO· stress-induced damage after ischemia in cultured hippocampal neurons. Authors attribute this beneficial effect rather to deoxidizing peroxynitrate/peroxynitrite to prevent NO· or hydroxy radical formation than to inhibition of iNOS. These mechanism may also be involved in our model of complex I inhibition. Whereas EGCG induced an increased formation of NO radicals, it could also reduce the rotenone-induced excess of O2 − formation to a large extent (Fig. 4).
The modes of action of EGCG might be multifaceted. On the one hand, low sensitivity of medium spiny neurons, the cells that form 90% of striatal neurons, to rotenone as well as radical scavenging effects of EGCG may underlie EGCG-protective effects in the striatal slice culture. On the other hand, other mechanisms rather than increasing ROS production may be mediating rotenone toxicity in EGCG-treated dissociated mesencephalic cultures. For instance, Newhouse et al. (2004) found that rotenone induced apoptosis in dopaminergic SH-SY5Y cells through activation of c-Jun N-terminals protein kinase (JNK), p38 mitogen activated protein (MAP) kinase and caspase-3. Moreover, Chung et al. (2007) observed that EGCG enhanced rotenone-induced activation of caspase-3 in SH-SY5Y cells.
In conclusion, rotenone significantly damaged both striatal slice and mesencephalic cultures. EGCG partially counteracted against the effects of rotenone effects in striatal slice cultures through reduction of NO·. However, the most prominent result of our study is that EGCG had no influence on the survival of dopaminergic neurons in mesencephalic cultures in order to prevent the toxicity of rotenone.
References
Alam M, Schmidt WJ (2002) Rotenone destroys dopaminergic neurons and parkinsonian symptoms in rats. Behav Brain Res 136:317–324
Benzie IF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239:70–76
Betarbet R, Sherer TB, Mackenzie G, Garcia-Osuna MV, Panov A, Greenamyre JT (2000) Chronic systemic pesticide exposure reproduces features of Parkinson`s disease. Nat Neurosci 3:1301–1306
Cao Y, Cao R (1999) Angiogenesis inhibited by drinking tea. Nature 398:381–381
Chung WG, Miranda CL, Maier CS (2007) Epigallocatechin gallate (EGCG) potentiates the cytotoxicity of rotenone in neuroblastoma SH-SY5Y cells. Brain Res 1176:133–142
Crespy V, Williamson G (2004) A review of the health effects of green tea catechins in in vivo animal models. J Nutr 134:3431S–3440S
Franke H, Schelhorn N, Illes P (2003) Dopaminergic neurons develop axonal projections to their target areas in organotypic co-cultures of the ventral mesencephalon and the striatum/prefrontal cortex. Neurochem Int 42:431–439
Gao HM, Hong JS, Zhang W, Liu B (2002) Distinct role for microglia in rotenone-induced degeneration of dopaminergic neurons. J Neurosci 22:782–790
Greenamyre JT, Mackenzie G, Peng TI, Stephans SE (1999) Mitochondrial dysfunction in Parkinson`s disease. Biochem Soc Symp 66:85–97
Grinberg LN, Newmark H, Kitrossky N, Rahamim E, Chevion M, Rachmilewitz EA (1997) Protective effects of tea polyphenols against oxidative damage to red blood cells. Biochem Pharmacol 54:973–978
Groppo FC, Bergamaschi Cde C, Cogo K, Franz-Montan M, Motta RH, de Andrade ED (2008) Use of phytotherapy in dentistry. Phytother Res 22:993–998
Lee SR, Im KJ, Suh SI, Jung JG (2003) Protective effect of green tea polyphenol (−)-epigallocatechin gallate and other antioxidants on lipid peroxidation in gerbil brain homogenates. Phytother Res 17:206–209
Levites Y, Weinreb O, Maor G, Youdim MB, Mandel S (2001) Green tea polyphenol (−)-epigallocatechin-3-gallate prevents N-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-induced dopaminergic neurodegeneration. J Neurochem 78:1073–1082
Levites Y, Youdim MB, Maor G, Mandel S (2002) Attenuation of 6 hydroxydopamine (6-OHDA)-induced nuclear factor-kappaB (NF-kappaB) activation and cell death by tea extracts in neuronal cultures. Biochem Pharmacol 63:21–29
Li R, Peng N, Du F, Li XP, Le WD (2006) Epigallocatechin gallate protects dopaminergic neurons against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity by inhibiting microglial cell activation. Nan Fang Yi Ke Da Xue Xue Bao 26:376–380
Lin JK, Liang YC, Lin-Shiau SY (1999) Cancer chemoprevention by tea polyphenols through mitotic signal transduction blockade. Biochem Pharmacol 58:911–915
Mandel S, Maor G, Youdim MB (2004) Iron and alpha-synuclein in the substantia nigra of MPTP-treated mice: effect of neuroprotective drugs R-apomorphine and green tea polyphenol (−)-epigallocatechin-3-gallate. J Mol Neurosci 24:401–416
Mandel S, Weinreb O, Reznichenko L, Kalfon L, Amit T (2006) Green tea catechins as brain-permeable, non toxic iron chelators to “iron out iron” from the brain. J Neural Transm Suppl 71:249–257
Miyazawa T (2000) Absorption, metabolism and antioxidative effects of tea catechin in humans. Biofactors 13:55–59
Moldzio R, Radad K, Duvigneau JC, Kranner B, Krewenka C, Piskernik C, Rausch WD (2006) Glutamate-induced cell death and formation of radicals can be reduced by lisuride in mesencephalic primary cell culture. J Neural Transm 113:1095–1105
Moldzio R, Piskernik C, Radad K, Rausch WD (2008) Rotenone damages striatal organotypic slice culture. Ann N Y Acad Sci 1148:530–535
Nagai K, Jiang MH, Hada J, Nagata T, Yajima Y, Yamamoto S, Nishizaki T (2002) (−)-Epigallocatechin gallate protects against NO stress-induced neuronal damage after ischemia by acting as an anti-oxidant. Brain Res 956:319–322
Newhouse K, Hsuan SL, Chang SH, Cai B, Wang Y, Xia Z (2004) Rotenone-induced apoptosis is mediated by p38 and JNK MAP kinases in human dopaminergic SH-SY5Y cells. Toxicol Sci 79:137–146
Qiong G, Baolu Z, Meifen L, Shengrong S, Wenjuan X (1996) Studies on protective mechanisms of four components of green tea polyphenols against lipid peroxidation in synaptosomes. Biochim Biophys Acta 1304:210–222
Radad K, Rausch WD, Gille G (2006) Rotenone induces cell death in primary dopaminergic culture by increasing ROS production and inhibiting mitochondrial respiration. Neurochem Int 49:379–386
Radad K, Moldzio R, Taha M, Rausch WD (2009) Thymoquinone protects dopaminergic neurons against MPP(+) and rotenone. Phytother Res 23:696–700
Schroeder EK, Kelsey NA, Doyle J, Breed E, Bouchard RJ, Loucks A, Harbison A, Linseman DA (2008) Green tea epigallocatechin 3-gallate accumulates in mitochondria and displays a selective anti-apoptotic effect against inducers of mitochondrial oxidative stress in neurons. Antioxid Redox Signal (Epub ahead of print)
Sherer TB, Kim JH, Betarbet R, Greenamyre JT (2003) Subcutaneus rotenone exposure causes highly selective dopaminergic degeneration and α-synuclein aggregation. Exp Neurol 179:9–16
Singer TB, Ramsay RR (1994) The reaction site of rotenone and ubichinone with mitochondrial NADH dehydrogenase. Biochim Biophys Acta 1187:198–202
Skrzydlewska E, Ostrowska J, Farbiszewski R, Michalak K (2002) Protective effect of green tea against lipid peroxidation in the rat liver, blood serum and the brain. Phytomedicine 9:232–238
Stewart AJ, Mullen W, Crozier A (2005) On-line high-performance liquid chromatography analysis of the antioxidant activity of phenolic compounds in green and black tea. Mol Nutr Food Res 49:52–60
Suganuma M, Okabe S, Oniyama M, Tada Y, Ito H, Fujiki H (1998) Wide distribution of [3H](−)-epigallocatechin gallate, a cancer preventive tea polyphenol, in mouse tissue. Carcinogenesis 19:1771–1776
Sun AY, Wang Q, Simonyi A, Sun GY (2008) Botanical phenolics and brain health. Neuromolecular Med 10:259–274
Wang LF, Kim DM, Lee CY (2000) Effects of heat processing and storage on flavanols and sensory qualities of green tea beverage. J Agric Food Chem 48:4227–4232
Yin ST, Tang ML, Su L, Chen L, Hu P, Wang HL, Wang M, Ruan DY (2008) Effects of epigallocatechin-3-gallate on lead-induced oxidative damage. Toxicology 249:45–54
Zaveri NT (2006) Green tea and its polyphenolic catechins: medicinal uses in cancer and noncancer applications. Life Sci 78:2073–2080
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moldzio, R., Radad, K., Krewenka, C. et al. Effects of epigallocatechin gallate on rotenone-injured murine brain cultures. J Neural Transm 117, 5–12 (2010). https://doi.org/10.1007/s00702-009-0284-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-009-0284-z