Abstract
This study examined the effects of downhill treadmill exercise on brain-derived neurotrophic factor (BDNF) protein on the hippocampus and striatum of mice. Twenty-four adult mice were assigned to three groups: non-runners control, level or downhill (16° decline) running exercise. The exercise schedule consisted of progressive treadmill running for 5 days week−1 over 8 weeks. Blood lactate levels classified exercise intensity as moderate to high. Both training types increased citrate synthase activity of the soleus muscle when compared to untrained controls. While level running increased BDNF levels selectively in the hippocampus (68.5%), the eccentric running resulted in a pronounced BDNF increase in both the hippocampus (137.0%) and the striatum (49.9%). Further studies will specify whether the observed alterations in BDNF are due to downhill-induced upregulation or complex learning-induced mechanisms that influence BDNF levels in these brain regions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The benefits that physical exercise can produce on the brain function and on mental health are well documented. For instance, exercise enhances hippocampal-dependent learning and memory and improves executive functioning (Cotman and Berchtold 2002; Hunsberger et al. 2007). These exercise effects seem to be associated with adaptive responses in the central nervous system (CNS) such as the upregulation of neurotrophic factors, most notably the brain-derived neurotrophic factor (BDNF) (Huang et al. 2006). BDNF is an important intercellular signal that mediates neurogenesis, synaptic plasticity and cell survival (for review see Hu and Russek 2008).
There is extensive literature indicating that wheel running programs, voluntary and low-intensity exercise, result in an upregulation of BDNF protein and mRNA levels in different brain areas of rodents such as the hippocampus (Berchtold et al. 2005; Ploughman et al. 2007; Vaynman et al. 2004) and the striatum (Cotman and Berchtold 2002; Pang et al. 2006). Moreover, Soya et al. (2007) have recently documented that moderate-intensity exercise and forced-treadmill schedule increase BDNF levels in the rat’s hippocampus. However, the effects of high-intensity forced-treadmill schedule on striatal BDNF remain unclear.
The effects of downhill exercise on the CNS has been fairly well studied, probably because exercise that includes a large eccentric component such as downhill training has been associated with muscle damage (Chapman et al. 2006) and intense muscle fatigue (Carmichael et al. 2005). In accordance with this notion, previous data from our laboratory have shown that high-intensity downhill training schedule results in brain mitochondrial dysfunction and decreased BDNF levels in the frontal cortex of mice (Aguiar et al. 2007, 2008). Here, we extended these results by using neurochemistry assays to show that downhill exercise upregulates BDNF protein levels in the striatum and hippocampus of mice.
Materials and methods
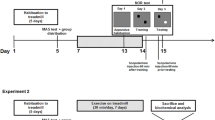
All procedures were performed in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC) and were approved by the Ethics Committee of the Universidade do Extremo Sul Catarinense, Brazil. Twenty-four male CF1 mice (6 weeks old and weighing 25–30 g at the beginning of the experiments) were used. They were maintained in a room under controlled temperature (20 ± 2°C) and were subjected to a 12-h light cycle (lights on 7:00 a.m.) with free access to food and water. The mice were randomly assigned to three groups (n = eight each group) designated: non-running controls, level runners and downhill runners. Each animal was weighed upon inclusion in the study and checked for weight loss.
The exercise training protocol was performed as described previously (Aguiar et al. 2007, 2008). Initially, all groups were habituated to a nine-channel motor-driven treadmill at a speed of 5 m min−1 for 10 min day−1 during 1 week (Table 1). During the following 8 weeks, the level (0° incline) and downhill (16° decline) trained groups performed a continuous running program once a day (7:00–8:00 p.m.), 5 days week−1, performing a total of 40 days (Table 1). Non-runner control animals were put on the switched-off treadmill during the same 8 weeks to equal environmental and noise stress. The exercise training protocol was discontinued 48 h before sacrifice. The mice were anesthetized with CO2 and sacrificed by cervical dislocation. The hippocampus, the striatum and the soleus muscle were removed, weighed, stored and frozen at −80°C until analysis.
Blood lactate level was acutely determined at end of the last session of exercise and was used to classify the exercise intensity. Therefore, to acquire sedentary responses to exercise, non-running control animals also performed this last session of physical training. A commercial kit from Roche, Germany, was used according to the manufacturers instructions to determine blood lactate levels from 15–50 μl of mice tail capillary blood by reflectance photometry at a wavelength of 657 nm, via colorimetric lactate-oxidase mediator reaction (Aguiar et al. 2007, 2008).
Due to collaborative tissue requirements and dissection time limitations, citrate synthase (CS) analysis was performed only on the soleus (Alp et al. 1976). The tissue was weighed and homogenized with a glass homogenizer on ice in 100 mM Tris–HCl at a constant weight-to-volume ratio. Sample homogenate was then added to a reaction mix of 100 mM Tris–HCl, 1.0 mM dithio-bis (2-nitrobenzoic acid), and 3.9 mM acetyl coenzyme A. After addition of 1.0 mM oxaloacetate, absorbance at 412 nm was recorded for a 2-min period. Mean absorbance change per minute was recorded for each sample, and CS activity in mmol min−1 g−1 was then calculated by using an extinction coefficient of 13.6.
BDNF protein was quantified using an enzyme-linked immunosorbent assay (ELISA) and standard protocols (ChemiKine™ brain-derived neurotrophic factor, sandwich ELISA, Chemicon, USA). Briefly, Nunc MaxiSorp 96-well plates were coated with 0.1 ml of a monoclonal antibody against BDNF in a buffer containing 0.025 M sodium bicarbonate and 0.025 M sodium carbonate (pH 9.7) for 16 h at 4°C. After washing in TBST [(20 mM Tris–HCl (pH 7.6), 150 mM NaCl, 0.05% Tween 20)], wells were incubated with 0.2 ml of a blocking buffer at room temperature for 1 h and then washed again in TBST. Samples, six serial dilutions of a BDNF standard (500 pg ml−1), and a blank (no BDNF) were added in triplicate into separate wells. Plates were incubated for 2 h at room temperature and washed five times in TBST. A polyclonal antibody against BDNF (1:500 dilution) was added into each well and plates were incubated for 2 h at room temperature. After five washes in TBST, 0.1 ml of a secondary anti-IgY antibody with a horseradish peroxidase conjugate was added to each well and plates were incubated for 1 h at room temperature. Wells were washed five times with TBST; hydrogen peroxidase solution with a peroxidase substrate was added and incubated for 10 min at room temperature. Reactions were stopped with 1 M phosphoric acid and absorbance at 450 nm was measured using an automated microplate reader. Standard curves were plotted for each plate. Triplicates were averaged and values were corrected for total amount of protein in the sample. The analysis of tissue protein was performed with protein binding after addition of folin-phenol reagent and colorimetric changes were read at 700 nm on a spectrophotometer (Lowry et al. 1951), with bovine serum albumin as standard.
Results were expressed as mean ± standard error of the mean (SEM). The statistical evaluation of the results was carried out using analysis of variance (ANOVA) followed by Tukey’s post hoc test. The accepted level of significance for the tests was P ≤ 0.05. All analyses were performed using the Statistical Package for the Social Sciences (SPSS) software in a compatible computer.
Results
At the end of the physical training, there was no significant difference in body weight among the three groups (approximately 37 g of body weight) (data not shown). Our results showed higher blood lactate levels in the sedentary control group than in both trained groups (Table 2). These data indicate a significantly lower (F 2,21 = 18.51, P ≤ 0.001) blood lactate content in trained animals than in untrained animals after a session of exercise, meaning improvement mainly of the muscle oxidative metabolism of the runners. The trained mice of our study performed the exercise around the lactate threshold (Table 2) of approximately 4.2 mmol l−1 for rodents (Voltarelli et al. 2002) meaning moderate to high-intensity physical training.
The soleus muscle CS activity in the level exercise and the downhill exercise groups was significantly higher (F 2,21 = 19.02, P ≤ 0.001) than in the non-runners control group (Table 2). These results indicate that the treadmill-training program used was sufficient to increase the oxidative metabolism in skeletal muscle of mice.
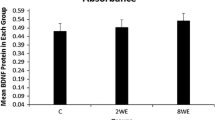
When compared to untrained animals, the treadmill level running and downhill running programs significantly increased (F 2,21 = 9.60, P ≤ 0.001) BDNF levels in the hippocampus (around 68.5 and 137.0%, respectively). Inter-comparisons between both treadmill trainings did not show significant differences in hippocampal BDNF levels (Table 2). In the striatum, physical training significantly increased (F 2,21 = 9.90, P ≤ 0.001) BDNF protein levels (approximately 49.9%) only in the downhill schedule when compared to untrained animals. Level treadmill training did not show significant differences from the untrained control and the downhill-trained groups (Table 2).
Discussion
The present findings reinforce the notion that the configuration of exercise induces different effects in the CNS plasticity. Corroborating with previous literature (Berchtold et al. 2005; Huang et al. 2006; Hunsberger et al. 2007; Vaynman et al. 2004), the current results indicate that low to moderate chronic level-running program results in BDNF enhancement in the hippocampus. On the other hand, previous studies investigating the effects of moderate exercise in BDNF levels in rodents striatum have been inconsistent, with some authors demonstrating increased BDNF levels (Dobrossy and Dunnet 2004, 2006), while others have reported no significant differences (Cechetti et al. 2008; Neeper et al. 1996) or even reduced striatal BDNF levels (Dobrossy and Dunnett 2006). Our findings indicate that striatal BDNF protein levels are differentially influenced by the exercise training program demonstrating that striatal BDNF levels are mainly responsive to a more complex physical exercise such as downhill running.
The downhill is generally used as a stressor model of exercise since it is associated with greater fiber damage, soreness, inflammation, fatigue, and other functional deficits (Chapman et al. 2006). The excessive repetition of the eccentric training stimulates muscle inflammation and can generate a systemic inflammatory response (Angeli et al. 2004). However, the effects of eccentric exercise on CNS plasticity are yet to be fully elucidated. Carmichael et al. (2005) showed increased concentration of plasma creatine kinase concentration, a marker of muscle damage, and brain inflammatory cytokine IL-1β lasting up to 48 h after downhill treadmill training. Moreover, previous data from our laboratory have shown that high-intensity downhill training schedule results in brain mitochondrial dysfunction and decreased BDNF levels in the frontal cortex of mice (Aguiar et al. 2007, 2008). Here, we have shown that the hippocampal and striatal BDNF levels are increased 48 h after the 8-week downhill training.
Although the exact molecular mechanisms and signaling pathways through which downhill training upregulates mice hippocampal and striatal BDNF levels remains to be elucidated, there is considerable evidence supporting the role of insulin-like growth factor I (IGF-I) in this response. IGF-I entrains similar downstream pathways to BDNF action (Yamada et al. 1997). Moreover, it has been previously suggested that peripheral sources of IGF-I supplied to the brain can mediate the effect of exercise on neuronal plasticity (Carro et al. 2001). More recently, Ding et al. (2006) have published a highlight study demonstrating that exercise increases IGF-I expression in the hippocampus and that the IGF-I receptor blockade abrogated the exercise-induced increase in the hippocampal BDNF mRNA and protein levels. However, it is important to emphasize that the hippocampus and the striatum are two structures that have been implicated as playing an important role in learning and memory processes (McDonald et al. 2004). Therefore, at this moment, it is not easy to specify whether the observed alterations in BDNF are due to downhill-induced upregulation or complex learning-induced mechanisms that influence BDNF levels in these brain regions, and this constitutes a very interesting field that requires additional research.
Consistent with the present data, several lines of evidence strongly suggest that CNS differentially modulates concentric and eccentric muscle actions. For example, eccentric contractions are more difficult to perform than concentric ones (Roland et al. 1980; Yue et al. 2000). This is supported by the result of higher eccentric than concentric errors in movement execution (Fang et al. 2001). Higher eccentric-force fluctuation seems consistent with the observation that high-threshold motor units are selectively recruited first and that they discharge action potentials at a lower rate during submaximal eccentric contractions (Howell et al. 1995; Nardone et al. 1989). Moreover, neuroimaging studies have shown an increased level of brain activation when motor tasks with a higher degree of difficulty are performed (Roland et al. 1980; Yue et al. 2000). Thus, the present findings suggest that to control a movement with a higher degree of intensity and difficulty the CNS may need more extensive neural network to participate in the controlling process. It must be conceded that additional research is needed to determine the exact mechanisms and cellular sites responsible for the selective striatal BDNF upregulation by downhill training.
In conclusion, it has long been speculated that the brain uses differentiated strategies for eccentric muscle actions. Several novel concepts emerge from this study. In particular, (1) downhill running is as effective as level running in increasing hippocampal BDNF protein levels, (2) BDNF protein is elevated in the striatum after downhill physical training.
References
Aguiar AS Jr, Tuon T, Pinho CA, Silva LA, Andreazza AC, Kapczinski F, Quevedo J, Streck EL, Pinho RA (2007) Mitochondrial IV complex and brain neurothrophic derived factor responses of mice brain cortex after downhill training. Neurosci Lett 426:171–174
Aguiar AS Jr, Tuon T, Pinho CA, Silva LA, Andreazza AC, Kapczinski F, Quevedo J, Streck EL, Pinho RA (2008) Intense exercise induces mitochondrial dysfunction in mice brain. Neurochem Res 33:51–58
Alp PR, Newsholme EA, Zammit VA (1976) Activities of citrate synthase and NAD+-linked and NADP+-linked isocitrate dehydrogenase in muscle from vertebrates and invertebrates. Biochem J 154:689–700
Angeli A, Minetto M, Dovio A, Paccotti P (2004) The overtraining syndrome in athletes: a stress-related disorder. J Endocrinol Invest 6:603–612
Berchtold NC, Chinn G, Chou M, Kesslak JP, Cotman CW (2005) Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience 133:853–861
Carmichael MD, Davis JM, Murphy EA, Brown AS, Carson JA, Mayer E, Ghaffar A (2005) Recovery of running performance following muscle-damaging exercise: relationship to brain IL-1β. Brain Behav Immun 19:445–452
Carro E, Nunez A, Busiguina S, Torres-Aleman I (2001) Circulating insulin-like growth factor I mediates effects of exercise on the brain. J Neurosci 20:2926–2933
Cechetti F, Fochesatto C, Scopel D, Nardin P, Gonçalves CA, Netto CA, Siqueira IR (2008) Effect of a neuroprotective exercise protocol on oxidative state and BDNF levels in the rat hippocampus. Brain Res 1188:182–188
Chapman D, Newton M, Sacco P, Nosaka K (2006) Greater muscle damage induced by fast versus slow velocity eccentric exercise. Int J Sports Med 27:591–598
Cotman CW, Berchtold NC (2002) Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci 25:295–301
Ding Q, Vaynman S, Akhavan M, Ying Z, Gomez-Pinilla F (2006) Insulin-like growth factor I interfaces with brain-derived neurotrophic factor-mediated synaptic plasticity to modulate aspects of exercise-induced cognitive function. Neuroscience 140:823–833
Dobrossy MD, Dunnett SB (2004) Environmental enrichment affects striatal graft morphological and functional recovery. Eur J Neurosci 19:159–168
Dobrossy MD, Dunnett SB (2006) Morphological and cellular changes within embryonic striatal grafts associated with enriched environment and involuntary exercise. Eur J Neurosci 24:3223–3233
Fang Y, Siemionow V, Sahgal V, Xiong F, Yue GH (2001) Greater movement-related cortical potential during human eccentric versus concentric muscle contractions. J Neurophysiol 86:1764–1772
Howell JN, Fuglevand AJ, Walsh ML, Bigland-Ritchie B (1995) Motor unit activity during isometric and concentric-eccentric contractions of the human first dorsal interosseous muscle. J Neurophysiol 74:901–904
Hu Y, Russek SJ (2008) BDNF and the diseased nervous system: a delicate balance between adaptive and pathological processes of gene regulation. J Neurochem 105:1–17
Huang AM, Jen CJ, Chen HF, Yu L, Kuo YM, Chen HI (2006) Compulsive exercise acutely upregulates rat hippocampal brain-derived neurotrophic factor. J Neural Transm 113:803–811
Hunsberger JG, Newton SS, Bennett AH, Duman CH, Russell DS, Salton SR, Duman RS (2007) Antidepressant actions of the exercise-regulated gene VGF. Nature Med 13:1476–1482
Lowry OH, Rosebough NG, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
McDonald JR, Hong NS, Devan BD (2004) The challenges of understanding mammalian cognition and memory-based behaviours: an interactive learning and memory systems approach. Neurosci Biobehav Rev 28:719–745
Nardone A, Romano C, Schieppati M (1989) Selective recruitment of high threshold human motor units during voluntary isotonic lengthening of active muscles. J Physiol 409:451–471
Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW (1996) Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res 726:49–56
Pang TYC, Stam NC, Nithianantharajah J, Howard ML, Hannan AJ (2006) Differential effects of voluntary physical exercise on behavioral and brain-derived neurotrophic factor expression deficits in Huntington’s disease transgenic mice. Neuroscience 141:569–584
Ploughman M, Granter-Button S, Chernenko G, Attwood Z, Tucker BA, Mearow KM, Corbett D (2007) Exercise intensity influences the temporal profile of growth factors involved in neuronal plasticity following focal ischemia. Brain Res 1150:207–216
Roland PE, Lassen B, Lassen NA, Skinhoj E (1980) Supplementary motor area and other cortical areas in organization of voluntary movements in man. J Neurophysiol 43:118–136
Soya H, Nakamura T, Deocaris CC, Kimpara A, Iimura M, Fujikawa T, Chang H, McEwen BS, Nishijima T (2007) BDNF induction with mild exercise in the rat hippocampus. Biochem Biophys Res Commun 358:961–967
Vaynman S, Ying Z, Gomez-Pinilla F (2004) Exercise induces BDNF and synapsin I to specific hippocampal subfields. J Neurosci Res 76:356–362
Voltarelli FA, Gobatto CA, Mello MAR (2002) Determination of anaerobic threshold in rats using the lactate minimum test. Braz J Med Biol Res 35:1389–1394
Yamada M, Ohnishi H, Sano S, Nakatani A, Ikeuchi T, Hatanaka H (1997) Insulin receptor substrate (IRS)-1 and IRS-2 are tyrosine phosphorylated and associated with phosphatidylinositol 3-kinase in response to brain-derived neurotrophic factor in cultured cerebral cortical neurons. J Biol Chem 272:30334–30339
Yue GH, Liu JZ, Siemionow V, Ranganathan VK, Ng TC, Sahgal V (2000) Brain activation during human finger extension and flexion movements. Brain Res 856:291–300
Acknowledgments
This research was supported by grants from CNPq/MCT (Brazil) and CAPES/MEC (Brazil).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aguiar, A.S., Speck, A.E., Prediger, R.D.S. et al. Downhill training upregulates mice hippocampal and striatal brain-derived neurotrophic factor levels. J Neural Transm 115, 1251–1255 (2008). https://doi.org/10.1007/s00702-008-0071-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-008-0071-2