Abstract
Background
Reherniation after lumbar discectomy is classified as a failure and occurs in 3 to 18% of cases. Various risk factors for reherniation such as age, sex, body mass index, smoking, and size of annular defect have been reported. The aim of this study was to identify risk factors for early reherniation after one-level lumbar discectomy with or without annular closure within 3 months after surgery.
Methods
This study is based on data analysis of a prospective, multicenter randomized controlled trial in Europe. Patients included underwent standard lumbar discectomy—with or without implantation of an annular closure device (ACD). Enrollment of 554 patients in 21 centers in Europe (Germany, Switzerland, Austria, Belgium, The Netherlands, and France) started in 2010 and was completed in October 2014. A total of 276 patients were randomized to the ACD group (ACG) and 278 patients to the control group (CG).
Results
Four (1.5%) symptomatic reherniations occurred in the ACG and 18 (6.5%) in the CG. In the overall population, a significant correlation was found with recurrent herniation for disc degeneration (Pfirrmann p = 0.009) and a trend for current smoker status (p = 0.07). In CG, age ≥ 50 years (p = 0.05) and disc degeneration (Pfirrmann p = 0.026, Kellgren and Lawrence p = 0.013) were predictive factors for reherniation.
Conclusion
In the current study, risk factors for early recurrent disc herniation after lumbar discectomy were age ≥ 50 years and moderate disc degeneration. The annular closure device reduced the risk of early reherniation.
Trial registration
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lumbar disc herniation is the most common spinal degenerative pathology, and discectomy is the most frequently performed spinal surgery [7, 13, 14]. Reherniation after lumbar discectomy (RLD) is a known frequent failure and has been described extensively in the literature [5, 9, 10, 12, 20, 23, 30, 31, 53]. The reported reherniation rate after lumbar discectomy ranges from 3 to 18% [3, 41, 55]. RLD is defined as recurrence of symptoms after a postoperative pain-free period caused by a new disc herniation at the same ipsilateral level [43]. If RLD is diagnosed, reoperation may be required. The SPORT study described reoperation rates of 2% at 90 days, 4% at 1 year, and 10% after 4 years post-surgery [54]. Moreover, only 78% patient satisfaction was reported at 1 year post-surgery in the Swedish Spine Registry [46]. The reoperation rate after 3 years in our current trial was 19.3% in the control group and 11% in the group with annular closure device implantation [21].
Many risk factors have already been reported, such as age, sex, body mass index (BMI), smoking, type and size of annular defect, amount of removed disc volume, grade of disc degeneration, disc height, and range of motion [1, 8, 25, 26, 32,33,34,35, 42, 51]. In the study by Meredith et al., obese patients were 12 times more likely to suffer RLD compared with non-obese patients [33]. Several authors have confirmed the findings that smoking and increasing age are associated with RLD [1, 22, 25, 32, 34, 42]. A Finnish study found no difference between genders concerning risk for reoperation [20]. Carragee et al. demonstrated a trend towards higher reherniation rates with limited discectomy compared with the more aggressive discectomy group [9]. This was confirmed in a review article by Watters et al. which described a significant increase in RLD rate following conservative versus aggressive discectomy [52]. Furthermore, it was shown that patients with annular defects > 6 mm account for most of the clinically relevant reherniations [8]. Kim et al. recently acknowledged the correlation between a wider defect and a higher reherniation rate [25]. Moreover, a larger mean annular defect area (46 mm ± 18 mm2) and a lower percentage of removed disc volume were obtained in patients with reherniations in the study by McGirt et al. [33].
A recently introduced annular closure device (Barricaid®, Intrinsic Therapeutics, Inc., Woburn, MA) was developed to occlude the annular defect and prevent reherniation [56]. It has been reported that Barricaid® might reduce the risk of reherniation in high-risk patients. These findings were observed in two prospective, single-arm trials with a total of 75 patients. A low rate (1.4%) of symptomatic RLD was reported, and Barricaid® use was associated with a good overall outcome [6]. The 2- and 3-year follow-up results of a European prospective, multicenter randomized controlled trial including 554 patients in 21 centers have already been published [21, 50]. The frequency of symptomatic reherniation after 2 years was lower in the annular closure device group compared with the control group (12% vs. 25%, p < 0.001) [50]. Similar results have been reported for the 3-year follow-up (14.8% vs. 29.5%) [21].
The aim of the present data analysis was to identify factors associated with very early reherniation within the first 3 months after surgery in patients undergoing one-level lumbar discectomy, with or without annular closure.
Methods
Trial design
This study includes data of a post-marketing, prospective, multicenter, randomized controlled trial (RCT) of limited discectomy—with and without use of an annular closure device (Clinicaltrials.gov NCT01283438). The study protocol was published previously [27].
Participants
This European study included 554 patients in 21 centers located in Germany, Switzerland, Austria, Belgium, The Netherlands, and France from 2010 to 2014 and was conducted in compliance with the Ethics Committee, GCP, and ISO requirements. A total of 276 patients were randomized to ACG and 278 patients to CG. Only 267 patients in the ACG with a device implanted (no implantation n = 4, failures to implant n = 5, implantation on second attempt n = 3) were included in our analysis. Informed consent was obtained for all patients. Study oversight regarding safety was provided by an independent data safety monitoring board.
Randomization and masking
Patients were randomized web-based intraoperatively 1:1 to either standard discectomy (control group, CG) or standard discectomy with implantation of an annular closure device (annular closure group, ACG). The main criteria for study enrollment were an annular defect of 4 to 6 mm in height and 6 to 10 mm in width. Neither the surgeons nor the participants were blinded for the treatment allocation.
Early reherniation after lumbar discectomy
RLD was reported as adverse event in the RCT. RLD was reported as ipsi- or contralateral new disc herniation at index level and had to be confirmed by either magnetic resonance imaging (MRI) or during reoperation.
Annular closure device
The ACD (Barricaid®, Intrinsic Therapeutics, Inc., Woburn, MA, USA) was developed to prevent reherniation after lumbar discectomy, through closure of the annular defect. European Conformity (CE) marking was received in 2009 and FDA approval in 2019. The device consists of a flexible mesh, a mechanical barrier to the annular defect, and a bone anchor.
Surgical technique
The majority of surgeons preferred intraoperative microscopy. A limited nucleotomy described by Spengler [45] was performed. The volume of removed disc material varied according to the surgeon’s implementation of limited discectomy and was measured in cubic centimeters. The height and width of the annular defect were measured using sizing paddles. Randomization occurred after discectomy. No further nucleus removal was allowed thereafter. Device implantation and correct alignment were verified by intra- and X-ray.
Follow-up
Clinical examination and X-ray were performed at the consultation after 6 weeks and 3 months. Assessment included Visual analog Scale (VAS) leg (LP-VAS) and low back pain (BP-VAS), Oswestry Disability Index (ODI), and Medical Outcomes Short Form-36 (SF-36) physical and mental component summary scales [15, 37].
Radiographic assessment
Preoperative imaging of the lumbar spine included anterior-posterior /lateral X-ray with extension/flexion, computed tomography (CT), and MRI. Analysis of the images was performed by a team of independent radiologists.
An MRI was performed in cases with clinical evidence of early RLD. Disc degeneration was graded according to Kellgren and Lawrence (K-L) [18] and Pfirrmann [38].
Statistical analysis
Descriptive statistics was used for demographic characteristics, intraoperative findings, and pain scores. Univariate logistic regression analysis was applied to investigate correlation between potential risk factors and early RLD in three different populations: (1) all subjects, (2) subjects implanted with the ACD, and (3) control subjects.
The multivariate logistic regression analysis was applied on the “all subjects” population only. Any variable with a p value < 0.1 in the univariate regression analysis was utilized in a multivariate logistic regression, stepwise, backward elimination. At each step, the variable with the highest insignificant p value was dropped, until all p values were less than 0.05.
Results
Preoperative demographic characteristics
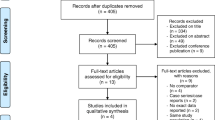
There were no significant differences between the groups (Table 1). Follow-up rates at 3 months were 97.4% for ACG and 95.3% for CG. Ten patients (CG n = 6, ACG n = 4) withdrew from the study before their 3-month visit (Fig. 1).
Preoperative radiological findings
The majority of patients had a Pfirrmann grade III (423 patients, 76.3%) or IV (100 patients, 18%) disc degeneration (DD). In comparison, DD classified according to Kellgren and Lawrence presented mostly as doubtful (235 patients, 42.4%) or minimal (229 patients, 41.3%) changes (Table 2). There was no significant difference in Pfirrmann grades (p = 0.398) or Kellgren and Lawrence classification (p = 0.441) between ACG and CG groups. Average disc height was 8.9 ± 2.1 mm (ACG: 8.9 ± 2.1 mm, CG: 8.8 ± 2.2 mm), translational range of motion (ROM) 0.4 ± 1.5 mm (ACG: 0.5 ± 1.4 mm, CG: 0.3 ± 1.6 mm) and angular ROM − 4.2 ± 4.5° (ACG: − 4.0 ± 4.5°, CG: − 4.4 ± 4.5°).
Intraoperative findings
A full thickness defect was present in 51.4% of patients. Approach through the existing annular defect was used in 62.4% of patients. The overall mean defect size was 38.8 mm2, and the mean amount of nucleus removed was 1.3 mL (Table 3).
Postoperative clinical outcome
We observed postoperative at the 3-month follow-up a significant improvement in VAS leg pain, VAS back pain, and ODI in ACG and CG compared with the preoperative baseline (< 0.0001).
Risk factors for RLD
Twenty-two patients (4%) suffered a symptomatic index level reherniation during the first 3 months, 4 (1.5%) occurred in ACG and 18 (6.5%) in CG. Type of RLD is shown in Table 4. RLD occurred ipsilateral in 91% (ACG 75%, CG 94%). Ipsilateral reherniations in ACG were found lateral to the device mesh. RLD due to device malfunction did not occur. No patient described an event that precipitated the recurrence. Reoperation due to RLD was necessary in 12 patients (n = 3 ACG, n = 9 CG). Median time from pain recurrence to reoperation was 17 days (1 day ACG, 20 days CG).
Regarding risk factors for RLD, a significant correlation was found for age ≥ 50 years and DD. Control subjects ≥ 50 years of age were more likely to have a symptomatic reherniation (CG p = 0.051, OR = 2.61) (Table 5). In CG, reherniation rate of patients aged < 50 years was 4.6% compared with 9.9% for patients aged ≥ 50 years. In ACG, all RLD occurred in patients < 50 years. Disc degeneration according to the K-L classification was a predictor for RLD in CG (CG p = 0.013, OR = 2.15). The RLD rate in CG was 13.2% for moderate degeneration. Pfirrmann grade IV degeneration showed a reherniation rate of 9% overall and 13.2% in CG. In summary, subjects with a 1 point higher Pfirrmann grade were 3.2 times more likely to have a symptomatic RLD.
All other variables including sex, BMI, smoking status, amount of nucleus removed, herniation or defect type, defect size, surgical approach, and radiological data—including range of motion and disc height—were not significant in the uni- and multivariate logistic regression analysis.
Smokers had a trend for a higher symptomatic RLD rate overall (p = 0.07, OR = 2.26). Although sex was not a significant predictor for reherniation, the RLD rate for females in CG was higher (F: 7.5% vs. M: 5.9%), and all reherniations in ACG occurred in females.
Implantation of ACD correlated with an overall reduction of symptomatic RLD (p = 0.007, OR = 0.23). In the multivariate logistic regression model, the following three variables remained after use of backward stepwise elimination: current smoker (p = 0.049, OR = 2.57), Pfirrmann grade (p = 0.010, OR = 3.26), and implantation of ACD (p = 0.007, OR = 0.23). Reherniation rate was 0.9% for non-smokers with grade III degeneration and implantation of ACD, compared with 19.2% for smokers with grade IV degeneration and no ACD device. In summary, the multivariate model predicts that a smoker with one point worse Pfirrmann grade without ACD is 36.4 (2.57 × 3.26 × 1/0.23) times more likely to suffer a symptomatic reherniation within 3 months.
Discussion
In our multicenter randomized controlled trial, the early RLD rate until the 3-month follow-up was 4% overall. These rates are in line with the literature [9, 20, 23, 30, 31, 49]. Implantation of an ACD was a significant predictor for RLD risk reduction in the overall population.
The median interval until RLD in the study by Moliterno et al. [35] and Wera et al. [55] was 3 and 2.8 month, respectively; McGirt et al. identified two postoperative time periods when RLD most frequently occurred, < 4 months or > 11 months after surgery [32].
In our current study, RLD reoperation rate was 2.2% within 3 months. The reported reoperation rate was 2% at 90 days in the SPORT trial [54] and 4.9% at 3 months after discectomy in the study by Kim et al. [23], where 46% of reoperations were performed within half a year. In Aizawa et al.’s trial, reoperation for RLD was performed significantly more often within the first 6 months [2].
Patients with grade IV Pfirrmann DD in CG and overall had the highest reherniation rate. Several authors found that patients with low to moderate DD were at increased risk of RLD [1, 11, 26]. Dora et al. presented a reduced RLD rate for each Pfirrmann grade increase [11].
A trend for RLD and increasing age has been reported [1, 23, 24, 32]. We confirmed these findings with a significant correlation between age ≥ 50 years and RLD in CG. Martin et al. presented results showing older age associated with higher short-term and lower long-term reoperation risk [31].
Our study identified smoking as a risk factor for RLD. Earlier studies have also shown that smokers have a higher recurrence rate [4, 19, 26, 34, 42, 47]. The lumbar disc space is known to be an avascular structure and absorbs all nutrients by diffusion [16, 17]. It was proposed that smoking-associated hypoxia inhibits closure of the discogenic and ligamentous defect after discectomy [28, 29, 34, 36, 44].
Similar to our findings, Martin et al. noted that female sex was associated with a higher risk for RLD and reoperation [31]. In contrast, Suk et al. reported male sex to be a risk factor for RLD [47]. However, other studies showed no difference between the genders [20, 33, 35, 48].
As with other studies [39, 40, 57], BMI did not prove to be a risk factor for RLD in our trial. However, patients with a BMI greater than 40 kg/m2 were excluded, and the mean BMI was 26.3 kg/m2. Several other authors have found higher BMIs to be associated with RLD rates [1, 24, 33, 42]. In contrast, Moliterno et al. found that patients with RLD had on average significantly lower BMIs [35].
It was shown by Carragee et al. that patients with large annular defects (> 6 mm wide) account for most of the clinically significant RLD [8]. Kim et al. [25] confirmed these results in a patient cohort of 467 patients, whereby large annular defect (> 6 mm) was a risk factor for RLD.
McGirt et al. [32] observed a greater mean annular defect area for patients with symptomatic RLD. In patients with early RLD (within 4 months), mean annular defect was larger compared with patients with RLD at a later time point [32]. An annular defect size of > 54 mm2 correlated with an 18% RLD rate, which is 4 times higher compared with 4.7% rate for patients with defect size of 36 mm2 [32].
A minimum of 6 mm and maximum of 10-mm defect width were necessary for our study enrollment. This might explain why we did not observe correlation between RLD and defect size. Awareness of a maximum allowed width and height made the surgeons more careful in their incision of the annulus. Only a small number (7%) of patients in our study had a defect size of ≥ 54 mm2, and the RLD rate in this group was 7.9%.
We were not able to observe any correlations between removed nucleus amount, disc height, type of herniation, sagittal ROM, and RLD rate.
Conclusion
Significant risk factors for early recurrent disc herniation after lumbar microdiscectomy in this study included age ≥ 50 years (OR = 2.6) and moderate disc degeneration (OR = 3.2). The annular closure device was a predictive factor that significantly reduced the risk of early reherniation. These results suggest that implantation of an ACD can prevent early reherniation after lumbar discectomy.
References
Abrishamkar S, Mahmoudkhani M, Aminmansour B, Mahabadi A, Jafari S (2014) Does disk space degeneration according to Los Angeles and Modic scales have relation with recurrent disk herniation? Adv Biomed Res 3:220. https://doi.org/10.4103/2277-9175.145125
Aizawa T, Ozawa H, Kusakabe T, Nakamura T, Sekiguchi A, Takahashi A, Sasaji T, Tokunaga S, Chiba T, Morozumi N, Koizumi Y, Itoi E (2012) Reoperation for recurrent lumbar disc herniation: a study over a 20-year period in a Japanese population. J Orthop Sci 17:107–113. https://doi.org/10.1007/s00776-011-0184-6
Ambrossi GL, McGirt MJ, Sciubba DM, Witham TF, Wolinsky JP, Gokaslan ZL, Long DM (2009) Recurrent lumbar disc herniation after single-level lumbar discectomy: incidence and health care cost analysis. Neurosurgery 65:574–578; discussion 578. https://doi.org/10.1227/01.NEU.0000350224.36213.F9
An HS, Silveri CP, Simpson JM, File P, Simmons C, Simeone FA, Balderston RA (1994) Comparison of smoking habits between patients with surgically confirmed herniated lumbar and cervical disc disease and controls. J Spinal Disord 7:369–373
Atlas SJ, Keller RB, Wu YA, Deyo RA, Singer DE (2005) Long-term outcomes of surgical and nonsurgical management of sciatica secondary to a lumbar disc herniation: 10 year results from the maine lumbar spine study. Spine (Phila Pa 1976) 30:927–935. https://doi.org/10.1097/01.brs.0000158954.68522.2a
Bouma GJ, Barth M, Ledic D, Vilendecic M (2013) The high-risk discectomy patient: prevention of reherniation in patients with large anular defects using an anular closure device. Eur Spine J 22:1030–1036. https://doi.org/10.1007/s00586-013-2656-1
Bruske-Hohlfeld I, Merritt JL, Onofrio BM, Stonnington HH, Offord KP, Bergstralh EJ, Beard CM, Melton LJ 3rd, Kurland LT (1990) Incidence of lumbar disc surgery. A population-based study in Olmsted County, Minnesota, 1950-1979. Spine (Phila Pa 1976) 15:31–35. https://doi.org/10.1097/00007632-199001000-00009
Carragee EJ, Han MY, Suen PW, Kim D (2003) Clinical outcomes after lumbar discectomy for sciatica: the effects of fragment type and anular competence. J Bone Joint Surg Am 85:102–108
Carragee EJ, Spinnickie AO, Alamin TF, Paragioudakis S (2006) A prospective controlled study of limited versus subtotal posterior discectomy: short-term outcomes in patients with herniated lumbar intervertebral discs and large posterior anular defect. Spine (Phila Pa 1976) 31:653–657. https://doi.org/10.1097/01.brs.0000203714.76250.68
Cauchoix J, Ficat C, Girard B (1978) Repeat surgery after disc excision. Spine (Phila Pa 1976) 3:256–259. https://doi.org/10.1097/00007632-197809000-00011
Dora C, Schmid MR, Elfering A, Zanetti M, Hodler J, Boos N (2005) Lumbar disk herniation: do MR imaging findings predict recurrence after surgical diskectomy? Radiology 235:562–567. https://doi.org/10.1148/radiol.2352040624
Findlay GF (1992) Minimally invasive lumbar disc surgery. Br J Neurosurg 6:405–408. https://doi.org/10.3109/02688699208995028
Frymoyer JW (1992) Lumbar disk disease: epidemiology. Instr Course Lect 41:217–223
Gray DT, Deyo RA, Kreuter W, Mirza SK, Heagerty PJ, Comstock BA, Chan L (2006) Population-based trends in volumes and rates of ambulatory lumbar spine surgery. Spine (Phila Pa 1976) 31:1957–1963; discussion 1964. https://doi.org/10.1097/01.brs.0000229148.63418.c1
Grevitt M, Khazim R, Webb J, Mulholland R, Shepperd J (1997) The short form-36 health survey questionnaire in spine surgery. J Bone Joint Surg Br 79:48–52. https://doi.org/10.1302/0301-620x.79b1.1269
Hampton D, Laros G, McCarron R, Franks D (1989) Healing potential of the anulus fibrosus. Spine (Phila Pa 1976) 14:398–401. https://doi.org/10.1097/00007632-198904000-00009
Holm S, Nachemson A (1988) Nutrition of the intervertebral disc: acute effects of cigarette smoking. An experimental animal study. Ups J Med Sci 93:91–99. https://doi.org/10.1517/03009734000000042
Kellgren JH, Lawrence JS (1957) Radiological assessment of osteo-arthrosis. Ann Rheum Dis 16:494–502. https://doi.org/10.1136/ard.16.4.494
Kelsey JL, Githens PB, O'Conner T, Weil U, Calogero JA, Holford TR, White AA 3rd, Walter SD, Ostfeld AM, Southwick WO (1984) Acute prolapsed lumbar intervertebral disc. An epidemiologic study with special reference to driving automobiles and cigarette smoking. Spine (Phila Pa 1976) 9:608–613. https://doi.org/10.1097/00007632-198409000-00012
Keskimaki I, Seitsalo S, Osterman H, Rissanen P (2000) Reoperations after lumbar disc surgery: a population-based study of regional and interspecialty variations. Spine (Phila Pa 1976) 25:1500–1508. https://doi.org/10.1097/00007632-200006150-00008
Kienzler JC, Klassen PD, Miller LE, Assaker R, Heidecke V, Frohlich S, Thome C, Annular Closure RCTSG (2019) Three-year results from a randomized trial of lumbar discectomy with annulus fibrosus occlusion in patients at high risk for reherniation. Acta Neurochir 161:1389–1396. https://doi.org/10.1007/s00701-019-03948-8
Kim CH, Chung CK, Park CS, Choi B, Hahn S, Kim MJ, Lee KS, Park BJ (2013) Reoperation rate after surgery for lumbar spinal stenosis without spondylolisthesis: a nationwide cohort study. Spine J 13:1230–1237. https://doi.org/10.1016/j.spinee.2013.06.069
Kim CH, Chung CK, Park CS, Choi B, Kim MJ, Park BJ (2013) Reoperation rate after surgery for lumbar herniated intervertebral disc disease: nationwide cohort study. Spine (Phila Pa 1976) 38:581–590. https://doi.org/10.1097/BRS.0b013e318274f9a7
Kim JM, Lee SH, Ahn Y, Yoon DH, Lee CD, Lim ST (2007) Recurrence after successful percutaneous endoscopic lumbar discectomy. Minim Invasive Neurosurg 50:82–85. https://doi.org/10.1055/s-2007-982504
Kim KT, Lee DH, Cho DC, Sung JK, Kim YB (2015) Preoperative risk factors for recurrent lumbar disk herniation in L5-S1. J Spinal Disord Tech 28:E571–E577. https://doi.org/10.1097/BSD.0000000000000041
Kim KT, Park SW, Kim YB (2009) Disc height and segmental motion as risk factors for recurrent lumbar disc herniation. Spine (Phila Pa 1976) 34:2674–2678. https://doi.org/10.1097/BRS.0b013e3181b4aaac
Klassen PDHGJ, Bouma S, Eustacchio M, Barth M, Kursumovic A et al (2016) A multicenter, prospective, randomized study protocol to demonstrate the superiority of a bone-anchored prosthesis for anular closure used in conjunction with limited discectomy to limited discectomy alone for primary lumbar disc herniation. Int J Clin Trials 3(3):120–131
Kuri M, Nakagawa M, Tanaka H, Hasuo S, Kishi Y (2005) Determination of the duration of preoperative smoking cessation to improve wound healing after head and neck surgery. Anesthesiology 102:892–896. https://doi.org/10.1097/00000542-200505000-00005
Little CP, Burston BJ, Hopkinson-Woolley J, Burge P (2006) Failure of surgery for scaphoid non-union is associated with smoking. J Hand Surg Br 31:252–255. https://doi.org/10.1016/j.jhsb.2005.12.010
Malter AD, McNeney B, Loeser JD, Deyo RA (1998) 5-year reoperation rates after different types of lumbar spine surgery. Spine (Phila Pa 1976) 23:814–820. https://doi.org/10.1097/00007632-199804010-00015
Martin BI, Mirza SK, Flum DR, Wickizer TM, Heagerty PJ, Lenkoski AF, Deyo RA (2012) Repeat surgery after lumbar decompression for herniated disc: the quality implications of hospital and surgeon variation. Spine J 12:89–97. https://doi.org/10.1016/j.spinee.2011.11.010
McGirt MJ, Eustacchio S, Varga P, Vilendecic M, Trummer M, Gorensek M, Ledic D, Carragee EJ (2009) A prospective cohort study of close interval computed tomography and magnetic resonance imaging after primary lumbar discectomy: factors associated with recurrent disc herniation and disc height loss. Spine (Phila Pa 1976) 34:2044–2051. https://doi.org/10.1097/BRS.0b013e3181b34a9a
Meredith DS, Huang RC, Nguyen J, Lyman S (2010) Obesity increases the risk of recurrent herniated nucleus pulposus after lumbar microdiscectomy. Spine J 10:575–580. https://doi.org/10.1016/j.spinee.2010.02.021
Miwa S, Yokogawa A, Kobayashi T, Nishimura T, Igarashi K, Inatani H, Tsuchiya H (2015) Risk factors of recurrent lumbar disk herniation: a single center study and review of the literature. J Spinal Disord Tech 28:E265–E269. https://doi.org/10.1097/BSD.0b013e31828215b3
Moliterno JA, Knopman J, Parikh K, Cohan JN, Huang QD, Aaker GD, Grivoyannis AD, Patel AR, Hartl R, Boockvar JA (2010) Results and risk factors for recurrence following single-level tubular lumbar microdiscectomy. J Neurosurg Spine 12:680–686. https://doi.org/10.3171/2009.12.SPINE08843
Moller AM, Pedersen T, Villebro N, Munksgaard A (2003) Effect of smoking on early complications after elective orthopaedic surgery. J Bone Joint Surg Br 85:178–181. https://doi.org/10.1302/0301-620x.85b2.13717
Patrick DL, Deyo RA, Atlas SJ, Singer DE, Chapin A, Keller RB (1995) Assessing health-related quality of life in patients with sciatica. Spine (Phila Pa 1976) 20:1899–1908; discussion 1909. https://doi.org/10.1097/00007632-199509000-00011
Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N (2001) Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976) 26:1873–1878. https://doi.org/10.1097/00007632-200109010-00011
Quah C, Syme G, Swamy GN, Nanjayan S, Fowler A, Calthorpe D (2014) Obesity and recurrent intervertebral disc prolapse after lumbar microdiscectomy. Ann R Coll Surg Engl 96:140–143. https://doi.org/10.1308/003588414X13814021676873
Rihn JA, Kurd M, Hilibrand AS, Lurie J, Zhao W, Albert T, Weinstein J (2013) The influence of obesity on the outcome of treatment of lumbar disc herniation: analysis of the spine patient outcomes research trial (SPORT). J Bone Joint Surg Am 95:1–8. https://doi.org/10.2106/JBJS.K.01558
Rogers LA (1988) Experience with limited versus extensive disc removal in patients undergoing microsurgical operations for ruptured lumbar discs. Neurosurgery 22:82–85. https://doi.org/10.1227/00006123-198801010-00013
Shimia M, Babaei-Ghazani A, Sadat BE, Habibi B, Habibzadeh A (2013) Risk factors of recurrent lumbar disk herniation. Asian J Neurosurg 8:93–96. https://doi.org/10.4103/1793-5482.116384
Shin BJ (2014) Risk factors for recurrent lumbar disc herniations. Asian Spine J 8:211–215. https://doi.org/10.4184/asj.2014.8.2.211
Sorensen LT, Karlsmark T, Gottrup F (2003) Abstinence from smoking reduces incisional wound infection: a randomized controlled trial. Ann Surg 238:1–5. https://doi.org/10.1097/01.SLA.0000074980.39700.31
Spengler DM (1982) Lumbar discectomy. Results with limited disc excision and selective foraminotomy. Spine (Phila Pa 1976) 7:604–607
Stromqvist B, Fritzell P, Hagg O, Jonsson B, Sanden B, Swedish Society of Spinal S (2013) Swespine: the Swedish spine register: the 2012 report. Eur Spine J 22:953–974. https://doi.org/10.1007/s00586-013-2758-9
Suk KS, Lee HM, Moon SH, Kim NH (2001) Recurrent lumbar disc herniation: results of operative management. Spine (Phila Pa 1976) 26:672–676. https://doi.org/10.1097/00007632-200103150-00024
Swartz KR, Trost GR (2003) Recurrent lumbar disc herniation. Neurosurg Focus 15:E10. https://doi.org/10.3171/foc.2003.15.3.10
Thome C, Barth M, Scharf J, Schmiedek P (2005) Outcome after lumbar sequestrectomy compared with microdiscectomy: a prospective randomized study. J Neurosurg Spine 2:271–278. https://doi.org/10.3171/spi.2005.2.3.0271
Thome C, Klassen PD, Bouma GJ, Kursumovic A, Fandino J, Barth M, Arts M, van den Brink W, Bostelmann R, Hegewald A, Heidecke V, Vajkoczy P, Frohlich S, Wolfs J, Assaker R, Van de Kelft E, Kohler HP, Jadik S, Eustacchio S, Hes R, Martens F, Annular Closure RCTSG (2018) Annular closure in lumbar microdiscectomy for prevention of reherniation: a randomized clinical trial. Spine J 18:2278–2287. https://doi.org/10.1016/j.spinee.2018.05.003
Watters WC 3rd, Bono CM, Gilbert TJ, Kreiner DS, Mazanec DJ, Shaffer WO, Baisden J, Easa JE, Fernand R, Ghiselli G, Heggeness MH, Mendel RC, O'Neill C, Reitman CA, Resnick DK, Summers JT, Timmons RB, Toton JF, North American Spine S (2009) An evidence-based clinical guideline for the diagnosis and treatment of degenerative lumbar spondylolisthesis. Spine J 9:609–614. https://doi.org/10.1016/j.spinee.2009.03.016
Watters WC 3rd, McGirt MJ (2009) An evidence-based review of the literature on the consequences of conservative versus aggressive discectomy for the treatment of primary disc herniation with radiculopathy. Spine J 9:240–257. https://doi.org/10.1016/j.spinee.2008.08.005
Weber H (1983) Lumbar disc herniation. A controlled, prospective study with ten years of observation. Spine (Phila Pa 1976) 8:131–140
Weinstein JN, Lurie JD, Tosteson TD, Tosteson AN, Blood EA, Abdu WA, Herkowitz H, Hilibrand A, Albert T, Fischgrund J (2008) Surgical versus nonoperative treatment for lumbar disc herniation: four-year results for the spine patient outcomes research trial (SPORT). Spine (Phila Pa 1976) 33:2789–2800. https://doi.org/10.1097/BRS.0b013e31818ed8f4
Wera GD, Dean CL, Ahn UM, Marcus RE, Cassinelli EH, Bohlman HH, Ahn NU (2008) Reherniation and failure after lumbar discectomy: a comparison of fragment excision alone versus subtotal discectomy. J Spinal Disord Tech 21:316–319. https://doi.org/10.1097/BSD.0b013e31813e0314
Wilke HJ, Ressel L, Heuer F, Graf N, Rath S (2013) Can prevention of a reherniation be investigated? Establishment of a herniation model and experiments with an anular closure device. Spine (Phila Pa 1976) 38:E587–E593. https://doi.org/10.1097/BRS.0b013e31828ca4bc
Yoo MW, Hyun SJ, Kim KJ, Jahng TA, Kim HJ (2014) Does obesity make an influence on surgical outcomes following lumbar microdiscectomy? Korean J Spine 11:68–73. https://doi.org/10.14245/kjs.2014.11.2.68
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
This study was approved by the local EC (Ethikkommission Nordwest- und Zentralschweiz, EKNZ, Nr. 2012-036). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was supported by the Intrinsic Therapeutics, Inc., Woburn MA, USA. None of the authors received financial compensation for the work related to the study. The coauthor G.J. Bouma has received research grants from the Intrinsic Therapeutics. All other authors declared that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Spine degenerative
Rights and permissions
About this article
Cite this article
Kienzler, J.C., Fandino, J., Van de Kelft, E. et al. Risk factors for early reherniation after lumbar discectomy with or without annular closure: results of a multicenter randomized controlled study. Acta Neurochir 163, 259–268 (2021). https://doi.org/10.1007/s00701-020-04505-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-020-04505-4