Abstract
Introduction
Spinal bronchogenic cysts are rare findings, with only four cases of lumbar bronchogenic cysts reported in the literature. All of these bronchogenic cysts involved the conus medullaris. We present the first case of a lumbar bronchogenic cyst and arachnoid cyst arising from the cauda equina in a 68-year-old male. Uniquely, this bronchogenic cyst also contained components of an arachnoid cyst.
Methods
Magnetic resonance imaging (MRI) demonstrated a compressive cystic lesion at the level of the L3 vertebra splaying the cauda equina. An L3/L4 laminectomy was performed with marsupialisation of the cyst.
Results
Histological examination revealed pseudostratified ciliated columnar epithelium confirming the diagnosis of a bronchogenic cyst, as well as a pleated fibrovascular tissue lined by sparsely spaced small monomorphic arachnoidal cells, indicating an arachnoid cyst.
Conclusion
We demonstrate that bronchogenic cysts can be successfully treated with marsupialisation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neurenteric cysts, or enterogenous cysts, are epithelial cysts lined by displaced epithelium. They occur most frequently in the pontomedullary region, craniocervical junction and along the spinal neuraxis, where they represent approximately 0.7% to 1.3% of all spinal cord tumours. Bronchogenic cysts are a subtype of neurenteric cyst that are lined by respiratory epithelium [1].
Spinal arachnoid cysts are uncommon protrusions of arachnoid that result in the collection of cerebrospinal fluid (CSF). The precise pathophysiology is not well understood but arachnoid cysts may be classified either as primary or secondary. Primary arachnoid cysts are thought to arise during development where they may gradually expand and compress spinal tissue. Secondary arachnoid cysts are thought to follow a variety of spinal traumas [7].
Similarly, the pathogenesis of bronchogenic cysts is not well understood. However, it is believed that these cysts develop around embryonic day 16 as a part of the spectrum of split cord malformations. Following this embryologic phase, a tube of mesoderm condenses to form the notochord. In normal development the neurenteric canal maintains a transient communication between the endoderm and ectoderm through the notochord. This neurenteric canal closes in the third embryonic week and it is hypothesised that a persistent embryologic communication between the endoderm and ectoderm results in neurenteric fistulas or cysts [5]. Spinal neurenteric cysts are almost pathognomonically associated with vertebral anomalies, spina bifida or diastematomyelia [3].
Case report
A 68-year-old retired plumber presented to the emergency department complaining of bilateral sciatica with a left foot drop. This was on a background of hypertension and gastric reflux. He had a Charcot joint of the right ankle due to a congenital ankle deformity complicated later by septic arthritis, which was managed with internal fixation. Since this event he had been unable to move his right foot.
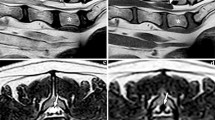
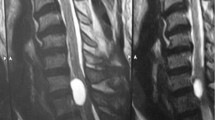
The relevant history was that he reported suffering from stress urinary incontinence for the previous 7 years, but he denied any faecal incontinence or saddle anaesthesia. He reported no previous spinal surgery or trauma. On clinical examination it was noted that the patient mobilised with the use of two walking sticks. His right foot was in an orthosis and was completely covered in bandages, and there was noticeable bilateral pitting oedema of his lower limbs to the level of his knees. Tone was normal, as was power bilaterally at the L2, L3 and S1 myotomes as well as the L4 and L5 myotomes on the right. However, power of the left L4 and L5 myotomes was MRC grade 2/5. Bilateral patellar reflexes and the left ankle reflex were diminished, as was pinprick sensation over the left L5 and S1 dermatomes. An MRI revealed a 5-cm cystic lesion posterior to the L3 and L4 vertebral bodies. The lesion was compressing the cauda equina and splaying the nerve roots to either side of the canal, which was thought to be a compressive arachnoid cyst (Fig. 1).
The patient underwent an L3/L4 laminectomy with marsupialisation of the presumed arachnoid cyst. Macroscopically there was a compressive, tense cystic lesion with cloudy cyst contents that was densely adherent to the spinal nerve roots. Because the cyst was adherent to the spinal nerve roots, the decision was made not to attempt complete resection because of safety concerns. The procedure was completed without any complications. Histology demonstrated pseudostratified ciliated columnar epithelium with an underlying fibrovascular stroma, indicative of a bronchogenic cyst (Figs. 2, 3 and 4). In addition, the pathologist discovered sections demonstrating a pleated fibrovascular tissue lined by sparsely spaced small monomorphic arachnoidal cells with several foci of mineralisation (Fig. 5). This was interpreted as an arachnoid cyst. Immunohistochemistry demonstrated positive CK7 with negative CK20, consistent with respiratory epithelium.
Post-operatively all of the patient’s neurological deficits resolved. The patient recovered well and his 12-month post-operative MRI demonstrated no recurrence of the cyst, and he remained symptom free.
Discussion
Due to the uncommon nature of bronchogenic and arachnoid cysts, they are usually discovered as part of routine investigations for lumbar radiculopathy. MRI is the best investigation for diagnosis as it enables delineation between the cyst and adjacent neural structures. It is important to investigate for other developmental abnormalities given the high association rate of other vertebral anomalies in patients with bronchogenic cysts.
Bronchogenic cysts are slow growing because of the tight junctions between the epithelial cells. Due to their indolent growth it is believed that the most effective treatment is surgical resection. Because arachnoid cysts can be either congenital or secondary due to trauma, it was difficult accurately to determine the aetiology of the arachnoid cyst in this patient. It would seem most likely that the arachnoid cyst developed secondary to the mass effect created by the slowly expanding bronchogenic cyst, as opposed to being a primary congenital lesion with the bronchogenic cyst developing coincidentally at the same location.
There are only four other cases of bronchogenic cysts occurring in the lumbar spine reported in the literature [2, 4, 8, 9]. These cases all occurred at the thoracolumbar junction and involved the conus medullaris. Importantly, all of these cases presented with new-onset neurology such as: continuous therapy resistant leg pain [2], progressive bilateral lower limb weakness with paresthesias [4], bilateral paresthesias alone [8] and unilateral leg weakness with sphincter dysfunction [9]. Three of the cases were treated with sub-total resection [2, 4, 8] and one was totally resected [9]. Given the limited number of cases it is difficult to recommend complete resection over subtotal resection as damage to adjacent neural structures can occur when complete removal of the cyst is attempted.
In the wider literature only 36% of neurenteric cysts are amenable to complete removal; however there are no reported cases of recurrence following gross total resection [6]. For cases that are partially resected the overall recurrence rate reported in the literature is 11.6%. It is unknown whether this is due to variations in technique such as fenestration versus marsupialisation or due to the inherent pathology of neurenteric cysts. Furthermore, due to the rarity of neurenteric cysts it is reasonable to assume that most surgeons would assume they were treating an arachnoid cyst solely and may not be as aggressive in their resection.
We present the first report of a bronchogenic cyst occurring within the cauda equina with what is likely a secondary arachnoid cyst. This case demonstrates that neurenteric cysts should be considered in cases of compressive spinal cysts where patients have presented because of new onset and/or progressive neurology. The presence of worsening neurology should encourage the surgeon to reconsider other differential diagnoses apart from arachnoid cyst as it is uncommon for arachnoid cysts to present with progressive neurology. Given the similarity in imaging characteristics between neurenteric cysts and arachnoid cysts the goal of gross total resection should always be considered if it is felt that this can be safely achieved. However safe dissection of the cyst from the adjacent neurological structures may be unachievable. In these cases, we believe marsupialisation may be a superior technique to fenestration in reducing the rate of recurrence. Accurate diagnosis will not likely occur until histological examination of surgical specimens has occurred.
The patient consented to submission of this case report to the journal.
References
Arnold PM, Neff LL, Anderson KK, Reeves AR, Newell KL (2009) Thoracic myelopathy secondary to intradural extramedullary bronchogenic cyst. J Spinal Cord Med 32:595–597
Baumann CR, Konu D, Glatzel M, Siegel AM (2005) Thoracolumbar intradural extramedullary bronchiogenic cyst. Acta Neurochir 147:317–319 discussion 319
Cai C, Shen C, Yang W, Zhang Q, Hu X (2008) Intraspinal neurenteric cysts in children. Can J Neurol Sci 35:609–615
Chongyi S, Meng Y, Dejun Y, Yingjie L, Qingpeng L (2008) Lumbar intradural extramedullary bronchiogenic cyst. Eur Surg Res 40:26–28
Garg N, Sampath S, Yasha TC, Chandramouli BA, Devi BI, Kovoor JM (2008) Is total excision of spinal neurenteric cysts possible? Br J Neurosurg 22:241–251
Paleologos TS, Thom M, Thomas DG (2000) Spinal neurenteric cysts without associated malformations. Are they the same as those presenting in spinal dysraphism? Br J Neurosurg 14:185–194
Ryan Dahlgren EB, Alexander Vaccaro (2007) Pathophysiology, diagnosis and treatment of spinal meningoceles and arachnoid cysts, in Department of Orthopaedic Surgery Faculty Papers: Jefferson digital commons, Vol Paper 4, pp 2–7
Yilmaz C, Gulsen S, Sonmez E, Ozger O, Unlukaplan M, Caner H (2009) Intramedullary bronchogenic cyst of the conus medullaris. J Neurosurg Spine 11:477–479
Zou MX, Hu JR, Kang YJ, Li J, Lv GH (2015) She XL: bronchogenic cyst of the conus medullaris with spinal cord tethering: a case report and review of the literature. Int J Clin Exp Pathol 8:3937–3942
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Candy, N., Young, A., Devadass, A. et al. Dual lumbar bronchogenic and arachnoid cyst presenting with sciatica and left foot drop. Acta Neurochir 159, 2029–2032 (2017). https://doi.org/10.1007/s00701-017-3284-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-017-3284-z