Abstract
Deep brain stimulation (DBS) in the thalamic ventral intermediate (Vim) or the subthalamic nucleus (STN) reportedly improves medication-refractory Parkinson’s disease (PD) tremor. However, little is known about the potential synergic effects of combined Vim and STN DBS. We describe a 79-year-old man with medication-refractory tremor-dominant PD. Bilateral Vim DBS electrode implantation produced insufficient improvement. Therefore, the patient underwent additional unilateral left-sided STN DBS. Whereas Vim or STN stimulation alone led to partial improvement, persisting tremor resolution occurred after simultaneous stimulation. The combination of both targets may have a synergic effect and is an alternative option in suitable cases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Deep brain stimulation (DBS) is an efficient therapeutic option for Parkinson’s disease (PD) tremor refractory to medical treatment. Both the thalamic ventral intermediate (Vim) and the subthalamic nucleus (STN) are reliable targets for alleviation of parkinsonian tremor [10, 14]. However, Vim DBS may be associated with loss of efficacy over time [3, 6, 10]. Moreover, poor outcomes were correlated with lead mislocation [1, 5, 8] and reoperation may be indicated. Little is known about the optimal target or the efficiency of dual stimulation. We report the case of a PD patient with a persistent disabling tremor after Vim DBS and discuss the possible mechanisms underlying the observed synergic effect of combined thalamic and subthalamic stimulation.
Case report
A 79-year-old right-handed man with an 11-years history of tremor-dominant PD received bilateral Vim electrode (model 3387; Medtronic, Minneapolis, MN, USA) implantation for DBS 2 years before presenting to the authors’ institution. Postoperatively, he exhibited a persisting right-sided tremor, which worsened with time. Stimulation with higher amplitudes led to intractable side effects, including dysarthria and gait disturbance. Clinical examination showed bradykinesia, rigidity, cognitive impairment and a significant bilateral resting tremor, partially improved on both sides under bilateral Vim stimulation, although still disabling on the right side. His scores according to the Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) were: part I (non-motor experiences of daily living), 1/42; difficulties in activities of daily living by modified MDS-UPDRS part II, 21/42; part III (motor examination), 45/142 (20 points for tremor); part IV (motor complications), 0.
Essential Tremor Rating Assessment Scale (TETRAS) tremor score (part 1) was 35/72 for tremor and 36/64 for functional score (part 2).
External postoperative 1.5-T magnetic resonance imaging (MRI) revealed suboptimal implantation of the left-sided lead (Fig. 1) and calculation of the coordinates of the stimulating electrodes related to the mid-commissural point (MCP) demonstrated medial and posterior displacement compared with conventional atlas-based coordinates [3, 5, 6, 9].
Owing to the insufficient stimulation effect and because the residual symptoms increasingly interfered with his daily life and did not respond well to medication, the patient underwent additional unilateral DBS.
Because of the partial effect of stimulation with the previously implanted left Vim electrode and the potential for bleeding if removed, we left the implanted electrode in place. Moreover, due to possible gliosis around this electrode, which could prevent an optimal DBS effect through a newly implanted lead in its vicinity, and the presence of slight bradykinesia and rigidity, a subsequent implantation in the left STN was preferred.
On the day of surgery, contrast-enhanced computed tomography (CT; Somatom Definition Edge; Siemens Healthcare, Forchheim, Germany) was performed under stereotactic conditions (field of view, 290 mm; slice increment, 0.7 mm; section thickness, 1 mm; matrix size, 512 × 512; no gap and no gantry tilt) with the patient’s head fitted with a Leksell stereotactic frame (model G; Elekta Instruments, Stockholm, Sweden). Images were fused to the MRI with stereotactic planning of the STN using iPLAN stereotaxy software (Brainlab, Feldkirchen, Germany) through a new trajectory. Furthermore, we verified the exact superposition of the previously implanted electrodes on CT and MRI during the image co-registration process. Two microelectrode trajectories (central, 2 mm medial) were chosen for recording (D.ZAP array insertion electrodes; FHC, Bowdoin, ME, USA) followed by clinical testing by STN stimulation with or without simultaneous Vim stimulation.
Both electrophysiological trajectories showed features of STN firing patterns. The medial trajectory showed a good stimulation effect and a complete disappearance of tremor with simultaneous Vim stimulation. No stimulation-induced side effects were observed. Finally, the definitive quadripolar DBS electrode (model 3389; Medtronic, Minneapolis, MN, USA) was implanted under C-arm fluoroscopy.
Direct postoperative stereotactic CT was performed and co-registered with the preoperative imaging to confirm lead locations. In addition to the initially implanted infraclavicular right-sided single-channel device (Activa SC; Medtronic, Minneapolis, MN, USA), a dual-channel primary cell neurostimulator (Activa PC; Medtronic, Minneapolis, MN, USA) was placed in the left infraclavicular region and connected to both the Vim and STN lead under general anaesthesia.
Monopolar stimulation was performed on the two ventral contacts of the left Vim lead (C0, C1; amplitude, 2.6 V; pulse width, 90 μs; frequency, 200 Hz), while bipolar stimulation was applied on the two middle contacts (C1, C2; amplitude, 1.6 V; pulse width, 60 μs; frequency, 200 Hz) of the left STN electrode.
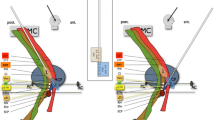
Position calculation of the stimulating electrodes in the anterior commissure–posterior commissure (AC–PC) reference standard, as well as volume estimation of tissue activated (VTA) were performed with iPLAN stereotaxy and Optivise software (Medtronic, Minneapolis, MN, USA) (Fig. 2). AC–PC coordinates of the two stimulating contacts of the previously implanted left Vim lead were (mm): C0: anteroposterior (AP) = −6.35, lateral (LAT) = 12.72, vertical (VERT) = −0.63; C1: AP = −4.67, LAT = 13.74, VERT = 2.05, and for the newly implanted STN lead (mm): C1: AP = −2.36, LAT = 13.95, VERT = −3.74; C2: AP = −1.47, LAT = 14.76, VERT = −2.39 related to the MCP, respectively.
Fused preimplanted preoperative coronal T2-weighted MRI with postoperative stereotactic CT showing the left-sided location of: a contacts (red) and simulation of VTA (grey) of the initially implanted Vim electrode and b the newly inserted lead in the STN. c Concomitant localisation, contacts and simulation of VTA of both the Vim and STN electrodes. The thalamus is shown in magenta, the Vim in blue and the anterior commissure–posterior commissure line in yellow. Lead 1 (not shown) represents the previously implanted right-sided Vim electrode, lead 2 the initially inserted Vim electrode and lead 3 the secondary implanted electrode in the STN (both electrodes are shown in red). Figures were created using Optivise computer software (Medtronic, Minneapolis, MN, USA). VTA volume of tissue activated, Vim thalamic ventral intermediate nucleus, STN subthalamic nucleus
Whereas the patient was assessed with and without Vim stimulation or levodopa before surgery, he was assessed with combined Vim and STM stimulation on usual 400 mg daily levodopa medication postoperatively. The severity of final preoperative and early postoperative right-sided tremor is shown in Table 1. After 2 months of bilateral Vim stimulation, scores for MDS-UPDRS parts I-IV were: 2/42, 22/42, 31/142 (11 points for tremor), 0, respectively. The corresponding parameters of stimulation were: Vim1 C+, 0-; 1.5 V, 60 μs, 120 Hz; Vim2 C+, 1-; 3.5 V, 90 μs, 120 Hz (right) and Vim1 C+; 3.5 V, 90 μs, 120 Hz; Vim2 C+, 0-; 1.5 V, 60 μs, 120 Hz (left). The TETRAS tremor scale was 15/72 for tremor and 21/64 for functional score. Off stimulation induced unbearable generalised tremor (rebound effect) with 5 cm amplitude tremor of all four extremities and chin. After 3 months of bilateral Vim and left STN DBS, scores for MDS-UPDRS scale were: part I, 2/42; part II, 17/42; part III, 28/142 (8 points for tremor); and part IV, 0. TETRAS score was 9/72 for tremor and 15/64 for function. The parameters of stimulation were: Vim1 C+, 0-; 1.4 V, 60 μs, 120 Hz; Vim2 C+, 1-; 3.1 V, 90 μs, 120 Hz (right) and Vim C+, 9-, 8-; 2.6 V, 90 μs, 200 Hz; STN C+, 1-, 2-; 1.6 V, 60 μs, 200 Hz (left). Off stimulation was not tolerated because of a rebound effect with more than 5 cm generalised tremor. Isolated DBS of the left-sided STN of different and varying stimulation parameters not only led to an intractable rebound effect but was also unable to sufficiently suppress the symptoms and was therefore rapidly discontinued.
The therapeutic benefits remained unchanged after surgery at 48 months’ follow-up
Discussion
Vim, STN and globus pallidus internus (GPi) are reportedly effective DBS targets for parkinsonian tremor control [6, 10, 14, 17, 18]. Few studies have directly compared the efficacies of DBS of the Vim, STN and GPi. However, previous data suggest that STN stimulation should be preferred over Vim or GPi DBS [6, 10, 14, 18].
Although single-electrode implantation usually provides satisfactory results, patients may sometimes require additional surgery. Apart from hardware-related complications, DBS failure could result from initial microlesional effects preventing reliable intraoperative clinical testing [8], lead misplacements [1, 5, 8], or efficacy loss through habituation or underlying disease progression [3, 6, 10]. In our patient, although a partial effect of Vim DBS persisted, the left Vim electrode had been suboptimally placed resulting in an incomplete Vim stimulation effect, and reimplantation appeared to lead to further improvement of clinical outcome.
Whether electrode replacement in the previous target or switching to an alternative target is more beneficial is unclear [1, 3, 5]. Beneficial results of STN [1] and of Vim reimplantation were reported in seven patients with PD and essential tremor (ET), respectively [5], whereas tremor improvement with caudal zona incerta (cZI) DBS in five ET patients initially implanted in the Vim was documented [3]. Fraix and co-workers [6] described 15 PD patients with previous Vim stimulation whose motor state worsened; in these patients, single STN DBS lastingly reduced tremor, especially in those with levodopa-induced motor complications or tremor recurrence. Both isolated resting tremor in PD and, even more likely, mixed components such as intention and postural tremor in ET [5], or all of these tremor types occurring in cases of Holmes tremor [20], can involve different nuclei and pathways and may require combined Vim and STN stimulation for sufficient symptom control.
Therefore, after worsening of bradykinesia and rigidity in our patient following the first operation, we proposed to target the STN.
Potential additive effects of multitarget DBS electrodes have already been suggested [12–16, 18–22]. Previous successfully combined PD targets include GPi and STN [15, 18, 22], pendunculopontine nucleus (PPN) [22] or intra-laminar complex thalamic nuclei comprising the centromedian and parafascicular nucleus (CM/Pf) [16, 22], as well as STN with CM/Pf [17, 22], PPN [11, 19, 21, 22] and cZI [13] or PPN in combination with the cZI [12, 13]. In the present case, although ultimately the exact complementary effect of dual stimulation is not completely clear, a synergic effect obtained through simultaneous Vim and STN stimulation would be in line with these previous observations. However, as the Vim electrode was recognised to be suboptimally located in the target, it is unlikely that the VTA reached the optimal Vim target via the thalamic electrode.
The vicinity of the two leads, the position of the stimulating contacts slightly inferior to the thalamus and in the superior aspect of the STN, as well as the supposition that the VTAs overlapped in the cZI, raise questions about the stimulation of this area as the underlying mechanism for successful tremor control when both electrodes are activated. The cZI was reported to be an effective target for parkinsonian tremor [2, 3]. Furthermore, stimulation of the subthalamic area involving the cZI might be more effective than Vim stimulation in patients with severe intentional tremor or ET [4, 5]. Effective active cathode locations reported include (mm): 12.6 ± 1.4 LAT, 7.0 ± 1.2 AP, 2.0 ± 1.8 VERT [2] and 12.7 ± 1.4 LAT, 7.0 ± 1.6 AP, 1.5 ± 2.0 VERT in relation to the MCP [9]. In the present case, the location of overlapping VTA between Vim and STN stimulation is in accordance with these previously reported coordinates (Fig. 1) and supports the hypothesis of an additional benefit of dual stimulation through cZI stimulation. As suggested by both anatomical investigations [7] and diffusion tensor imaging studies [4], the dentato-rubro-thalamic tract seems to be most closely associated with DBS-induced improvement of tremor.
Conclusions
Combined Vim and STN DBS showed a synergic effect in the treatment of parkinsonian tremor. Although an additive effect of the stimulation of two different targets might be the mechanism underlying tremor suppression, we postulate that the combined stimulation of the posterior subthalamic area (PSA) including the cZI affected by both electrodes, could better explain this phenomenon. Inclusion of the cZI is expected based on the location of the stimulating contacts as well as their respective VTA.
References
Anheim M, Batir A, Fraix V, Silem M, Chabardès S, Seigneuret E, Krack P, Benabid AL, Pollak P (2008) Improvement in Parkinson disease by subthalamic nucleus stimulation based on electrode placement: effects of reimplantation. Arch Neurol 65:612–616
Blomstedt P, Fytagoridis A, Åström M, Linder J, Forsgren L, Hariz MI (2012) Unilateral caudal zona incerta deep brain stimulation for Parkinsonian tremor. Parkinsonism Relat Disord 18:1062–1066
Blomstedt P, Lindvall P, Linder J, Olivecrona M, Forsgren L, Hariz MI (2012) Reoperation after failed deep brain stimulation for essential tremor. World Neurosurg 78:7554.e1–7554.e5
Coenen VA, Allert N, Paus S, Kronenbürger M, Urbach H, Mädler B (2014) Modulation of the cerebello-thalamo-cortical network in thalamic deep brain stimulation for tremor: a diffusion tensor imaging study. Neurosurgery 75:657–670
Ellis TM, Foote KD, Fernandez HH, Sudhyadhom A, Rodriguez RL, Zeilman P, Jacobson CE 4th, Okun MS (2008) Reoperation for suboptimal outcomes after deep brain stimulation surgery. Neurosurgery 63:754–761
Fraix V, Pollak P, Moro E, Chabardes S, Xie J, Ardouin C, Benabid AL (2005) Subthalamic nucleus stimulation in tremor dominant parkinsonian patients with previous thalamic surgery. J Neurol Neurosurg Psychiatry 76:246–248
Gallay MN, Jeanmonod D, Liu J, Morel A (2008) Human pallidothalamic and cerebellothalamic tracts: anatomical basis for functional stereotactic neurosurgery. Brain Struct Funct 212:443–463
Granziera C, Pollo C, Russmann H, Staedler C, Ghika J, Villemure JG, Burkhard PR, Vingerhoets FJ (2008) Sub-acute delayed failure of subthalamic DBS in Parkinson’s disease: the role of micro-lesion effect. Parkinsonism Relat Disord 14:109–113
Hamel W, Herzog J, Kopper F, Pinsker M, Weinert D, Müller D, Krack P, Deuschl G, Mehdorn HM (2007) Deep brain stimulation in the subthalamic area is more effective than nucleus ventralis intermedius stimulation for bilateral intention tremor. Acta Neurochir (Wien) 149:749–758
Hariz MI, Krack P, Alesch F, Augustinsson LE, Bosch A, Ekberg R, Johansson F, Johnels B, Meyerson BA, N’Guyen JP, Pinter M, Pollak P, von Raison F, Rehncrona S, Speelman JD, Sydow O, Benabid AL (2008) Multicentre European study of thalamic stimulation for parkinsonian tremor: a 6 years follow-up. J Neurol Neurosurg Psychiatry 79:694–699
Khan S, Gill SS, Mooney L, White P, Whone A, Brooks DJ, Pavese N (2012) Combined pedunculopontine-subthalamic stimulation in Parkinson disease. Neurology 78:1090–1095
Khan S, Javed S, Mooney L, White P, Plaha P, Whone A, Gill SS (2012) Clinical outcomes from bilateral versus unilateral stimulation of the pedunculopontine nucleus with and without concomitant caudal zona incerta region stimulation in Parkinson’s disease. Br J Neurosurg 26:722–725
Khan S, Mooney L, Plaha P, Javed S, White P, Whone AL, Gill SS (2011) Outcomes from stimulation of the caudal zona incerta and pedunculopontine nucleus in patients with Parkinson’s disease. Br J Neurosurg 25:273–280
Kim HJ, Jeon BS, Paek SH, Lee JY, Kim HJ, Kim CK, Kim DG (2010) Bilateral subthalamic deep brain stimulation in Parkinson disease patients with severe tremor. Neurosurgery 67:626–632
Mazzone P, Brown P, Dilazzaro V, Stanzione P, Oliviero A, Peppe A, Santilli V, Insola A, Altibrandi M (2005) Bilateral implantation in globus pallidus internus and in subthalamic nucleus in Parkinson’s disease. Neuromodulation 8:1–6
Mazzone P, Stocchi F, Galati S, Insola A, Altibrandi MG, Modugno N, Tropepi D, Brusa L, Stefani A (2006) Bilateral implantation of centromedian-parafascicularis complex and GPi: a new combination of unconventional targets for deep brain stimulation in severe Parkinson disease. Neuromodulation 9:221–228
Peppe A, Gasbarra A, Stefani A, Chiavalon C, Pierantozzi M, Fermi E, Stanzione P, Caltagirone C, Mazzone P (2008) Deep brain stimulation of CM/PF of thalamus could be the new elective target for tremor in advanced Parkinson’s disease? Parkinsonism Relat Disord 14:501–504
Peppe A, Pierantozzi M, Bassi A, Altibrandi MG, Brusa L, Stefani A, Stanzione P, Mazzone P (2004) Stimulation of the subthalamic nucleus compared with the globus pallidus internus in patients with Parkinson disease. J Neurosurg 101:195–200
Peppe A, Pierantozzi M, Chiavalon C, Marchetti F, Caltagirone C, Musicco M, Stanzione P, Stefani A (2010) Deep brain stimulation of the pedunculopontine tegmentum and subthalamic nucleus: effects on gait in Parkinson’s disease. Gait Posture 32:512–518
Romanelli P, Brontë-Stewart H, Courtney T, Heit G (2003) Possible necessity for deep brain stimulation of both the ventralis intermedius and subthalamic nuclei to resolve Holmes tremor. Case report. J Neurosurg 99:566–571
Stefani A, Lozano AM, Peppe A, Stanzione P, Galati S, Tropepi D, Pierantozzi M, Brusa L, Scarnati E, Mazzone P (2007) Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson’s disease. Brain 130:1596–1607
Stefani A, Peppe A, Pierantozzi M, Galati S, Moschella V, Stanzione P, Mazzone P (2009) Multi-target strategy for parkinsonian patients: the role of deep brain stimulation in the centromedian-parafascicularis complex. Brain Res Bull 78:113–118
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Informed consent
The patient has consented to submission of this case report to the journal.
Additional information
Comments
The authors present the case of a patient with tremor-dominant Parkinson’s disease (PD) who did not improve sufficiently from bilateral DBS targeted to the Vim subnucleus of the thalamus. MR revealed a possibly misplaced left lead. In consideration of the presence of a partial treatment effect a decision was taken not to relocate this lead but instead supplement it with another electrode lead in the STN. Improvement of stimulation equipment has made it possible to retain a lead with partial effect and supplement it with another lead without having to implant yet another pulse generator. Follow-up examinations demonstrated a synergistic effect of these two leads.
This presentation strengthens the argument to retain a partially effective electrode and supplement it with another lead in some other possibly effective target, rather than relocating a single misplaced lead. Furthermore, this article adds to the body of evidence concerning simultaneous stimulation in several target areas, a strategy that subsequent research efforts in the future may prove to be of value, not only as a salvage effort for insufficient initial results but also in select cases as a first-line treatment possibility.
Goran Lind
Stockholm, Sweden
Rights and permissions
About this article
Cite this article
Oertel, M.F., Schüpbach, W.M.M., Ghika, JA. et al. Combined thalamic and subthalamic deep brain stimulation for tremor-dominant Parkinson’s disease. Acta Neurochir 159, 265–269 (2017). https://doi.org/10.1007/s00701-016-3044-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-016-3044-5