Abstract
Background
It remains unclear how intracranial pressure (ICP) measures are associated with brain biopsies and radiological markers. Here, we aim to investigate associations between ICP and radiological findings, brain biopsies, and shunt surgery outcome in patients with suspected idiopathic normal pressure hydrocephalus (iNPH).
Method
In this study, we retrospectively analyzed data from 73 patients admitted with suspected iNPH to Kuopio University Hospital. Of these patients, 71% underwent shunt surgery. The NPH registry included data on clinical and radiological examinations, 24-h intraventricular pressure monitoring, and frontal cortical biopsy.
Results
The mean ICP and mean ICP pulse wave amplitude were not associated with the shunt response. Aggregations of Alzheimer’s disease (AD)-related proteins (amyloid-β, hyperphosphorylated tau) in frontal cortical biopsies were associated with a poor shunt response (P = 0.014). High mean ICP was associated with Evans’ index (EI; P = 0.025), disproportional sylvian and suprasylvian subarachnoid spaces (P = 0.014), and focally dilated sulci (P = 0.047). Interestingly, a high pulse wave amplitude was associated with AD-related biopsy findings (P = 0.032), but the mean ICP was not associated with the brain biopsy. The ICP was not associated with medial temporal lobe atrophy, temporal horn widths, or white matter changes. ICP B waves were associated with less atrophy of the medial temporal lobe (P = 0.018) and more severe disproportionality between the sylvian and suprasylvian subarachnoid spaces (P = 0.001).
Conclusions
The EI and disproportional sylvian and suprasylvian subarachnoid spaces were associated with mean ICP. Disproportionality was also associated with ICP B waves. These associations, although rather weak, with elevated ICP in 24-h measurements, support their value in iNPH diagnostics and suggest that these radiological markers are potentially related to the pathogenesis of iNPH. Interestingly, our results suggested that elevated pulse wave amplitude might be associated with brain amyloid accumulation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The formation and resorption of cerebrospinal fluid (CSF) regulates intracranial pressure (ICP) [3]. Normal pressure hydrocephalus (NPH) manifests in a triad of symptoms, including impaired gait, declined cognition, and urinary incontinence. Cognitive impairment is the least likely to be restored, particularly in prolonged cases [22]; moreover, cognition may deteriorate from various causes, including comorbid Alzheimer’s disease (AD) [19].
In idiopathic NPH (iNPH), there is no clear underlying cause, such as subarachnoid (SA) hemorrhage, head injury, or intracranial surgery [32]. The specific pathophysiology is unknown, but CSF circulation is disturbed, which leads to occasional increases in ICP. The diagnosis is based on clinical examinations and brain imaging with computed tomography (CT) or magnetic resonance imaging (MRI). Additional diagnostic tests for iNPH include a high-volume lumbar puncture, extended lumbar drainage, infusion test, cisternography, and ICP monitoring [29].
Measuring ICP in an invasive manner for at least 24 h provides information about daily pressure fluctuations. Although the monitoring is demanding for the hospital staff and the patient, this test gives the most accurate information about daily mean pressure and pulse pressure values [3, 9]. A simultaneous brain biopsy may assist in the differential diagnosis and prognostic evaluation of patients with suspected iNPH [13, 25, 26]. ICP A waves, i.e., plateau waves, and B waves, i.e., rhythmic oscillations, were first described by Lundberg in cases with intracranial lesions and intracranial hypertension of other origin [27]. Similar waves can also be seen in NPH [36].
Patients with intermittently elevated ICP, elevated pulse pressure and enlarged cerebral ventricles have shown the best response to shunt treatments [4, 7, 12]. In one study, shunt response was predicted by a volumetric disproportionality between the ventricles and cortical sulci [39]. However, our previous study showed that a disproportionality between sylvian and suprasylvian SA spaces was not useful in predicting the shunt response [21]. Nevertheless, other previous studies found that spatial disproportionalities could provide differentiation between iNPH and AD [14, 15, 17, 28]. Periventricular and deep white matter changes are also common features of iNPH [23], but they have no prognostic value in predicting the shunt response [30, 38]. However, a relationship between ICP measurements and radiological indicators, such as obliterated cortical sulci, have not been well established in iNPH.
This retrospective study aimed to investigate associations between ICP and radiological findings, brain biopsies, and shunt responses in 73 patients with suspected iNPH, based on data retrieved from the Kuopio NPH registry.
Methods and materials
Study population
Over 700 patients with suspected NPH are currently registered in the Kuopio University Hospital (KUH) NPH registry (www.uef.fi/nph), which started in 1991. All primary clinical examinations were performed by a neurologist. All registered patients had one to three symptoms potentially related to NPH (impaired cognition, gait disorder, and/or urinary incontinence) and finding of enlarged brain ventricles (Evans’ index >0.3) on a CT and/or MRI of the brain. All patients were referred for further neurosurgical examinations. The registry included extensive patient information at baseline and follow-up, including ICP measurements, brain biopsy findings, medications, causes of death, and radiological findings.
This retrospective KUH NPH registry study was approved by the KUH Research Ethics Committee, The Finnish National Supervisory Authority for Welfare and Health, and the Finnish Ministry of Social Affairs and Health, and informed consents were obtained. The study was conducted in accordance with the Declaration of Helsinki.
We identified 75 consecutive patients who were registered in the KUH NPH registry with suspected NPH between 2008 and 2010. All patients underwent clinical evaluations, brain CT or MR imaging, and 24-h ICP monitoring. Two patients with known etiologies (secondary NPH) were excluded from the analyses. Seventy-three patients with suspected iNPH and available ICP monitoring data (Fig. 1) were included in this study. In addition, CT scans were available for 23 (32%) patients, MRIs for 19 (26%) patients, and both CT and MRI scans for 31 (42%) patients.
Brain biopsies and 24-h ICP measurements
Under local anesthesia and sedation, all patients received a right frontal 12-mm burr hole, approximately 3 cm from the midline and close to the coronal suture of the skull. Biopsy forceps were inserted through the hole to obtain one to three cylindrical cortical brain biopsies of 2 to 5 mm in diameter and 3 to 7 mm in length. Next, a catheter was passed through the hole into the right lateral ventricle for ICP monitoring. ICP was measured with an arterial blood pressure measurement system, implemented with in-house registration and analysis software. Patients were in a horizontal position during the entire measurement. The mean ICP and mean pulse wave amplitude were measured for each patient with a reference point on the level of the forehead. Continuous waveforms were digitized at a 1-kHz sampling frequency and analyzed with in-house software. Cardiac beat-induced pressure waves were averaged over 6-s time intervals, and we computed the mean pulse wave amplitude and mean ICP wave amplitude for each 6-s interval. These mean values were used to calculate mean pressure values over a 24-h period [6]. Presence of A waves [27] (yes or no) and frequency of B waves [36] (none, <10, 10–20, or >30%) were evaluated visually. No Lundberg A waves were detected in this study population.
Paraffin-embedded biopsy samples were sectioned (7 μm) and stained with hematoxylin-eosin. Sections were immunostained with monoclonal antibodies directed at amyloid beta (Aβ) (6F/3D, M0872; Dako; dilution 1:100; pre-treatment, 80% formic acid for 1 h) and hyperphosphorylated tau (HPτ) (AT8, 3Br-3; Innogenetics; dilution 1:30), as described previously [24]. In histological evaluations, a neuropathologist graded all samples in terms of the presence or absence of immunoreactivity to Aβ and HPτ.
The indications for a shunt were: (1) a baseline ICP that remained continuously above 10 mmHg; if the baseline ICP was below 10 mmHg the indications were: (2) multiple B waves that comprised >30% of the pressure waves in 24-h ICP monitoring or (3) the presence of any A waves [19, 34].
Clinical and radiological evaluations
The response to a shunt (improvement) or no response (no change or deterioration) was determined by evaluating the patient’s gait, memory, and urinary continence at the outpatient clinic and 2 to 3 months after the shunt operation. Other clinical data were obtained from patient records. After that, patients were then followed up by the local neurologist, geriatrician, or general practitioner. Final clinical diagnoses were determined according to all available medical records until death or until the end of 2014 during a median follow-up time of 5.3 years, as described previously [20].
For the present study, CT and MRI images acquired prior to possible shunt surgery were evaluated retrospectively, with a structured form, by a neuroradiologist. CT and MRI scans were primarily performed prior to ICP measurements (median 3.7 months, IQR: −0.03 to 6.2 months). All radiological assessments were evaluated with a Sectra-PACS workstation (IDS7, version 15.1.20.2, Sectra AB, Linköping, Sweden).
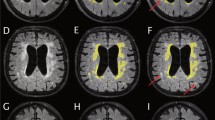
For this study, the following NPH and AD-related radiological markers were visually evaluated: SA spaces in the superior convexity and medial regions (narrowed or normal/mildly enlarged); disproportionality between sylvian and suprasylvian SA spaces (none, mild, or severe; Fig. 2); sylvian fissures (normal, mildly enlarged, enlarged, or severely enlarged); focally dilated sulci (no or yes); basal cisterns (normal, mildly enlarged, or enlarged/severely enlarged); and periventricular and deep white matter changes (Fazekas scale [11]). The medial temporal lobe atrophy was assessed visually on coronal T1-weighted MRI scans with Scheltens’ scale (n = 51) [35]. Evans’ index (EI) was defined as the ratio of the maximal width of the frontal horns of the lateral ventricles to the maximal inner diameter of the skull [10, 32, 37]. The widths of the left and right temporal horns of the lateral ventricles were measured on the axial plane.
a–c Ventricles and sulci of a non-NPH-patient primarily suspected with NPH. d–f Mild disproportionality between sylvian and suprasylvian subarachnoid spaces in an idiopathic normal pressure hydrocephalus patient. g–i Strong disproportionality between sylvian and suprasylvian subarachnoid spaces in an idiopathic normal pressure hydrocephalus patient
Statistical analyses
Values for continuous variables are expressed as the mean ± SD. Categories of radiological markers with fewer than three subjects were combined for statistical analyses. Pearson’s correlation coefficients are reported. Differences between groups were analyzed with an ANOVA or t-test for continuous variables and with the Fisher exact test for categorical variables. Forward and backward stepwise linear regression modeling was used to find the best model for predicting the mean ICP by including only the variables (excluding shunt status) that were significantly associated with the mean ICP in univariate analyses. P-values <0.05 were considered significant. All statistical analyses were performed with IBM SPSS Statistics 23.0 software.
Results
The patients were divided into three groups: shunt responsive, no shunt response, and no shunt. The baseline characteristics of patients are shown in Table 1. The patients with no shunt response were older than shunt-responsive ones (P = 0.049). BMI was significantly higher in shunt-responsive than in non-shunted patients (P = 0.033). There was no difference in gender among the three study groups. Final clinical diagnoses determined during the follow-up (median 5.3 years) are presented in Table 2.
The mean ICP was not correlated with the mean ICP pulse wave amplitude. Mean ICP, but not mean pulse wave amplitude, was higher if there were more B waves (P < 0.001). The mean ICP was higher in the shunt-responsive group than in the no shunt group (4.0 ± 1.8 vs. 2.1 ± 1.4 mmHg, P < 0.001) (Fig. 3a), but there was no significant difference in mean ICP between the shunt-responsive and non-responsive groups. These results were expected, because ICP was used as a criterion for the shunt operation. The mean ICP pulse wave amplitude was similar among the three study groups. As expected, shunt-responsive patients had more ICP B waves (P < 0.001) than non-shunted, because these were used as shunting criteria. Gait impairment was the main symptom in the shunt-responsive group more frequently than in the no shunt group (70% vs. 29%, P = 0.005; Table 1). There was no difference in other symptoms (gait/ cognition/ incontinence) or symptom duration among the three groups. An AD-related brain biopsy finding (Aβ+HPτ+) was more common in the no shunt response group than in the shunt-responsive group (56% vs. 12%, P = 0.014). The frequency of narrowed superior medial SA spaces in the shunt-responsive group was significantly higher than in the no shunt group (93% vs. 57%, P = 0.001), and the frequency tended to be higher compared to the no shunt response group (93% vs. 67%, P = 0.057). In addition, SA spaces in the superior convexity were more often narrowed in the shunt-responsive group than in the no shunt group (67% vs. 24%, P = 0.001). Disproportionality between the sylvian and suprasylvian SA spaces was more frequently severe in the shunt-responsive group than in the no shunt group (67% vs. 10%, P < 0.001) and the no shunt response group (67% vs. 33%, P = 0.043). Focally dilated sulci were reported more frequently in the shunt-responsive group than in the no shunt group (49% vs. 14%, P = 0.012). There were more periventricular white matter changes in the shunt-responsive than in the no shunt group (P = 0.035) and more deep white matter changes in the shunt-responsive than non-responsive group (P = 0.012). Medial temporal lobe atrophy was more progressed in the no shunt group than in the shunt-responsive group (P = 0.021).
A high mean ICP was associated with increased disproportionality (P = 0.014; Fig. 3b) and the presence of focally dilated sulci (P = 0.047; Fig. 3c). Also, a high ICP tended to be associated with more frequent narrowing of the SA spaces in the superior convexity (P = 0.064) and more frequent enlargement of the basal cisterns (P = 0.061). A high EI was correlated with a high mean ICP (r = 0.26, P = 0.025; Fig. 4a). This association was strongest in the shunt-responsive group (r = 0.36, P = 0.017). Patients with a high mean ICP also had a high BMI (r = 0.24, P = 0.042; Fig. 4b). Positive Aβ findings in cortical brain biopsies were associated with a high mean ICP pulse wave amplitude (P = 0.032; Fig. 5). Other variables (Table 1) were not associated with either the mean ICP or the pulse wave amplitude.
In a stepwise linear regression, only disproportionality (P = 0.005) and EI (P = 0.013) were useful in predicting the mean ICP; they explained 16% of the variation in the mean ICP. Each increase to a higher level of disproportionality was associated with a 0.75 mmHg (CI 95%: 0.23–1.26 mmHg) elevation in mean ICP. Similarly, a 0.1 increase in EI was associated with a 1.18 mmHg (CI 95%: 0.26–2.09 mmHg) elevation in mean ICP.
Significant associations with B waves are shown in Table 3. Higher frequency of B waves (P = 0.022) was associated with gait impairment as a main symptom. More frequent B waves (P = 0.010) were associated with less atrophy of the medial temporal lobe. Of radiological markers only narrowed superior medial and convexity SA spaces and more severe disproportionality between the sylvian and suprasylvian SA spaces were associated with B waves (P = 0.002, P = 0.005, P = 0.001, respectively). Other radiological markers (mean width of the temporal horns, EI, Sylvian fissure, focally dilated sulci, basal cisterns, periventricular white matter changes or deep white matter changes) were not associated with B waves. Gender, age, BMI, gait disturbance, impaired cognition, urinary incontinence, symptom duration, and brain biopsy were not associated with B waves.
Discussion
To the best of our knowledge, this study was the first to investigate relationships between 24-h ICP measurements and radiological markers, brain biopsy findings, and shunt surgery outcomes in patients with suspected iNPH. The major finding was that a high mean ICP was associated with increased disproportionality between sylvian and suprasylvian SA spaces, the presence of focally dilated sulci, and a high EI. ICP B waves were associated with less atrophy of the medial temporal lobe, more narrowed superior medial and convexity SA spaces, and more severe disproportionality. Furthermore, AD-related brain biopsy findings were associated with a high mean ICP pulse wave amplitude.
The ICP and radiological markers
The two most important radiological iNPH-markers, increased EI and a disproportionality between the sylvian and suprasylvian SA spaces, were associated with an elevated ICP, probably because a high ICP causes expansion in the ventricles, sylvian subarachnoid (SA) spaces, and basal cisterns. In conjunction with a suprasylvian SA block, this results in diminished superior SA spaces [17], which are included in disproportionality evaluations. Moreover, focally dilated sulci and the SA spaces in the superior convexity, which contribute to the suprasylvian block phenomenon, tended to be associated with a high ICP [17].
Recently, Kim et al. [16] found that the ventricle indices did not correlate significantly with the CSF opening pressure in the lateral ventricle, measured during shunt surgery, in patients with NPH or communicating hydrocephalus. However, it is notable that their correlation coefficient for EI (r = 0.20) was similar to that found in the present study for the correlation between EI and ICP (r = 0.26) in patients with NPH; moreover, in the same study the ventricular index correlated nearly significantly with the mean ICP (r = 0.25). Such relatively weak correlations are expected, because atrophy may also cause ventricular enlargement without increasing the mean ICP. Indeed, we found stronger correlations between EI and the mean ICP in the shunt-responsive group (r = 0.36) than in the entire study population, probably because shunt responders were likely to have less atrophy. Another previous study found an inverse correlation between EI and the 24-h ICP measurement. However, this result might be explained by the much younger study population, which even included children, and the different types of hydrocephalus included, with much higher ICP levels and shorter symptom durations than in the present study [2]. Our results were consistent with previous studies, suggesting that radiological markers could not be used to predict ICP in clinical practice, based on rather weak associations and inconsistent results. Nevertheless, our results implied that these radiological markers were directly related to the pathophysiology of iNPH.
ICP and biopsy findings
An increased mean ICP pulse wave amplitude has been associated with a better shunt outcome. This association is suggested to be a feature of iNPH, and it may result from decreased compliance in patients with increased mean ICP [5, 7]. In this study, the pulse wave amplitude did not predict the shunt response. In our study ICP pulse wave amplitude was relatively high in all three groups [shunt responsive (4.8 mmHg), non-responsive (4.1 mmHg), and no shunt group (4.8 mmHg)], whereas in the Eide and Sorteberg study [8], the criterion for abnormal pulse wave amplitude was 4 mmHg [shunt responsive (5.7 mmHg), non-responsive (3.6 mmHg), and no shunt group (3.7 mmHg)]. The exact mean ICP values are not fully comparable between different studies because of technical variations. For example, we had ten times higher sampling frequency; an intraventricular catheter with a fluid column and pressure sensor was used to measure intra-arterial blood pressure. Our zero level was on the forehead but the actual pressure in the ventricle is lower than that. Some of the previous studies have used an intraparenchymal sensor with a different zeroing protocol. These differences can explain the small differences in the mean pressure values but also the differences in the pulse amplitude values. Also, we did not use ICP pulse wave amplitude as a selection criterion for shunting.
The mean ICP pulse wave amplitude was only associated with brain biopsy findings. Even this finding was unexpected, since we found that a high mean pulse wave amplitude was associated with AD-related biopsy findings (Aβ+HPτ+). Although AD is a common comorbidity in patients with iNPH [28], we found no indication that the pulse wave amplitude was associated with medial temporal lobe atrophy. It has been hypothesized that the pulsations in intracranial arteries and CSF could be related to the development of both AD and NPH [1]. In support of this hypothesis, in experimental models, kaolin-induced hydrocephalus was associated with amyloid accumulation. Thus, this hypothesis may explain our unexpected result [18]. In addition, a previous study showed that the expression of genes that regulate amyloid processing in the brain was altered in patients with iNPH compared to controls without dementia [24]. Therefore, amyloid pathology may be related to both AD and iNPH.
Neurodegeneration and shunt outcome
We also found that AD-related brain biopsy findings, i.e., amyloid-β or hyperphosphorylated tau, were more common in the no shunt response group than in the shunt-responsive group. This finding was not surprising, because shunting would have minimal effects on cognitive deterioration, whether it was caused by iNPH or AD [33]. Also, shunt-responsive patients had less medial temporal lobe atrophy than non-shunted patients. Shunt response was instead associated with more severe disproportionality, in line with a previous study [39], which is not directly related to neurodegeneration. Our results are in agreement with a previous study that found that iNPH patients with AD had worse shunt outcomes than patients with only iNPH [31]. On the other hand periventricular and deep white matter changes were common in the shunt-responsive group, which indicates that white matter changes should not prevent shunting [38]. Also, white matter changes in iNPH might be at least partially related to leakage of the CSF into brain parenchyma and therefore not necessarily indicate vascular changes.
Strengths and limitations
One limitation of this study was that we could not determine whether the ICP pulse wave amplitude was increased before or after the amyloid accumulation because the ICP measurement and the brain biopsy were performed at the same time. A retrospective study design is another limitation, but on the other hand the NPH registry aims to include all the patients who had undergone ICP measurement. The radiological images were evaluated retrospectively, but this was done blind to other patient data. Both CT and MRI scans were used in this study, which can be seen as a weakness, but on the other hand this is common in clinical practice. Study population size, especially the non-responders to shunt group, was relatively small. In addition, shunt response was assessed after a short period (2 to 3 months) after the operation. Also, the shunt response was based on a clinical evaluation, which did not include standardized, objective criteria. Moreover, this study could not identify definite mechanisms for the observed associations. The possibility that AD-related protein accumulated in iNPH should be studied further. The primary strength of this study was the broad extent of the analysis, which included multiple radiological markers, cortical biopsy findings, and a 24-h ICP measurement. Another strength was that, for comparisons, our control, patients in the no shunt group, were not healthy controls but were patients with symptoms and findings that were close to similar to those of the iNPH patients; thus, our study design reflected the clinical setting.
Conclusions
In conclusion, we found that EI and disproportionality between the sylvian and suprasylvian SA spaces were associated with high ICP, which suggested that these radiological markers were potentially related to the pathogenesis of iNPH. However, these associations were rather weak, which suggests that these markers cannot be used to predict the mean ICP or ICP pulse wave amplitude in clinical settings. Interestingly, a high pulse wave amplitude was related to AD-related biopsy findings, which suggested that CSF pulsatility, rather than ICP alone, may be related to increases in amyloid accumulation.
Abbreviations
- Aβ:
-
Amyloid beta
- AD:
-
Alzheimer’s disease
- CSF:
-
Cerebrospinal fluid
- CT:
-
Computed tomography
- EI:
-
Evans’ index
- HPτ:
-
Hyperphosphorylated tau
- ICP:
-
Intracranial pressure
- iNPH:
-
Idiopathic normal pressure hydrocephalus
- KUH:
-
Kuopio University Hospital
- MMSE:
-
Mini-Mental State Examination
- MRI:
-
Magnetic resonance imaging
- NPH:
-
Normal pressure hydrocephalus
- SA:
-
Subarachnoid
References
Bateman GA (2004) Pulse wave encephalopathy: a spectrum hypothesis incorporating Alzheimer’s disease, vascular dementia and normal pressure hydrocephalus. Med Hypotheses 62:182–187
Borgesen SE, Gjerris F (1987) Relationships between intracranial pressure, ventricular size, and resistance to CSF outflow. J Neurosurg 67:535–539
Czosnyka M, Pickard JD (2004) Monitoring and interpretation of intracranial pressure. J Neurol Neurosurg Psychiatry 75:813–821
Eide PK (2008) Demonstration of uneven distribution of intracranial pulsatility in hydrocephalus patients. J Neurosurg 109:912–917
Eide PK (2006) Intracranial pressure parameters in idiopathic normal pressure hydrocephalus patients treated with ventriculo-peritoneal shunts. Acta Neurochir (Wien) 148:21–29
Eide PK (2006) A new method for processing of continuous intracranial pressure signals. Med Eng Phys 28:579–587
Eide PK, Sorteberg W (2016) Outcome of surgery for idiopathic normal pressure hydrocephalus: role of preoperative static and pulsatile intracranial pressure. World Neurosurg 86:186–193
Eide PK, Sorteberg W (2010) Diagnostic intracranial pressure monitoring and surgical management in idiopathic normal pressure hydrocephalus: a 6-year review of 214 patients. Neurosurgery 66:80–91
Eide PK, Stanisic M (2010) Cerebral microdialysis and intracranial pressure monitoring in patients with idiopathic normal-pressure hydrocephalus: association with clinical response to extended lumbar drainage and shunt surgery. J Neurosurg 112:414–424
Evans WA (1942) An encephalographic ratio for estimating ventricular enlargement and cerebral atrophy. Arch Neurol Psych 47:931
Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA (1987) MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol 149:351–356
Foss T, Eide PK, Finset A (2007) Intracranial pressure parameters in idiopathic normal pressure hydrocephalus patients with or without improvement of cognitive function after shunt treatment. Dement Geriatr Cogn Disord 23:47–54
Hamilton R, Patel S, Lee EB, Jackson EM, Lopinto J, Arnold SE, Clark CM, Basil A, Shaw LM, Xie SX, Grady MS, Trojanowski JQ (2010) Lack of shunt response in suspected idiopathic normal pressure hydrocephalus with Alzheimer disease pathology. Ann Neurol 68:535–540
Hashimoto M, Ishikawa M, Mori E, Kuwana N, Study of INPH on neurological improvement (SINPHONI) (2010) Diagnosis of idiopathic normal pressure hydrocephalus is supported by MRI-based scheme: a prospective cohort study. Cerebrospinal Fluid Res 7:18
Holodny AI, Waxman R, George AE, Rusinek H, Kalnin AJ, de Leon M (1998) MR differential diagnosis of normal-pressure hydrocephalus and Alzheimer disease: significance of perihippocampal fissures. AJNR Am J Neuroradiol 19:813–819
Kim E, Lim YJ, Park HS, Kim SK, Jeon YT, Hwang JW, Lee YS, Park HP (2015) The lack of relationship between intracranial pressure and cerebral ventricle indices based on brain computed tomography in patients undergoing ventriculoperitoneal shunt. Acta Neurochir (Wien) 157:257–263
Kitagaki H, Mori E, Ishii K, Yamaji S, Hirono N, Imamura T (1998) CSF spaces in idiopathic normal pressure hydrocephalus: morphology and volumetry. AJNR Am J Neuroradiol 19:1277–1284
Klinge PM, Samii A, Niescken S, Brinker T, Silverberg GD (2006) Brain amyloid accumulates in aged rats with kaolin-induced hydrocephalus. Neuroreport 17:657–660
Koivisto AM, Alafuzoff I, Savolainen S, Sutela A, Rummukainen J, Kurki M, Jääskeläinen JE, Soininen H, Rinne J, Leinonen V, Kuopio NPH Registry (www.uef.finph) (2013) Poor cognitive outcome in shunt-responsive idiopathic normal pressure hydrocephalus. Neurosurgery 72:1–8
Koivisto AM, Kurki MI, Alafuzoff I, Sutela A, Rummukainen J, Savolainen S, Vanninen R, Jääskeläinen JE, Soininen H, Leinonen V (2016) High risk of dementia in ventricular enlargement with normal pressure hydrocephalus related symptoms1. J Alzheimers Dis 52:497–507
Kojoukhova M, Koivisto AM, Korhonen R, Remes AM, Vanninen R, Soininen H, Jaaskelainen JE, Sutela A, Leinonen V (2015) Feasibility of radiological markers in idiopathic normal pressure hydrocephalus. Acta Neurochir (Wien) 157:1709–1718
Kondziella D, Sonnewald U, Tullberg M, Wikkelso C (2008) Brain metabolism in adult chronic hydrocephalus. J Neurochem 106:1515–1524
Krauss JK, Droste DW, Vach W, Regel JP, Orszagh M, Borremans JJ, Tietz A, Seeger W (1996) Cerebrospinal fluid shunting in idiopathic normal-pressure hydrocephalus of the elderly: effect of periventricular and deep white matter lesions. Neurosurgery 39:292–299
Laitera T, Kurki MI, Pursiheimo JP, Zetterberg H, Helisalmi S, Rauramaa T, Alafuzoff I, Remes AM, Soininen H, Haapasalo A, Jääskeläinen JE, Hiltunen M, Leinonen V (2015) The expression of transthyretin and amyloid-beta protein precursor is altered in the brain of idiopathic normal pressure hydrocephalus patients. J Alzheimers Dis 48:959–968
Leinonen V, Koivisto AM, Alafuzoff I, Pyykkö OT, Rummukainen J, von Und Zu Fraunberg M, Jääskeläinen JE, Soininen H, Rinne J, Savolainen S (2012) Cortical brain biopsy in long-term prognostication of 468 patients with possible normal pressure hydrocephalus. Neurodegener Dis 10:166–169
Leinonen V, Koivisto AM, Savolainen S, Rummukainen J, Tamminen JN, Tillgren T, Vainikka S, Pyykkö OT, Molsa J, Fraunberg M, Pirttila T, Jääskeläinen JE, Soininen H, Rinne J, Alafuzoff I (2010) Amyloid and tau proteins in cortical brain biopsy and Alzheimer’s disease. Ann Neurol 68:446–453
Lundberg N (1960) Continuous recording and control of ventricular fluid pressure in neurosurgical practice. Acta Psychiatr Scand Suppl 36:1–193
Malm J, Graff-Radford NR, Ishikawa M, Kristensen B, Leinonen V, Mori E, Owler BK, Tullberg M, Williams MA, Relkin NR (2013) Influence of comorbidities in idiopathic normal pressure hydrocephalus—research and clinical care. A report of the ISHCSF task force on comorbidities in INPH. Fluids Barriers CNS 10:22
Marmarou A, Black P, Bergsneider M, Klinge P, Relkin N, International NPH Consultant Group (2005) Guidelines for management of idiopathic normal pressure hydrocephalus: progress to date. Acta Neurochir Suppl 95:237–240
Mori E, Ishikawa M, Kato T, Kazui H, Miyake H, Miyajima M, Nakajima M, Hashimoto M, Kuriyama N, Tokuda T, Ishii K, Kaijima M, Hirata Y, Saito M, Arai H, Japanese Society of Normal Pressure Hydrocephalus (2012) Guidelines for management of idiopathic normal pressure hydrocephalus: second edition. Neurol Med Chir (Tokyo) 52:775–809
Pomeraniec IJ, Bond AE, Lopes MB, Jane JAS (2016) Concurrent Alzheimer’s pathology in patients with clinical normal pressure hydrocephalus: correlation of high-volume lumbar puncture results, cortical brain biopsies, and outcomes. J Neurosurg 124:382–388
Relkin N, Marmarou A, Klinge P, Bergsneider M, Black PM (2005) Diagnosing idiopathic normal-pressure hydrocephalus. Neurosurgery 57:S4–S16
Savolainen S, Hurskainen H, Paljarvi L, Alafuzoff I, Vapalahti M (2002) Five-year outcome of normal pressure hydrocephalus with or without a shunt: predictive value of the clinical signs, neuropsychological evaluation and infusion test. Acta Neurochir (Wien) 144:515–523
Savolainen S, Paljarvi L, Vapalahti M (1999) Prevalence of Alzheimer’s disease in patients investigated for presumed normal pressure hydrocephalus: a clinical and neuropathological study. Acta Neurochir (Wien) 141:849–853
Scheltens P, Leys D, Barkhof F, Huglo D, Weinstein HC, Vermersch P, Kuiper M, Steinling M, Wolters EC, Valk J (1992) Atrophy of medial temporal lobes on MRI in “probable” Alzheimer’s disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry 55:967–972
Spiegelberg A, Preuß M, Kurtcuoglu V (2016) B-waves revisited. Interdisc Neurosurg 6:13–17
Toma AK, Holl E, Kitchen ND, Watkins LD (2011) Evans’ index revisited: the need for an alternative in normal pressure hydrocephalus. Neurosurgery 68:939–944
Tullberg M, Jensen C, Ekholm S, Wikkelso C (2001) Normal pressure hydrocephalus: vascular white matter changes on MR images must not exclude patients from shunt surgery. AJNR Am J Neuroradiol 22:1665–1673
Virhammar J, Laurell K, Cesarini KG, Larsson EM (2014) Preoperative prognostic value of MRI findings in 108 patients with idiopathic normal pressure hydrocephalus. AJNR Am J Neuroradiol 35:2311–2318
Acknowledgments
The authors thank Marita Voutilainen, RN, for maintenance of the KUH NPH register and biostatistician Tuomas Selander for statistical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The Fund of Mauri and Sirkka Wiljasalo, KUH VTR Fund, and The Finnish Medical Foundation provided financial support in the form of grant funding. The sponsors had no role in the design or conduct of this research.
Conflicts of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Comments
Kojoukhova and colleagues have retrospective analyzed 73 patients with suspected idiopathic normal pressure hydrocephalus to find potential associations of intracranial pressure with brain biopsy, radiological findings, and shunt surgery outcome.
As the authors point out, in idiopathic normal pressure hydrocephalus the pathophysiology is still not completely understood, and a multifactorial disease seems to be highly probable.
Thus, the present article comparing ICP data with neuropatholocical, neuroradiological and clinical post-shunt-surgery results could be highly relevant. The limitations of the present article—most importantly the retrospective design of the study, relatively small number of patients, and lack of standardized, objective criteria to assess shunt response—have been adequately discussed.
Marcus Reinges
Giessen, Germany
Anna Sutela and Ville Leinonen shared last authorship
Rights and permissions
About this article
Cite this article
Kojoukhova, M., Vanha, KI., Timonen, M. et al. Associations of intracranial pressure with brain biopsy, radiological findings, and shunt surgery outcome in patients with suspected idiopathic normal pressure hydrocephalus. Acta Neurochir 159, 51–61 (2017). https://doi.org/10.1007/s00701-016-3025-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-016-3025-8