Abstract
Background
Carotid endarterectomy (CEA) is the procedure of choice for reducing the risk of stroke in both symptomatic and asymptomatic carotid artery stenoses. Stroke is associated with significant morbidity and mortality peri-operatively (2–3 %). Our primary aim is to evaluate the etiology of these strokes after CEA and their impact on morbidity by comparing the length of stay in the hospital.
Methods
A total of 584 patients with documented neurological status evaluations who underwent CEAs were included in the study. Neurophysiological monitoring data was obtained during CEA for carotid stenosis included eight-channel electroencephalography (EEG) and upper extremity somatosensory evoked potentials (SSEPs).
Results
Twenty-one (3.595 %) patients had strokes in the perioperative period and they were more likely to have left-sided surgery (p = 0.008), intraoperative monitoring (IOM) changes (p < 0.001), an intraoperative shunt placed (p = 0.0002) or a hospital stay longer than 5 days (p = 0.0042). Unilateral anterior circulation ischemic stroke were the most common in our series. In a logistic regression model, left-sided surgery was shown to be 4.78 times more likely to be associated with perioperative stroke (1.50–15.27; p = 0.008) while intraoperative shunts were 11.85 times more likely to have strokes (3.97–35.34; p < 0.0001). Patients with stenosis greater than 70 % were 6.67 times less likely to have a stroke (0.04–0.59; p = 0.007).
Conclusions
Ischemic anterior circulation strokes are the most common type of post-operative neurological changes in patients undergoing CEA. Intraoperative shunt placement was a strong predictor of perioperative strokes. Since shunts are only placed following intraoperative monitoring changes, SSEPs and EEG can therefore function as a biomarker of cerebral hypo-perfusion.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Carotid endarterectomy (CEA) is the procedure of choice for reducing the risk of stroke in both symptomatic and selected asymptomatic carotid artery stenoses. Even though carotid endarterectomy is a safe procedure when performed by an experienced neurological or vascular surgeon, it is still associated with risks inherent to any surgical intervention, especially vascular surgeries [3, 10, 13–16, 20, 23–26]. Although uncommon, stroke in the perioperative period is associated with significant morbidity and mortality in this patient population [16]. The rate of symptomatic peri-procedural stroke ranges from 2 to 3 % in CEA [10], while this rate ranges between 0.08 and 2.9 % for any surgical procedure, with a slightly more elevated rate for high-risk and vascular procedures [19, 25, 28]. The etiology of the perioperative stroke has been shown to have a significant impact on the ultimate morbidity and mortality in patients, with patients who have strokes after their surgical interventions having poorer long-term survival [16, 17]. In addition to the estimated impact on mortality, perioperative stroke is also thought to have an impact on quality of life and overall morbidity burden [17]. In this manuscript, we evaluate the etiology of perioperative strokes after CEA, as well as evaluate the impact of these perioperative strokes on mortality and morbidity.
Clinical material and methods
Patient population and materials
We have conducted an observational retrospective case control study with data from patients who underwent CEA at the University of Pittsburgh Medical Center. Data from a database of patients who underwent CEA surgery for carotid stenosis with intraoperative neurophysiological monitoring (IOM) through the years 2007 to 2012 was collected for this study. Five hundred and eighty four patients, included in the final analysis, had documented preoperative and postoperative neurological status evaluations performed by a surgeon and/or a neurologist, who was consulted in the case of any presumed new postoperative neurological deficit. The surgeon documented the surgical procedure, and commented whether it was associated with any technical difficulties. Neurological status before and after CEA and co-morbid conditions were documented using medical records. We defined stroke as “rapidly developing clinical signs of focal disturbance of cerebral function, lasting more than 24 h or leading to death with no apparent cause other than that of vascular origin” [9, 21, 27]. Patient data collected included age, gender, ethnicity, presence of ipsilateral and contralateral carotid stenosis, vertebral artery disease (unilateral/ bilateral and percentage of occlusion), repeat CEA, diabetes mellitus, body mass index (BMI), hypertension, coronary artery disease, hyperlipidemia, smoking status, symptomatic carotid stenosis defined as a cerebrovascular accident in the 6 months prior to the procedure and length of hospital stay. Length of stay in the hospital has been used in previous epidemiological studies as a surrogate measure for morbidity for acute injury [22]. The patients with documented new neurological deficit/status change after CEA received an MRI including a diffusion-weighted imaging sequence to document the presence of a stroke. Other metrics collected from the MRI included the anatomical location of the new DWI change, the size of the infarct, which correlates with severity, as well as the type of stroke: ischemic or hemorrhagic. The study was approved by the IRB for retrospective review of data on human subjects at the University of Pittsburgh (MOD08120394-04 / PRO08120394).
Intraoperative neurophysiological monitoring
Neurophysiological monitoring data were obtained during CEA for carotid stenosis included eight-channel electroencephalography (EEG) and upper extremity somatosensory evoked potentials (SSEPs), both standard at UPMC. EEG scalp electrodes were applied using the 10-20 International systems. EEG amplitude attenuation of fast frequencies by more than 50 %, or an increase in the theta or delta amplitude by more than 50 % was considered a significant intraoperative event. Somatosensory evoked potentials from bilateral median nerve stimulation were collected as described previously [2]. We considered either a persistent and consistent (>2 average trials) 50 % reduction in the primary somatosensory cortical N20 to P30 peak-to-peak amplitude or a prolongation of the N20 response latency > 10 % from baseline in > 2 consecutive averaged trials to be a significant change. Significant changes in either the EEG or SSEPs within 3 min of cross clamping of the ICA were an indication for selective bypass shunting of the ICA during CEA. Following placement of the shunt, flow through the artery was checked using intraoperative ultrasound. All the shunts had to be patent with good flow as well as restoration of IOM parameters partially or completely to baseline in order for the operative procedure to continue. Data concerning significant EEG or SSEP change from baseline EEG and the type of change (temporary/permanent) was garnered from the neurophysiology records. EEG or SSEP changes that did or did not return to the baseline values at the end of the procedure were labeled as temporary and permanent respectively.
Statistical analysis
Descriptive characteristics are reported as mean ± SD or as number of cases and percentages. Differences among groups were tested using the two-tailed Chi-square or Fisher’s exact test for categorical variables, and the Student’s t test for continuous variables. Multivariate logistic regression models were used to assess the effect of baseline predictors on the outcome. The data were analyzed with SAS statistical software.
Results
Twenty-one (3.595 %) patients in our cohort had strokes in the perioperative period. The baseline characteristics of the patients are summarized in Table 1. The severity of the neurological deficits included numbness and weakness of the upper or lower extremities, aphasia as well as facial droop. Patients who had carotid stenosis greater than 70 % (p = 0.05) or who had their operation at a peripheral community hospital (p = 0.041) experienced less perioperative strokes. On the other hand, patients who had left-sided surgery (p = 0.008), intraoperative neurophysiology monitoring changes (p < 0.0001) or were documented to receive a shunt intra-operatively (p = 0.0002) experienced increased perioperative strokes. Using a Wilcoxon rank sum test, mean length of stay for patients who did not experience strokes was 4.36 ± 4.81 days, while patients who had strokes in the perioperative period stayed in the hospital for 5.9 ± 4.95 days. The difference in length of stay between both groups was significant with p = 0.042. In our patient population, there were no statistically significant differences with regard to coronary artery disease, hypertension, hyperlipidemia, diabetes mellitus, prior myocardial ischemia, age, gender, race or smoking status. There was no significant correlation between the modality that reflected the IOM change (temporary or permanent in the EEG or SSEP) and the location, severity, and type of stroke (Table 1).
When the patients were stratified according to their preoperative symptomatology, patients who had elevated LDLs (p = 0.026) or were smokers (p = 0.037) were more likely to have presented with symptoms, whereas patients who underwent their surgeries at peripheral or community hospitals presented for the operative intervention without any symptoms (p = 0.012) (Table 2).
Etiology of strokes
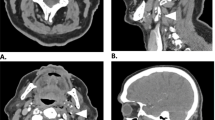
Among the 21 patients who presented with clinically detectable perioperative strokes, 19 (90.5 %) had unilateral ischemic strokes in the anterior circulation (73.6 %), as well as bilateral or multi-territory infarcts (15.7 %) and posterior circulation strokes (10.5 %). There were two hemorrhagic strokes reported (9.5 %) with one patient converting to a hemorrhage from an ischemic stroke. The results are summarized in Table 3. Figure 1 shows an MRI scan of a patient with a new onset ischemic stroke of the internal carotid artery vascular domain following carotid endarterectomy. The pattern of stroke distribution was mostly cortical (75 %), followed by sub-cortical infarcts (18.75 %) and scattered embolic infarcts (6.25 %).
Surgical parameters
All our patients underwent carotid endarterectomy surgery under general endotracheal anesthesia. Fourteen (60.8 %) of the patients who experienced perioperative strokes had an eversion carotid endarterectomy, while nine (39.2 %) had a primary longitudinal arteriotomy. Three patients (13 %) were reported to have a difficult exposure by the primary surgeon due to aberrant anatomy in two patients and scar tissue formation following prior radiation therapy to the neck in one patient. Thirteen (56.5 %) patients underwent endarterectomies emergently while ten (43.5 %) patients had elective operations.
Placement of intraoperative shunts
Twenty-seven patients received intraoperative shunts in our series, of which 21 patients did not have perioperative strokes while six patients did experience them in the perioperative period. Shunts were placed distal to the carotid plaque in all cases.
In the group that did not experience strokes but did receive intraoperative shunts, six patients (28.6 %) showed a mild decrease of signal on IOM during intraoperative monitoring, 11 patients (52.8 %) had a significant drop in their IOM signal during the procedure and four patients (19 %) showed complete loss of IOM signal. All the patients who experienced a mild decrease in IOM signal, four (36.4 %) of those with significant decreases in signal and three (75 %) of the patients with complete loss of signal had complete restoration of the signal following shunt placement. The remaining patients in this group experienced a partial recovery of the signal. No patient maintained the loss of IOM signal after placement of a patent shunt.
Amongst the six patients that did experience perioperative strokes, four patients (66.7 %) had a significant but incomplete drop in their IOM signal while the other two (33.3 %) showed complete loss of signal. Following insertion of the patent shunt, two of the patients (33.3 %) had a partial recovery while the remaining four patients (66.7 %) had full recovery of their signal. To note, both patients who had complete loss of signal prior to shunt placement made full recovery of their signal.
Predictive model
In a multivariate logistic regression model, various factors and co-morbidities were assessed for their predictive value with stroke as the primary outcome measure. CEA surgery on patients with carotid stenosis is greater than 70 % was shown to be 6.67-fold less likely to be associated with perioperative stroke (0.04–0.59; p = 0.007). However, patients who had IOM changes were 5.4 times more likely to have new post-operative strokes (0.96–30.32; p = 0.05) following CEA, while patients with left-sided surgery were 4.78 times more at risk of perioperative strokes (1.5–15.27; p = 0.008). Patients who received intraoperative shunts were 11.85 times more likely to experience perioperative strokes. Among those with asymptomatic carotid stenosis, patients who had ipsilateral stenosis were 14.28 times less likely to experience strokes following the procedure (0.01–0.76; p = 0.03) while patients who received intraoperative shunts were 29.73 times more likely to experience strokes (4.04–219; p = 0.001). On the other hand, amongst patients with symptomatic carotid stenosis, those who underwent their procedure at a peripheral community hospital were four times less likely to experience a post-operative stroke event (0.08–0.83; p = 0.024), while left-sided surgery and intraoperative shunts were 4.3 times and 7.08 times more likely to predict strokes in the patient population respectively (1.15–16.08; p = 0.03; 1.83–27.45; p = 0.005). The results of the logistic regression model are summarized in Tables 4 and 5.
Discussion
Perioperative stroke is an uncommon occurrence after CEA [16]. Our series is comparable to the recently reported rates of stroke in the literature. In the CREST trial, which included 2502 patients, the rate of stroke was 3.24 % [16]. The Carotid Atherosclerosis Study (ACAS), the Asymptomatic Carotid Surgery Trial (ACST), and other trials have also reported very similar rates (3.1–3.8 %) [14, 23, 24].
Perioperative strokes in our series were predominantly localized to anterior cerebral circulation territories (>80 %). In terms of etiology, it is reasonable to classify the source for stroke in our series into three different categories: scattered embolic events in the distribution of the involved artery (6.25 %), which are likely due to the manipulation of the intraluminal plaque, propelling emboli into the distal branches of the cerebral circulation [16, 17]; while the majority of the anterior circulation ischemic strokes involved cortical (75 %) and sub-cortical infarcts (18.75 %). A possible mechanism of action could be the dislodging of emboli, which occlude small distal branches of the involved arteries, but cannot be detected by IOM [16, 17]. Some of our cases showed a large wedge-shaped stroke of the MCA distribution, which has been shown in previous literature to be due to hypo-perfusion [16, 17].
Defining the etiology of large MCA infarcts, posterior circulation, bilateral or multi-territory stroke is more challenging, as it could relate to a number of factors that are unclear. However, in some cases of ischemic embolic stroke, it is possible that post-operative cardio-embolic events could be contributory to the neurological damage [16, 17, 24]. Previous studies have shown that acute stress, including surgery, leads to systematic inflammation, which might contribute to atherosclerotic plaque rupture and thrombosis leading to platelet activation and platelet leukocyte interaction [4, 12, 29]. This response has been shown to be due to an increase in local and systemic cytokines, which leads to the activation of the coagulation cascade causing post-operative non-embolic thrombosis of vasculature and cerebral infarction [4, 11, 12, 18, 29].
Surgical technical parameters could possibly be involved in the incidence of perioperative strokes. In our series, more patients with eversion endarterectomies had strokes. However, this is limited by our sample size, especially given that various studies have not found significant differences between the different techniques [1, 6–8].
Prevention of stroke, whether in the perioperative period or long term, is the primary endpoint of carotid revascularization surgery [16] and minimizing morbidity and mortality is an important factor to consider when the procedure is to be performed.
Given that length of hospital stay has been shown to be a reliable epidemiological marker of morbidity in this patient population [22], perioperative strokes are associated with significant compounded morbidity, especially given the fact that carotid endarterectomy is a low-morbidity procedure [16]. In an effort to identify various predictive factors and their relative weights for perioperative strokes in carotid endarterectomies, our multivariate logistic regression model showed that intraoperative neurophysiological changes, left-sided surgery, and selective intraoperative shunt placement were significant predictors of increased perioperative strokes, while stenosis greater than 70 % was a significant predictor of decreased perioperative stroke. It is our contention that left-sided surgery is not a true predictive factor given that eloquent left-sided strokes are more likely to manifest in explicit neurological symptoms, as compared to right-sided strokes. Stenosis greater than 70 % being a protective factor is an interesting finding. IOM changes generally trigger the surgeon to place a shunt during the procedure. Therefore, the occurrence of IOM changes should warrant increased scrutiny and additional monitoring following the procedure. Although neurophysiological changes were not found to be significant in our model as predictive factors of perioperative stroke, shunt placement remained a very significant predictor of increased stroke. This is still dependent on the prior occurrence of IOM changes. Intraoperative neurophysiological changes are generally interpreted as indicating brain hypo-perfusion. The first response to IOM disturbances intra-operatively is blood pressure support, followed by shunt placement if the monitoring disturbances do not subside.
It is worth noting that, in our series, procedures conducted in peripheral community hospitals were shown to be a significant predictor of decreased perioperative strokes in patients with symptomatic stenosis (OR 0.25; p = 0.024). This finding is probably related to patient selection. Patients who get treatment in community hospitals tend to be healthier with less co-morbidities, thereby needing less specialized care, available at larger academic centers. This correlates with the significantly higher proportion of patients who present to community hospitals for endarterectomies with asymptomatic carotid stenoses [5].
Limitations
The present study has some limitations, namely that the imaging sequences were obtained based on the clinical history rather than at predetermined time points, which intentionally selects for patients who had changes in their neurological status. In addition, this was a retrospective survey of outcomes after CEA, which does not allow us to longitudinally follow the patients post-operatively and therefore lacks long-term data. Our data did not include NIH stroke scales to comprehensively assess the severity of the stroke burden.
Conclusions
Ischemic anterior circulation strokes are the most common type of post-operative neurological changes in patients undergoing CEA. Shower emboli, hemorrhagic, and hypoperfusion-induced strokes are uncommon. IOM changes, selective intraoperative shunt placement as well as left-sided surgery were all predictors of increased perioperative stroke. SSEPs and EEG can function as a biomarker of cerebral hypoperfusion and perioperative strokes during CEA, especially whenever the disturbances do not respond to first-line measures such as blood pressure support.
References
Avgerinos ED, Chaer RA, Naddaf A, El-Shazly OM, Marone L, Makaroun MS (2016) Primary closure after carotid endarterectomy is not inferior to other closure techniques. J Vasc Surg 678–683
Balzer JR, Tomycz ND, Crammond DJ, Habeych M, Thirumala PD, Urgo L, Moossy JJ (2011) Localization of cervical and cervicomedullary stimulation leads for pain treatment using median nerve somatosensory evoked potential collision testing. J Neurosurg 114:200–205
Barnett HJ, Taylor DW, Eliasziw M, Fox AJ, Ferguson GG, Haynes RB, Rankin RN, Clagett GP, Hachinski VC, Sackett DL, Thorpe KE, Meldrum HE, Spence JD (1998) Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 339:1415–1425
Bastian D, Tamburstuen MV, Lyngstadaas SP, Reikeras O (2008) Systemic and local cytokine kinetics after total hip replacement surgery. Eur Surg Res 41:334–340
Bradac O, Mohapl M, Kramar F, Netuka D, Ostry S, Charvat F, Lacman J, Benes V (2014) Carotid endarterectomy and carotid artery stenting: changing paradigm during 10 years in a high-volume centre. Acta Neurochir 156:1705–1712
Cao P, De Rango P, Zannetti S (2002) Eversion vs conventional carotid endarterectomy: a systematic review. Eur J Vasc Endovasc Surg 23:195–201
Cao P, Giordano G, De Rango P, Zannetti S, Chiesa R, Coppi G, Palombo D, Peinetti F, Spartera C, Stancanelli V, Vecchiati E (2000) Eversion versus conventional carotid endarterectomy: late results of a prospective multicenter randomized trial. J Vasc Surg 31:19–30
Cao PG, de Rango P, Zannetti S, Giordano G, Ricci S, Celani MG (2001) Eversion versus conventional carotid endarterectomy for preventing stroke. Cochrane Database Syst Rev:CD001921
Capildeo R, Haberman S, Rose FC (1978) The definition and classification of stroke. A new approach. Q J Med 47:177–196
Carotid Stenting Trialists C, Bonati LH, Dobson J, Algra A, Branchereau A, Chatellier G, Fraedrich G, Mali WP, Zeumer H, Brown MM, Mas JL, Ringleb PA (2010) Short-term outcome after stenting versus endarterectomy for symptomatic carotid stenosis: a preplanned meta-analysis of individual patient data. Lancet 376:1062–1073
DeGraba TJ (2006) How and when do we alter inflammatory mechanisms in stroke? Will it help? Semin Neurol 26:75–87
Elkind MS (2010) Inflammatory mechanisms of stroke. Stroke; a J Cereb Circulat 41:S3–8
Ferguson GG, Eliasziw M, Barr HW, Clagett GP, Barnes RW, Wallace MC, Taylor DW, Haynes RB, Finan JW, Hachinski VC, Barnett HJ (1999) The North American Symptomatic Carotid Endarterectomy Trial : surgical results in 1415 patients. Stroke; a J Cereb Circulat 30:1751–1758
Halliday A, Harrison M, Hayter E, Kong X, Mansfield A, Marro J, Pan H, Peto R, Potter J, Rahimi K, Rau A, Robertson S, Streifler J, Thomas D, Asymptomatic Carotid Surgery Trial Collaborative G (2010) 10-year stroke prevention after successful carotid endarterectomy for asymptomatic stenosis (ACST-1): a multicentre randomised trial. Lancet 376:1074–1084
Halliday A, Mansfield A, Marro J, Peto C, Peto R, Potter J, Thomas D, Group MRCACSTC (2004) Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet 363:1491–1502
Hill MD, Brooks W, Mackey A, Clark WM, Meschia JF, Morrish WF, Mohr JP, Rhodes JD, Popma JJ, Lal BK, Longbottom ME, Voeks JH, Howard G, Brott TG, Investigators C (2012) Stroke after carotid stenting and endarterectomy in the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST). Circulation 126:3054–3061
International Carotid Stenting Study i, Ederle J, Dobson J, Featherstone RL, Bonati LH, van der Worp HB, de Borst GJ, Lo TH, Gaines P, Dorman PJ, Macdonald S, Lyrer PA, Hendriks JM, McCollum C, Nederkoorn PJ, Brown MM (2010) Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): an interim analysis of a randomised controlled trial. Lancet 375:985–997
Jin R, Yang G, Li G (2010) Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol 87:779–789
Limburg M, Wijdicks EF, Li H (1998) Ischemic stroke after surgical procedures: clinical features, neuroimaging, and risk factors. Neurology 50:895–901
Mayberg MR, Wilson SE, Yatsu F, Weiss DG, Messina L, Hershey LA, Colling C, Eskridge J, Deykin D, Winn HR (1991) Carotid endarterectomy and prevention of cerebral ischemia in symptomatic carotid stenosis. Veterans Affairs Cooperative Studies Program 309 Trialist Group. JAMA 266:3289–3294
Mehndiratta P, Chapman Smith S, Worrall BB (2015) Etiologic stroke subtypes: updated definition and efficient workup strategies. Curr Treat Options Cardiovasc Med 17:357
Newgard CD, Fleischman R, Choo E, Ma OJ, Hedges JR, McConnell KJ (2010) Validation of length of hospital stay as a surrogate measure for injury severity and resource use among injury survivors. Acad Emerg Med 17:142–150
No A (1995) Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA 273:1421–1428
No A (1998) Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet 351:1379–1387
Pappada G, Vergani F, Parolin M, Cesana C, Pirillo D, Pirovano M, Santoro P, Landi A, Ferrarese C (2010) Early acute hemispheric stroke after carotid endarterectomy. Pathogenesis and management. Acta Neurochir 152:579–587
Rhee-Moore SJ, DeRubertis BG, Lam RC, Hynecek RL, Lee L, McKinsey JF, Morrissey NJ, Karwowski J, Mureebe L, Kent KC, Faries PL (2008) Periprocedural complication rates are equivalent between symptomatic and asymptomatic patients undergoing carotid angioplasty and stenting. Ann Vasc Surg 22:233–237
Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, Elkind MS, George MG, Hamdan AD, Higashida RT, Hoh BL, Janis LS, Kase CS, Kleindorfer DO, Lee JM, Moseley ME, Peterson ED, Turan TN, Valderrama AL, Vinters HV, American Heart Association Stroke Council CoCS, Anesthesia, Council on Cardiovascular R, Intervention, Council on C, Stroke N, Council on E, Prevention, Council on Peripheral Vascular D, Council on Nutrition PA, Metabolism (2013) An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke; a J Cereb Circulat 44:2064–2089
Szeder V, Torbey MT (2008) Prevention and treatment of perioperative stroke. Neurologist 14:30–36
Zeller JA, Lenz A, Eschenfelder CC, Zunker P, Deuschl G (2005) Platelet–leukocyte interaction and platelet activation in acute stroke with and without preceding infection. Arterioscler Thromb Vasc Biol 25:1519–1523
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this research.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Additional information
Comments
I have been over and over this manuscript. The basic message seems to be that indwelling shunts placed during CEA are dangerous. This has not been my experience, and I disagree with this, but I do accept that these authors’ data show it in their experience.
I was taught to practice universal shunting for CEA. I soon abandoned this practice and switched to selective monitoring-dependent shunting when it became clear that universal shunting placed many patients at embolic risk who did not need shunt flow during surgery. This was the work of Halsey et. al. (1), not cited by these authors. What is also clear, from Halsey, above, and others like Gary Ferguson (2), is that there are clearly patients who need a shunt and have bad outcomes from cross-clamp ischemia if one is not used. So I do not personally agree with the proponents of a “never-shunt” strategy.
Regarding the current paper, the perioperative stroke rate is higher in shunted cases with eversion endarterectomy for reasons that I cannot discern, since I do not practice this technique. Stroke rate is also higher in this series in left-sided cases. There is no doubt in my mind that for right-handed surgeons a left CEA is more difficult to do, but with experience we learn to deal with this, and in my personal experience there is no difference at all.
So what can we take away? These authors report their retrospective data from a highly mixed patient series with different surgeons and different techniques. There are inherent compromises with this approach. I respect what they have done, but as for me, one surgeon with one constant technique, shunts are useful and lifesaving devices when called for, and we place them without hesitation when the monitoring changes (3). To their credit, they conclude that the need for shunting is a biomarker of cerebral hypoperfusion. While this seems intuitive, this is also the problem that a properly placed shunt, quickly inserted within a minute or less, and with audible confirmation of shunt flow (Doppler) is intended to, and in our experience effective at, solving. It is imperative that the monitoring returns at least partially to baseline after shunt placement. If it does not, the surgeon needs to audibly ascertain shunt flow, or else remove and replace it.
Similarly, in my own experience we approach left CEA with more caution, but the results are identical for us, and we do not hesitate to recommend surgery on the left when the clinical criteria are aligned.
Christopher M. Loftus
IL, USA
1. Halsey JH(1992) Risks and benefits of shunting in carotid endarterectomy. Stroke 23: 1583–1587.
2. Ferguson GG (1984) Protection of the brain during carotid endarterectomy. IV. Shunt almost never. Int Anesthesiol Clin. 22(3):147–52.
3. Loftus CM(2006) Carotid Artery Surgery: Principles and Technique. 2nd edition. New York, Informa Publishing
Rights and permissions
About this article
Cite this article
Khattar, N.K., Friedlander, R.M., Chaer, R.A. et al. Perioperative stroke after carotid endarterectomy: etiology and implications. Acta Neurochir 158, 2377–2383 (2016). https://doi.org/10.1007/s00701-016-2966-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-016-2966-2