Abstract
Background
The mortality rate of patients with brain oedema after malignant middle cerebral artery (MCA) infarction approaches 80 % without surgical intervention. Surgical treatment with ipsilateral decompressive hemicraniectomy (DHC) has been shown to dramatically improve survival rates. DHC currently lacks established inclusion criteria and additional research is needed to assess the impact of prognostic factors on functional outcome. The aim of this study was to assess the impact of prognostic factors on functional outcome.
Method
A retrospective cohort study was carried out including 46 patients who underwent DHC at the Karolinska University Hospital between 2004 and 2014. The maximum time to surgery was 5 days after symptom debut. The primary endpoint was a dichotomised score on the modified Rankin Scale (mRS) 3 months after surgery, with favourable outcome defined as mRS ≤ 4.
Results
When the study population was dichotomised according to the primary endpoint, a significant difference between the groups was seen in preoperative Glasgow Coma Score (GCS), blood glucose levels and the infarction’s involvement of the basal ganglia (p < 0.05). In a logistic regression model, preoperative GCS contributed significantly with a 59.6 % increase in the probability of favourable outcome for each point gained in preoperative GCS (p = 0.035).
Conclusions
The results indicate that preoperative GCS, blood glucose and the infarction’s involvement of the basal ganglia are strong predictors of clinical outcome. These factors should be considered when assessing the probable outcome of DHC, and additional research based on these factors may contribute to improved inclusion criteria for DHC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Space-occupying brain oedema is a life-threatening complication of middle cerebral artery (MCA) infarction [8]. The term “malignant” infarction was introduced to reflect the poor prognosis for this patient group. The brain oedema causes a critical increase in intracranial pressure (ICP) culminating 2–5 days after the infarction. Rising ICP may cause transtentorial brain herniation, which is the leading cause of death in MCA infarction [5]. Conventional medical treatments of elevated ICP include hyperventilation, mannitol therapy, diuretics and barbiturate coma [19]. However, these medical treatments have proved ineffective at reducing ICP after an MCA infarction [9]. The mortality rate of malignant MCA infarction is nearly 80 % without surgical intervention despite maximal non-surgical medical care [1, 5, 10].
Decompressive hemicraniectomy (DHC) is a neurosurgical procedure where ICP is reduced by removing a bone flap from the cranium and opening the dura mater [17]. The benefit of DHC in malignant MCA infarction has been demonstrated in three European randomised clinical trials: DESTINY [10], HAMLET [6] and DECIMAL [22]. A pooled analysis of these trials showed that 1-year mortality was reduced from 71 to 22 % when DHC was performed within 48 h of symptom onset in patients under the age of 60 [21]. A follow-up study, DESTINY II, found a reduction of 1-year mortality from 76 to 43 % in patients above the age of 60 [11].
Despite a proven reduction in mortality, DHC remains controversial among neurosurgeons and neurologists since more patients are left with a high degree of disability and dependency [18]. In the randomised trials, DHC increased the percentage of patients who survived with mRS (modified Rankin Scale) 2–3. However, the percentage of patients who survived with mRS 4 also increased more than 12 times, from 2.5 to 31 % [21]. DHC currently lacks established inclusion criteria for surgery. The current Swedish National Guidelines for Stroke Care recommend that DHC for MCA infarction be carried out within 48 h of symptom onset in patients under the age of 60 [20]. However, it has been hypothesised that bone flap size [3], side of the infarction, patient characteristics [4, 7] and preoperative status [2] also impact functional outcome. The influence of these factors remains uncertain and additional research is needed to establish evidence-based inclusion criteria for DHC in MCA infarction.
The decision to perform DHC must be based on a careful evaluation of the risks and benefits for each individual patient. However, it is difficult to assess the risks of DHC without knowing which factors impact outcome. We, therefore, performed a retrospective study at the Karolinska University Hospital aimed at assessing the impact of prognostic factors on the functional outcome of DHC in malignant MCA infarction.
Methods
Study design and patient population
A retrospective cohort study was performed on a consecutive sample of 46 patients who underwent DHC due to MCA infarction. The primary endpoint was a dichotomised mRS score 3 months after surgery with favourable outcome defined as mRS ≤4. The study was approved by the Ethical Review Board in Stockholm, Sweden. Patients were selected according to the following inclusion criteria:
-
1.
Age ≥18 years
-
2.
Acute onset of neurological deficits suggestive of an MCA infarction
-
3.
Manifest infarction on a computed tomography (CT) scan within the MCA territory, with or without involvement of the anterior or posterior cerebral artery
-
4.
DHC at the Karolinska University Hospital between 2004 and 2014
Surgical treatment
All patients were surgically treated with an ipsilateral hemicraniectomy performed according to currently available protocols [17]. After reflecting the scalp, burr holes were drilled and the dura was freed. A large bone flap was removed and the dura was opened in a stellate fashion. No infarcted brain tissue was removed. A dural substitute was then used to cover any defects before the scalp was reflected and sutured.
Data sources
Comprehensive electronic medical records and imaging from all patients were analysed. No patients were excluded due to missing data. Patients were cross-referenced with the Swedish national stroke register Riks-Stroke where additional information was collected on the same patients. Data was compiled on preoperative neurological status according to GCS, National Institutes of Health Stroke Scale (NIHSS) score, symptoms, age, sex, location of the infarction, S-100B and blood glucose levels. In addition, surgery timing and bone flap size was evaluated. Finally, outcome according to mRS was assessed at 3 and 12 months after DHC.
The bone flap size of all patients was measured on a postoperative CT scan. Since the actual bone flap was not included in the CT scan, its size was inferred from the size of the craniotomy. The length of the craniotomy was measured in the axial plane by dividing the surface of the dura into five linear segments. Correspondingly, the height of the craniotomy was measured in the coronal plane by dividing the surface into five linear segments as shown in Fig. 1. Measurements in both the axial and coronal planes were taken on the level where the craniotomy was largest. The length and height of the craniotomy were then multiplied with each other to yield an approximation of the bone flap area. While the bone flap was not rectangular as this model implies, multiplying the length with the height provided a rough approximation of its area that enabled comparison between patients.
Statistical methods
The study population was divided into two groups based on functional outcome with favourable outcome defined as mRS 0–4 and unfavourable outcome defined as mRS 5–6. Potential prognostic factors and patient characteristics were compared between the outcome groups and the results were compared to data available from previously published randomised trials [6, 10, 21, 22]. The study population was then divided into subgroups based on age (<60 years versus >60 years), sex, side of the infarction and involvement of the basal ganglia. Mann-Whitney U tests were performed to compare mRS scores between subgroups. Spearman’s rank-order correlation was also run to assess the correlation between prognostic factors and functional outcome. Lastly, a logistic regression model was created to quantify the impact of potential prognostic factors. A p value <0.05 was prospectively considered significant in all statistical analysis.
Results
Patient characteristics
A total of 46 patients met the inclusion criteria. The patient characteristics in each outcome category are presented in Table 1.
Preoperative status
A clinical neurological evaluation, CT scan and laboratory blood work was performed on all patients prior to surgery. A summary of preoperative status in each outcome category is displayed in Table 2.
Bone flap size
No statistically significant difference in bone flap length, height or length × height was found between the outcome groups. Bone flap size in each outcome category is displayed in Table 3.
Mortality and functional outcome
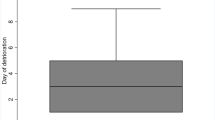
The mortality rate at 30 days post-surgery was 17.4 % (n = 8). An evaluation of mRS was done at 3 and 12 months, yielding the results in Fig. 2. A valid mRS score was available for all patients at 3 months post-surgery, whereas outcome scores were missing for six patients at 12 months. Favourable outcome (defined as mRS ≤4) was achieved by 56.5 % of patients after 3 months.
Univariate analysis
The 3-month mRS scores for patients aged ≤60 (n = 39) were not statistically significantly different from patients over the age of 60 (n = 7), U = 180.5, z = 1.406, p = 0.183. To further compare patients in the outermost age groups, a post hoc analysis was done to compare the mRS scores of patients aged ≤40 (n = 10) to patients aged ≥60 (n = 20). No statistically significant difference was found, U = 62.5, z = 0.985, p = 0.353.
No statistically significant difference in mRS scores was found between males (n = 31) and females (n = 15), U = 275.5, z = 1.053, p = 0.293. Additionally, no significant difference in outcome was found between patients with left-sided infarctions (n = 14) and right-sided infarctions (n = 32), U = 176, z = −1.197, p = 0.231. Finally, no significant difference in mRS score was found between surgery within 48 h (n = 30) and after 48 h (n = 16), U = 300, z = 1.446, p = 0.148.
The functional outcome of patients with an infarction involving the basal ganglia was compared to the functional outcome of patients without involvement of the basal ganglia. The mRS scores of patients with an infarction involving the basal ganglia (n = 38) were significantly higher compared to patients without involvement of the basal ganglia (n = 8), U = 254, z = 3.088, p = 0.002.
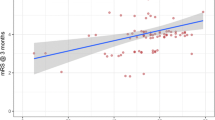
A Spearman’s rank-order correlation found a strong and significant positive correlation between preoperative blood glucose and mRS at 3 months, r s(39) = 0.473, p = 0.002. Higher blood glucose was thus associated with a higher mRS score.
Logistic regression analysis
A logistic regression model was created to predict the outcome category based on potential prognostic factors. The dependent variable was outcome according to mRS dichotomised into two groups at mRS ≤4. The independent variables in the regression model were blood glucose, GCS score, midline shift and preoperative S100B levels. The logistic regression model was statistically significant, χ2(4) = 12.572, p = 0.014. The model explained 56.5 % (Nagelkerke R 2) of the variance in outcome and correctly classified 78.3 % of cases. Sensitivity was 84.6 %, specificity was 70 %, positive predictive value was 78.5 % and negative predictive value was 77.8 %. The only statistically significant independent variable was preoperative GCS score, which resulted in a 59.6 % increase in the probability of favourable outcome for every point gained in preoperative GCS score (p = 0.035, standard error = 0.222). Higher preoperative GCS score was thus associated with an increased likelihood of favourable functional outcome.
Comparison to international results
The results from the present study were compared to results from the prospective randomised clinical trials DECIMAL [22], DESTINY [10] and HAMLET [6] in Table 4.
Discussion
In an effort to assess the impact of preoperative factors on the functional outcome of DHC for MCA infarction, we performed a retrospective cohort study of 46 patients who underwent DHC for an MCA infarction. When functional outcome was dichotomised at mRS ≤4 at 3 months, our results indicate that preoperative GCS, involvement of the basal ganglia and blood glucose levels are the factors most likely to impact functional outcome. No other preoperative factor or patient characteristic analysed in the study was found to have a statistically significant impact on functional outcome. These results largely support the results from the randomised trials DECIMAL, HAMLET and DESTINY [6, 10, 22].
Preoperative GCS was the preoperative factor most strongly associated with functional outcome. The logistic regression model indicates that the probability of favourable outcome increases with 59.6 % for every point gained in preoperative GCS. However, the standard error was also considerable. While the true impact of preoperative GCS may not be quite as large as the regression model suggests, it remains a significant preoperative factor. This is in line with clinical rationale since a low GCS score reflects a more critical patient with manifest neurological deterioration.
Involvement of the basal ganglia was also strongly correlated with functional outcome. The basal ganglia are instrumental in voluntary motor control, cognition and emotion. It is, therefore, reasonable from a clinical standpoint that impaired basal ganglia function be associated with a poorer functional outcome.
The results showed a significant difference in preoperative blood glucose levels between the outcome groups. The median glucose level was 8.2 mmol/l in the unfavourable outcome group compared with 6.3 mmol/l in the favourable outcome group. Spearman’s rank-order correlation also confirmed a strong positive correlation between preoperative blood glucose and mRS at 3 months. A systematic review by Kagansky et al. [12] found that hyperglycaemia in stroke patients was associated with worse clinical outcome than normoglycaemic patients. The correlation between blood glucose levels and outcome seen in our study supports these findings.
While there was a clear correlation between blood glucose and functional outcome in our study, the relationship may not necessarily be a causal one. Diabetes is a common cause of hyperglycaemia that also increases the risk for stroke [15]. It is, therefore, plausible that some patients had elevated blood glucose levels without it necessarily being associated with the degree of brain damage or oedema. Interestingly, studies have shown that hyperglycaemia remains a significant independent predictor of higher mortality even in non-diabetic patients with stroke [13]. One hypothesis is that hyperglycaemia is the result of increased stress hormones due to a more extensive brain injury. If elevated blood glucose was indicative of a severe infarction, it would likely covariate with GCS and involvement of the basal ganglia as seen in our study.
The present study found no statistically significant difference in outcome based on age. While functional outcome may be slightly worse in older patients, the outcome may still be acceptable when compared to the high probability of death. Surgery timing also failed to display a significant impact on functional outcome. Current guidelines recommend DHC within 48 h for MCA infarction in patients up to the age 60 [20]. It is, therefore, remarkable that neither age nor surgery timing showed any statistically significant correlation with functional outcome.
While the difference in surgery timing failed to reach statistical significance (p = 0.074), the median time to surgery in the favourable outcome category was 42.9 h compared with 24.4 h in the unfavourable outcome category. It is probable that patients with a massive infarction displaying signs of impending herniation likely received surgery earlier than patients displaying mild neurological symptoms. A similar trend was seen in the HAMLET trial, where DHC had no effect on the outcome of patients randomised after 48 h [6]. The trend in surgery timing in our study likely reflects the decisions made by the neurosurgical team to delay surgery as opposed to indicating that longer time to surgery leads to better outcome.
The present study found no correlation between functional outcome and bone flap size. However, the volume of decompression is mathematically correlated to the diameter of the craniotomy. A study by Flechsenhar et al. [3] found that a craniotomy should be at least 12 cm in diameter in order to achieve sufficient decompression. The results in Table 3 show that the majority of patients in the present study received a craniotomy larger than 12 cm in diameter. This suggests that bone flap size failed to impact outcome since it varied within an acceptable range.
Speech is generally lateralised to the left hemisphere in right-handed individuals [14]. Due to the high degree of lateralisation, it has been hypothesised that infarctions in the speech-dominant hemisphere be associated with poorer functional outcome. A major source of concern has been that an MCA infarction and subsequent DHC in the speech-dominant hemisphere may result in poor quality of life (QoL) due to aphasia. However, studies have found no indication that the side of the infarction impacts QoL [4, 18].
In the present study, significantly more patients with right-sided infarctions underwent DHC. However, the incidence of MCA infarctions is approximately equal between the left and right hemisphere [5]. This indicates that a bias has taken place. More patients with left-sided infarctions may have been treated conservatively in belief that they be associated with poorer QoL. Both our study and previously published studies found no difference in outcome depending on the side of the infarction. According to the current state of knowledge, there is no reason that patients with left-sided infarctions should be treated more conservatively.
A recent retrospective study by Merenda et al. [16] concluded that strokectomy may be considered when there is reason to believe that DHC alone will result in insignificant decompression. However, this additional decompressive treatment was not applied in our study. Further, the study identified a preoperative non-reactive pupil as the only significant predictor of clinical failure of DHC. A non-reactive pupil was also associated with poor outcome in our study but failed to reach statistical significance. In line with our study, a strong trend was seen between lower GCS score and clinical failure.
Favourable outcome in our study was defined as mRS ≤4. While a moderately severe disability may not seem like a favourable outcome in many other settings, it may be an acceptable outcome when the alternative is near certain death. A systematic review by Rahme et al. [18] found that 77 % of patients and/or caregivers were satisfied and would consent to DHC again. Currently available evidence suggests that the vast majority of patients are satisfied with their life after DHC despite physical disabilities.
The recovery process is still ongoing after 3 months and one would, therefore, expect the functional outcome to improve after an additional 9 months of recovery and rehabilitation [10, 22]. At 12 months postoperatively, both mRS 1 and mortality increased slightly while mRS 3, 4 and 5 decreased compared to the 3-month outcome. However, the true significance of these differences remains uncertain due to a degree of missing outcome data at 12 months.
A strength of the present study is that it included 46 consecutive patients, a larger population than the surgery groups in all three randomised trials [6, 10, 22]. However, the retrospective study design also carries inherent limitations. The lack of a control group necessitates caution in drawing conclusions about the relative effect of DHC compared to no DHC, in this population.
Clinical implications
While established guidelines on DHC are currently lacking, available recommendations on the inclusion criteria for DHC are based on age and time to surgery [20]. Our results indicate that preoperative GCS, blood glucose and involvement of the basal ganglia may be of greater importance. Preoperative GCS appears to be the strongest predictor of outcome and it should, therefore, be included in the recommendations for DHC. The probability of favourable outcome is already reduced once GCS has begun to decline, which calls for a more proactive approach to surgery. However, it is also unethical to perform an invasive neurosurgical procedure on a patient who may not need it. While pre-emptive DHC is therefore questionable, the neurosurgical team must be exceptionally sensitive to neurological deterioration to enable prompt surgery at the first signs of declining neurological status. This underscores the importance of high-quality neurological monitoring.
Conclusions
The present study indicates that preoperative GCS, blood glucose levels and involvement of the basal ganglia are significantly correlated with functional outcome. Our results indicate that the inclusion criteria for DHC can be improved by including these significant prognostic factors. The present study contributes to an evidence-based analysis of probable functional outcome based on preoperative factors. This in turn enables an informed discussion of the possible outcome of DHC with the patient and their significant others.
References
Berrouschot J, Sterker M, Bettin S, Köster J, Schneider D (1998) Mortality of space-occupying (‘malignant’) middle cerebral artery infarction under conservative intensive care. Intensive Care Med 24(6):620–623
Chen C-C, Cho D-Y, Tsai S-C (2007) Outcome of and prognostic factors for decompressive hemicraniectomy in malignant middle cerebral artery infarction. J Clin Neurosci 14(4):317–321
Flechsenhar J, Woitzik J, Zweckberger K, Amiri H, Hacke W, Jüttler E (2013) Hemicraniectomy in the management of space-occupying ischemic stroke. J Clin Neurosci 20(1):6–12
Gupta R, Connolly ES, Mayer S, Elkind MSV (2004) Hemicraniectomy for massive middle cerebral artery territory infarction: a systematic review. Stroke 35(2):539–543
Hacke W, Schwab S, Horn M, Spranger M, De Georgia M, von Kummer R (1996) “Malignant” middle cerebral artery territory infarction: clinical course and prognostic signs. Arch Neurol 53(4):309–315
Hofmeijer J, Kappelle LJ, Algra A, Amelink GJ, van Gijn J, van der Worp HB (2009) Surgical decompression for space-occupying cerebral infarction (the Hemicraniectomy After Middle Cerebral Artery infarction with Life-threatening Edema Trial [HAMLET]): a multicentre, open, randomised trial. Lancet Neurol 8(4):326–333
Holtkamp M, Buchheim K, Unterberg A, Hoffmann O, Schielke E, Weber JR, Masuhr F (2001) Hemicraniectomy in elderly patients with space occupying media infarction: improved survival but poor functional outcome. J Neurol Neurosurg Psychiatry 70(2):226–228
Jüttler E, Bösel J, Amiri H, Schiller P, Limprecht R, Hacke W, Unterberg A (2011) DESTINY II: DEcompressive Surgery for the Treatment of malignant INfarction of the middle cerebral arterY II. Int J Stroke 6(1):79–86
Jüttler E, Schellinger PD, Aschoff A, Zweckberger K, Unterberg A, Hacke W (2007) Clinical review: therapy for refractory intracranial hypertension in ischaemic stroke. Crit Care 11(5):231
Jüttler E, Schwab S, Schmiedek P, Unterberg A, Hennerici M, Woitzik J, Witte S, Jenetzky E, Hacke W (2007) Decompressive Surgery for the Treatment of Malignant Infarction of the Middle Cerebral Artery (DESTINY): a randomized, controlled trial. Stroke 38(9):2518–2525
Jüttler E, Unterberg A, Woitzik J, Bösel J, Amiri H, Sakowitz OW, Gondan M, Schiller P, Limprecht R, Luntz S, Schneider H, Pinzer T, Hobohm C, Meixensberger J, Hacke W (2014) Hemicraniectomy in older patients with extensive middle-cerebral-artery stroke. N Engl J Med 370(12):1091–1100
Kagansky N, Levy S, Knobler H (2001) The role of hyperglycemia in acute stroke. Arch Neurol 58(8):1209–1212
Kasner SE, Demchuk AM, Berrouschot J, Schmutzhard E, Harms L, Verro P, Chalela JA, Abbur R, McGrade H, Christou I, Krieger DW (2001) Predictors of fatal brain edema in massive hemispheric ischemic stroke. Stroke 32(9):2117–2123
Knecht S (2000) Handedness and hemispheric language dominance in healthy humans. Brain 123(12):2512–2518
Luitse MJA, Biessels GJ, Rutten GEHM, Kappelle LJ (2012) Diabetes, hyperglycaemia, and acute ischaemic stroke. Lancet Neurol 11(3):261–271
Merenda A, Perez-Barcena J, Frontera G, Benveniste RJ (2015) Predictors of clinical failure of decompressive hemicraniectomy for malignant hemispheric infarction. J Neurol Sci 355(1-2):54–58
Quinn TM, Taylor JJ, Magarik JA, Vought E, Kindy MS, Ellegala DB (2011) Decompressive craniectomy: technical note. Acta Neurol Scand 123(4):239–244
Rahme R, Zuccarello M, Kleindorfer D, Adeoye OM, Ringer AJ (2012) Decompressive hemicraniectomy for malignant middle cerebral artery territory infarction: is life worth living? J Neurosurg 117(4):749–754
Rangel-Castilla L, Rangel-Castillo L, Gopinath S, Robertson CS (2008) Management of intracranial hypertension. Neurol Clin 26(2):521–541
Socialstyrelsen (2009) Hjärninfarkt med expansiv effekt (malign mediainfarkt) hos patienter under cirka 60 år. Nationella riktlinjer för strokesjukvård 2009—Stöd för styrning och ledning.The National Board of Health and Welfare, Stockholm
Vahedi K, Hofmeijer J, Juettler E, Vicaut E, George B, Algra A, Amelink GJ, Schmiedeck P, Schwab S, Rothwell PM, Bousser M-G, van der Worp HB, Hacke W (2007) Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet Neurol 6(3):215–222
Vahedi K, Vicaut E, Mateo J, Kurtz A, Orabi M, Guichard J-PP, Boutron C, Couvreur G, Rouanet F, Touzé E, Guillon B, Carpentier A, Yelnik A, George B, Payen D, Bousser M-GG (2007) Sequential-design, multicenter, randomized, controlled trial of early decompressive craniectomy in malignant middle cerebral artery infarction (DECIMAL Trial). Stroke 38(9):2506–2517
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Karolinska Institutet provided financial support in the form of funding for the Department of Neurosurgery. The sponsor had no role in the design or conduct of this research.
Conflicts of interest
None.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required. The study was approved by the Ethical Review Board in Stockholm, Sweden.
Additional information
Comment
This is a quality manuscript and worthy of our consideration. It is gratifying to see that such good information can be mined from a retrospective study design, and I commend the authors for their rigorous and scientific approach to the subject.
There is useful information in this report that impacts our clinical decision making, specifically, that in cases of malignant MCA infarction, surgery is worthwhile, and that favorable surgical outcome can be predicted by three preoperative factors—a higher GCS score, lower blood glucose, and lack of basal ganglia involvement. Equally important to us is that other factors which we traditionally hold sacred don’t seem to impact outcome, like left/right infarct laterality, size of bone flap, age, sex, or time to surgery.
For our own practice in the USA we customarily let the patient’s family make the final decision. We have no hesitation to perform DHC for MCA infarcts, and we do it routinely. When we do it we try our best to do it early, before a falling GCS sets in, and before frank cerebral herniation has occurred.
We believe in this treatment as a strategy for patient salvage and we believe that this report extends our knowledge base regarding patient selection and the path to a successful outcome. The alternative in malignant MCA infarct, absent surgery, is customarily death, which we seek to avoid when we can.
Christopher M. Loftus
Illinois, USA
Rights and permissions
About this article
Cite this article
von Olnhausen, O., Thorén, M., von Vogelsang, AC. et al. Predictive factors for decompressive hemicraniectomy in malignant middle cerebral artery infarction. Acta Neurochir 158, 865–873 (2016). https://doi.org/10.1007/s00701-016-2749-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-016-2749-9