Abstract
Background
The aim of this study was to review our experience with surgical clipping and endovascular treatment (EVT) of unruptured intracranial aneurysms (UIAs), with a special focus on complications.
Methods
We retrospectively analyzed clinical and radiological data from patients who underwent surgery or EVT. Surgery was performed by one neurosurgeon, and EVT was performed by two neurointerventionists according to one hybrid neurosurgeon’s decision. Adverse events included the following: (1) decline of the modified Rankin Scale (mRS) score from 1 to 2 and (2) any unexpected neurological deficit or imaging finding affecting the prognosis and/or requiring additional procedures, medication, or prolonged hospital stay.
Results
Of the 1231 UIAs in 1124 patients, 625 (50.7 %) aneurysms were treated with surgery, and 606 (49.3 %) aneurysms were treated with EVT. The overall complication rate of UIA treatment was 3.2 %. The rate of adverse events was 2.4 %, and the rates of morbidity and mortality were 0.6 and 0.2 %, respectively. The rates of adverse events, morbidity, and mortality were not significantly different between surgery and EVT. The rate of hospital use for EVT was stationary over the years of the study. Posterior circulation in surgery, large aneurysms (>15 mm) in EVT, and stent- or balloon-assisted procedures in EVT were associated with the occurrence of complications. Poor clinical outcome (mRS of 3–6) was 0.8 % at hospital discharge.
Conclusions
Both UIA treatment modalities decided by one hybrid neurosurgeon showed low complication rates and good clinical outcomes in this study. These results may serve as a point of reference for clinical decision-making for patients with UIA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Unruptured intracranial aneurysms (UIAs) account for about 80–85 % of nontraumatic subarachnoid hemorrhages (SAHs). The overall prevalence rate of UIAs in the general population is 3.2 %, ranging from 0.4 to 7 % [31, 32]. The relative advantages of treatment remain controversial. UIAs are increasingly diagnosed using noninvasive imaging techniques, including computed tomography (CT) and magnetic resonance (MR) angiography. UIAs have a relatively benign natural history, with an annual incidence of rupture of 8–10 per 100,000 in the overall population [21, 27, 28], but rupture resulting in SAH is associated with mortality and morbidity rates of 30–67 and 15–30 %, respectively [26, 28].
The decision of whether to treat UIAs depends on various factors, including risks associated with the natural history of UIAs and those associated with preventive treatment. The International Study of Unruptured Intracranial Aneurysms (ISUIA), which provides the most recent evidence of the natural history and risk of rupture, suggests low overall rupture rates [11]. On the other hand, a precise evaluation of the risk of preventive treatment of UIA is difficult. The latest meta-analysis of cost-effectiveness [33] reports mortality and morbidity rates of 1.2–2.5 % and 4.4–7.0 % after surgery and 0.2–1.0 % and 4.7–9.9 % after EVT, respectively.
Several definitions for morbidity have been proposed, such as a modified Rankin Scale (mRS) score of 3, 4, or 5 [11], a Glasgow Outcome Scale score of less than 4 [16], an excellent, good, fair, poor scale score of ‘fair’ or ‘poor’ [16], permanent morbidity not present before surgery [26], or impairment of higher brain function as indicated by a Mini-Mental State Examination (MMSE) score less than 24 [11]. These definitions do not include minor adverse events such as cranial nerve palsy, fracture, transient ischemic attack, or epilepsy, which may strongly influence decisions regarding UIA treatment. Treatment of UIA is preventive and administered to premorbid healthy persons. Therefore, surgeons should consider all sequelae after UIA treatment, although few existing reports describe minor adverse events. In this study, we retrospectively investigated the surgical and endovascular risks for UIA at our hospital, paying particular attention to the risk of complications, including minor adverse events.
Materials and methods
Patients and management strategies
This study was approved by our institutional review board, which waived the requirement to obtain written informed consent from patients. From September 2008 to November 2014, a total of 1495 consecutive patients with 1687 aneurysms were treated at our institution. We included patients with UIA who underwent surgery or EVT. Remote SAH related to a different aneurysm (more than 30 days before) and retreated cases with a remotely ruptured aneurysm were also included. We excluded 371 patients with no imaging follow-up, extracranial aneurysms, follow-up loss less than 30 days after the procedure, or having an mRS score of greater than 2. The remaining 1124 patients harboring 1231 aneurysms (625 treated with surgery and 606 treated with EVT) were included in this study (Table 1). We retrospectively reviewed patient data from medical records and our neurosurgical database. The neurosurgical database was recorded by neurosurgical fellows and included the following information: sex, age, presence of hypertension, presence of diabetes, smoking status, family history, previous SAH, multiplicity of aneurysm, aneurysm location, size of aneurysm, procedure-related complications, image findings from postoperative CT or magnetic resonance imaging (MRI), mRS score at hospital discharge, and occurrence of morbidity and mortality. There were no significant differences in the basic characteristics of patients or their aneurysms between the included and excluded cases (p < 0.05).
The treatment procedure was decided by one hybrid neurosurgeon (Y.S. Shin). Preventive treatment with surgery or EVT was recommended based on the following considerations: (1) aneurysm size >5 mm, (2) aneurysm ≤5 mm if the patient had a family history of intracranial aneurysm, multiple aneurysms, a previously ruptured aneurysm, an aneurysm with a dangerous morphology including a daughter sac, or if the patient was worried about their aneurysm, and (3) general medical condition, complications, location and size of aneurysm(s), and the wishes of the patient if he or she was over 60 years of age. Surgery or EVT was selected depending on the aneurysm location, shape, vascular anatomy, and patient’s preference. Surgery was preferred for the middle cerebral artery or anterior choroidal aneurysms, whereas EVT was preferred for paraclinoid or basilar apex aneurysms.
Surgery was performed by a single neurosurgeon (Y.S. Shin), and EVT was performed by two neurointerventionists (Y.S. Shin and B.S. Kim). Both treatments were performed under general anesthesia. Beginning in 2013, surgery was performed under electrophysiological monitoring for motor or sensory function. Doppler flowmetry and/or indocyanine green angiography was used to prevent ischemic complications. The presence of ischemic complications, contusion, or epidural hemorrhage after surgery was assessed with CT immediately after surgery. Cerebral infarction and contusion were defined as hypodense and mixed density areas on CT, respectively. Follow-up CT occurred 5 days after surgery to evaluate any additional changes. EVT was performed under perioperative administration of antiplatelet agents for 5–7 days and heparin during the procedure. Coil placement proceeded until angiographically complete obliteration was achieved or no additional coils could be placed safely in the aneurysm. On the day after embolization, MRI (including T1, T2, fluid-attenuated inversion recovery, and diffusion-weighted images) was performed to evaluate ischemic or hemorrhagic complications, and skull X-ray was used to evaluate further coil compaction. MRI with MR angiography and skull X-ray were performed to check coil compaction 3–6 months after treatment.
Definition of adverse event, morbidity, and mortality
Mortality was defined as any death within 30 days of treatment. Adverse events and morbidity were evaluated at discharge and at follow-up appointments 1 and 6 months after treatment (for delayed complications, the reference point for mRS was the event day). Morbidity was defined as an mRS score of 3–5. Any adverse events or abnormal neuroangiographic findings related to the procedures were recorded regardless of symptom development. In cases of adverse events, procedural records and charts were extensively reviewed by the neurosurgeon.
Adverse events included the following:
-
(1)
decline of mRS from 1 to 2;
-
(2)
any unexpected neurological deficit or imaging finding affecting prognosis and requiring additional procedures, medication, or prolonged hospital stay, including cranial nerve palsy-associated coil mass effect, transient neurologic deficit resolving before discharge, postoperative seizure, subdural hygroma or epidural hemorrhage requiring an additional procedure (i.e., burr hole trephination or craniotomy for hematoma removal), thromboembolic infarct requiring an additional procedure (i.e., diagnostic angiography or intra-arterial thrombolysis), or wound infection.
We did not include the following adverse events:
-
(1)
minimal epidural hemorrhage (n = 8) or subdural hygroma/hemorrhage (n = 2) that did not present with clinical symptoms or require an additional procedure;
-
(2)
small contusion (n = 1), intracranial hemorrhage (n = 4), cerebellar hemorrhage (n = 6), or silent infarct (n = 8) that did not affect a patient’s symptoms or prognosis;
-
(3)
puncture site or wound hematoma/bleeding resolving without complication (n = 4);
-
(4)
intraprocedural rupture (n = 1) or vessel dissection (n = 2) that did not affect a patient’s prognosis.
Statistical analysis
Data were analyzed using the Statistical Package for the Social Sciences (SPSS version 19.0, SPSS Inc., Chicago, IL, USA). Continuous data were expressed as mean ± standard deviation (SD). The mean ages of patients in the surgery and EVT groups were compared using Student’s t-tests. χ 2 or Fisher’s exact tests were used to compare categorical values between surgery and EVT groups. Logistic regression models were used to evaluate independent associations between significant variables and treatment type. Odds ratio (ORs) and 95 % confidence intervals (CIs) were calculated. A p-value < 0.05 was considered statistically significant.
Results
Patient population, aneurysm characteristics, and modalities of treatment
Basic demographics of the patients are presented in Table 1. A total of 1231 UIAs from 1124 patients were treated; 625 (50.7 %) aneurysms were treated with surgical clipping, and 606 (49.3 %) aneurysms were treated with EVT. The patient population consisted of 823 women and 301 men (mean age: 56.19 ± 10.1 years, range: 19–81). The two groups showed no significant difference in age. A majority of patients (467/1124, 41.5 %) were in their 6th decade of age. The proportion of aneurysms ≤5 mm in size was 46.4 % (571/1231). Eight cases of giant aneurysm were treated by surgery; two cases were treated by direct clipping, and six cases were treated by trapping (surgical or endovascular) and low-flow bypass. Middle cerebral artery, anterior communicating artery, and anterior choroidal artery aneurysms were frequently treated by surgery, whereas the posterior communicating artery, vertebrobasilar system, and other internal carotid artery aneurysms were frequently treated with EVT. Thirty-two patients required retreatment because of aneurysm recurrence [6 patients (1.1 %) who had originally undergone surgery and 26 patients (4.7 %) who originally underwent EVT]; all of these patients were treated by EVT.
Complications
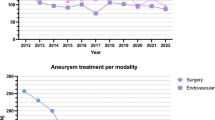
As shown in Fig. 1, the proportion of UIA patients treated with EVT was stable across the years of this study, ranging from 46 % in 2012 to 62 % in 2010 (mean: 49.3 %, p = 0.135). The overall complication rate of UIA treatment was 3.2 % [2.6 % for surgery (16/625) and 4.0 % for EVT (23/606)]. The rate of adverse events following UIA treatment was 2.4 % (30/1231). The rates of morbidity and mortality following UIA treatment were 0.6 % (7/1231) and 0.2 % (2/1231), respectively. As presented in Table 2, the percentage of overall complications of UIA treatment was also stable, ranging from 0 % in 2008 to 6 % in 2010 (p = 0.536). The rates of adverse events, morbidity, and mortality were not significantly different between the surgery and EVT groups. Details of complications are described in Table 3. Posterior circulation in surgery (p = 0.001), EVT other than catheter-based techniques, and large aneurysms in EVT cases (p = 0.013) were associated with the occurrence of complications as reported in Table 4.
Clinical outcomes
Thirty-nine (3.2 %, 39/1231) patients experienced complications associated with UIA treatment (16 surgery patients and 23 EVT patients). Among these patients, 30 (76.9 %, 11 surgery patients and 19 EVT patients) showed good outcomes (mRS scores of 0–2) at hospital discharge, and 31 patients (79.5 %, 12 surgery patients and 19 EVT patients) showed good outcomes at the 6-month follow-up. Table 5 summarizes the clinical outcomes of patients at discharge, 1-month follow-up, and 6-month follow-up. Poor clinical outcome (mRS scores of 3–6) was observed in 0.8 % of patients at discharge and 0.7 % of patients at the 6-month follow-up. There was no statistically significant difference in poor outcome rate between surgery and EVT patients at each follow-up (p = 0.604 at discharge, p = 0.358 at 1-month, p = 0.481 at 6-month).
Discussion
There are no prospective, randomized trials comparing the outcomes of conservative management of UIAs with preventive surgery or EVT. In the ISUIA, periprocedural morbidity and mortality rates were 12 % for surgery and 10 % for EVT [11]. However, these results should be interpreted with the understanding that the ISUIA was not designed to compare treatment modalities, and patients who received EVT were at a higher risk of morbidity and mortality than those who received surgery because of more advanced patient age, greater aneurysm size, and more aneurysms in the posterior circulation. Recent large studies also report relatively low rupture rates for small UIAs [12, 14, 29]. In the present study, the proportion of patients with aneurysms ≤5 mm in size was 46.4 %. These patients were treated because of a family history of intracranial aneurysm, multiple aneurysms, a previously ruptured aneurysm, an aneurysm with dangerous morphology, or because the patient was worried about their aneurysm. A recent study in Korea reports a similar proportion [17]. Since the publication of these studies, management strategies for UIAs have tended to be conservative. The number of patients undergoing radical treatment has been increasing in developed countries [13], partly because of an increase in the incidence of UIAs revealed by advanced image analysis such as 3-T MRI. However, there is no epidemiological evidence that radical treatment of UIA prevents SAH. Although the Japan Neurosurgical Society’s registry reports a 7 % reduction in radical treatment for SAH in the past decade [10, 30], the preventive effects of antihypertensive drugs and smoking cessation may have contributed to this reduction [30]. Because the prevention of SAH with UIA treatment cannot be proven at the national level, risk-benefit analyses at the individual patient level should be performed. Therefore, a comprehensive investigation of overall adverse events is required to justify these invasive treatments and to establish proper risk-benefit analyses for individual patients, considering the natural history of UIA and the risk of radical treatment.
Several studies report comparisons of the outcomes of EVT and surgery in single centers and multicenters. The overall mortality rate reported by Qureshi et al. varied from 1.36 to 6.3 %, and there was no significant difference in mortality between clipped and coiled populations [25]. There has been a growing interest in EVT because several studies suggest that it is associated with a lower procedural mortality rate, higher 1-year survival, lower incidence of vasospasm, and greater cost-effectiveness [2, 5, 8, 22, 31]. Brinjikji et al. [4] reviewed 64,043 patients with UIA using a national inpatient sample from 2001 to 2008 in the USA. Compared with surgical clipping, EVT was associated with significantly less morbidity (14 % for clipping vs. 4.9 % for EVT, p < 0.0001) and mortality (1.2 % for clipping vs. 0.6 % for coiling, p < 0.0001). Adverse outcomes (e.g., in-hospital death, discharge to a nursing home or rehabilitation hospital) were more frequent after surgery (25 %) than after EVT (10 %). Across this time period, the fraction of UIAs treated with coiling increased from 20 to 63 %, and adverse outcomes declined for EVT but not for surgery. The increasing use of EVT was associated with decreasing periprocedural morbidity and mortality. Although EVT is more frequently performed than surgery in the USA [1], in Japan, surgery was performed twice as often as coiling in 2010 [30]. In our series, the mortality rate was 0.2 % (2/1231), and the morbidity rate was 0.6 % (8/1231 patients), with no significant differences between treatment modalities, and the number of adverse events remained stable across the study period. The proportion of EVT-treated cases also remained stable across the investigation period. In addition to major morbidity, physicians should pay attention to minor adverse events, as the treatment of UIA is a preventive technique performed on premorbid patients. Therefore, even minor adverse events may influence quality of life or patient satisfaction. The few existing reports that describe minor adverse events after UIA treatment estimate that their frequency is 18–28 % [3, 9, 23, 30]. In our series, the overall complication rate of UIA treatment was 3.2 % (40/1231), and the rate of any unexpected events (including the cases that met our exclusion criteria) was 6.2 % (76/1231). The complications of EVT were associated with stent- or balloon-assisted techniques. For balloon-assisted techniques, the presence of a balloon in the parent vessel promotes stasis and can lead to thrombus formation or platelet aggregation or can be a nidus for thrombus formation [19]. For stent-assisted techniques, the gap between the stent and blood vessel acts as a thromboembolic hub and is associated with the occurrence of thromboembolic complications [7]. And it requires pre- and post procedural antiplatelet therapy, which has an additional risk of hemorrhage. With the use of these protective devices, an increased risk of thromboembolic complications is expected because of additional catheter movement [20].
In a prospective multicenter observational study, Brilstra et al. [3] measured the effect of surgery or EVT on functional health, quality of life, anxiety, and depression among UIA patients. At 3 months post-surgery, quality of life was worse than before treatment, and at 12 months post-surgery, quality of life had improved but had not completely returned to baseline. In the EVT group, quality of life after 3 months and 1 year was similar to that before treatment. Thus, in the short term, surgical treatment but not EVT seems to have a negative effect on functional health and quality of life. Previous single-center studies report shorter hospital stays and lower hospital costs associated with EVT, lending support to the use of this treatment in selected patients [24]. EVT seems more economical with lower complication rates than those previously indicated [3, 6]. Surgery results in lower retreatment rates but is associated with higher complication rates and higher initial costs. However, overall costs at 2 or 5 years are similar between treatment modalities because of frequent follow-up angiograms and retreatment in EVT patients [18].
Several guidelines have been proposed for facilities offering both treatment modalities. One of them suggests that surgery is preferred for young patients, low-risk patients, and patients with anterior circulation aneurysms [15]. Others suggest that EVT could be an initial treatment option for all ruptured and unruptured intracranial aneurysms [24]. Cooperation between surgery and EVT in one unit is emphasized to determine the most appropriate treatment modality for each patient. In the present study, one skilled hybrid neurosurgeon decided whether or not to treat patients and precisely chose treatment modalities considering the patient’s medical condition and preference, the shape and location of the aneurysm, and the patient’s vascular anatomy based on his long experience and thorough understanding of disease. Our results show that the relative proportions of chosen treatments remained stable during the study period, overall complication rates were similar between EVT and surgery, and the complication rate was very low even after including adverse events.
Treatment strategies for UIA are rapidly evolving. Advances in neurosurgical techniques (e.g., the development of operating microscopes, microsurgical instruments, improved clips, neuroanesthesia, and perioperative management of complications such as hydrocephalus and symptomatic vasospasm) enable neurosurgeons to treat most cerebral aneurysms, and surgery has been the predominant treatment for almost four decades. Since the development of detachable coils by the US Food and Drug Administration in 1995, treatment of intracranial aneurysms has evolved rapidly, mainly due to an increasing role of EVT with new developments in detachable coils, balloons, and stents. A skilled neurointerventionist can treat almost all intracranial aneurysms using EVT. However, one procedure cannot be said to be better than another without knowledge of the baseline characteristics that affect outcome. The risks of treating UIA by any procedure might be greater than the risk of rupture without treatment [24]. Physicians should choose among surgery, EVT, and observation for each patient based on their thorough knowledge of the natural history of UIA and objective data on the complication rates of treatments.
Limitations
This study has several limitations. First, it was based on patients who were treated in a combined unit at a single institute, and surgery or EVT selection was not randomized but precisely selected by a physician. Therefore, the findings cannot be used to determine the superiority of treatment modalities. Second, we did not investigate MMSE scores or quality of life because of a lack of data. Third, because the data were prospectively collected and retrospectively reviewed by a neurosurgeon, some minor neurological deficits could have been missed during data collection. Finally, our study is retrospective and susceptible to selection bias. Despite these limitations, this study provides important information about the determinants of mortality and complications associated with UIA treatment strategies.
Conclusions
The selection of treatment modality should be based on several factors including aneurysm characteristics as well as the patient’s medical and socioeconomic factors and individual preference. Physicians should freely choose between treatment modalities based on the patient’s best interests in a combined unit. In the present study, both types of UIA treatments decided by one skilled hybrid neurosurgeon showed low complication rates and good clinical outcomes. These results may serve as a point of reference for clinical decision-making in patients with UIA.
Abbreviations
- UIA:
-
unruptured intracranial aneurysm
- EVT:
-
endovascular treatment
- SAH:
-
subarachnoid hemorrhage
- mRS:
-
modified Rankin Scale
- CT:
-
computed tomography
- MR:
-
magnetic resonance
- MRI:
-
magnetic resonance imaging
- ISUIA:
-
International Study of Unruptured Intracranial Aneurysms
- MMSE:
-
Mini-Mental State Examination
- SD:
-
standard deviation
- OR:
-
odds ratio
- CI:
-
confidence interval
References
Alshekhlee A, Mehta S, Edgell RC, Vora N, Feen E, Mohammadi A, Kale SP, Cruz-Flores S (2010) Hospital mortality and complications of electively clipped or coiled unruptured intracranial aneurysm. Stroke 41:1471–1476
Barker FG 2nd, Amin-Hanjani S, Butler WE, Hoh BL, Rabinov JD, Pryor JC, Ogilvy CS, Carter BS (2004) Age-dependent differences in short-term outcome after surgical or endovascular treatment of unruptured intracranial aneurysms in the United States, 1996–2000. Neurosurgery 54:18–28, discussion 28–30
Brilstra EH, Rinkel GJ, van der Graaf Y, Sluzewski M, Groen RJ, Lo RT, Tulleken CA (2004) Quality of life after treatment of unruptured intracranial aneurysms by neurosurgical clipping or by embolisation with coils. A prospective, observational study. Cerebrovasc Dis 17:44–52
Brinjikji W, Rabinstein AA, Nasr DM, Lanzino G, Kallmes DF, Cloft HJ (2011) Better outcomes with treatment by coiling relative to clipping of unruptured intracranial aneurysms in the United States, 2001–2008. AJNR Am J Neuroradiol 32:1071–1075
Greving JP, Rinkel GJ, Buskens E, Algra A (2009) Cost-effectiveness of preventive treatment of intracranial aneurysms: new data and uncertainties. Neurology 73:258–265
Halkes PH, Wermer MJ, Rinkel GJ, Buskens E (2006) Direct costs of surgical clipping and endovascular coiling of unruptured intracranial aneurysms. Cerebrovasc Dis 22:40–45
Heller R, Calnan DR, Lanfranchi M, Madan N, Malek AM (2013) Incomplete stent apposition in Enterprise stent-mediated coiling of aneurysms: persistence over time and risk of delayed ischemic events. J Neurosurg 118:1014–1022
Hohlrieder M, Spiegel M, Hinterhoelzl J, Engelhardt K, Pfausler B, Kampfl A, Ulmer H, Waldenberger P, Mohsenipour I, Schmutzhard E (2002) Cerebral vasospasm and ischaemic infarction in clipped and coiled intracranial aneurysm patients. Eur J Neurol 9:389–399
Houkin K, Baba T, Minamida Y, Nonaka T, Koyanagi I, Iiboshi S (2009) Quantitative analysis of adverse events in neurosurgery. Neurosurgery 65:587–594, discussion 594
Igase K, Matsubara I, Igase M, Miyazaki H, Sadamoto K (2012) Initial experience in evaluating the prevalence of unruptured intracranial aneurysms detected on 3-tesla MRI. Cerebrovasc Dis 33:348–353
International Study of Unruptured Intracranial Aneurysms Investigators (1998) Unruptured intracranial aneurysms—risk of rupture and risks of surgical intervention. N Engl J Med 339:1725–1733
Investigators UJ, Morita A, Kirino T, Hashi K, Aoki N, Fukuhara S, Hashimoto N, Nakayama T, Sakai M, Teramoto A, Tominari S, Yoshimoto T (2012) The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med 366:2474–2482
Ishibashi T, Murayama Y, Saguchi T, Ebara M, Arakawa H, Irie K, Takao H, Abe T (2013) Justification of unruptured intracranial aneurysm repair: a single-center experience. AJNR Am J Neuroradiol 34:1600–1605
Ishibashi T, Murayama Y, Urashima M, Saguchi T, Ebara M, Arakawa H, Irie K, Takao H, Abe T (2009) Unruptured intracranial aneurysms: incidence of rupture and risk factors. Stroke 40:313–316
Komotar RJ, Mocco J, Solomon RA (2008) Guidelines for the surgical treatment of unruptured intracranial aneurysms: the first annual J. Lawrence pool memorial research symposium—controversies in the management of cerebral aneurysms. Neurosurgery 62:183–193, discussion 193–184
Kotowski M, Naggara O, Darsaut TE, Nolet S, Gevry G, Kouznetsov E, Raymond J (2013) Safety and occlusion rates of surgical treatment of unruptured intracranial aneurysms: a systematic review and meta-analysis of the literature from 1990 to 2011. J Neurol Neurosurg Psychiatry 84:42–48
Kwon SC, Kwon OK, Korean Unruptured Cerebral Aneurysm Coiling I (2014) Endovascular coil embolization of unruptured intracranial aneurysms: a Korean multicenter study. Acta Neurochir (Wien) 156:847–854
Lad SP, Babu R, Rhee MS, Franklin RL, Ugiliweneza B, Hodes J, Nimjee SM, Zomorodi AR, Smith TP, Friedman AH, Patil CG, Boakye M (2013) Long-term economic impact of coiling vs clipping for unruptured intracranial aneurysms. Neurosurgery 72:1000–1011, discussion 1011–1003
Layton KF, Cloft HJ, Gray LA, Lewis DA, Kallmes DF (2007) Balloon-assisted coiling of intracranial aneurysms: evaluation of local thrombus formation and symptomatic thromboembolic complications. AJNR Am J Neuroradiol 28:1172–1175
Lee JY, Seo JH, Cho YD, Kang HS, Han MH (2011) Endovascular treatment of wide-neck intracranial aneurysms using a microcatheter protective technique: results and outcomes in 75 aneurysms. AJNR Am J Neuroradiol 32:917–922
Menghini VV, Brown RD Jr, Sicks JD, O’Fallon WM, Wiebers DO (1998) Incidence and prevalence of intracranial aneurysms and hemorrhage in Olmsted County, Minnesota, 1965 to 1995. Neurology 51:405–411
Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA, Sandercock P, International Subarachnoid Aneurysm Trial Collaborative G (2005) International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet 366:809–817
Morita A (2010) Natural course, management risks and quality of life of patients with unruptured cerebral aneurysms: Japan unruptured cerebral aneurysm study II (UCAS II). J Neurosurg 113:A414–A414
Qureshi AI, Janardhan V, Hanel RA, Lanzino G (2007) Comparison of endovascular and surgical treatments for intracranial aneurysms: an evidence-based review. Lancet Neurol 6:816–825
Qureshi AI, Suri MF, Nasar A, Kirmani JF, Divani AA, He W, Hopkins LN (2005) Trends in hospitalization and mortality for subarachnoid hemorrhage and unruptured aneurysms in the United States. Neurosurgery 57:1–8, discussion 1–8
Raaymakers TW, Rinkel GJ, Limburg M, Algra A (1998) Mortality and morbidity of surgery for unruptured intracranial aneurysms: a meta-analysis. Stroke 29:1531–1538
Rinkel GJ, Djibuti M, Algra A, van Gijn J (1998) Prevalence and risk of rupture of intracranial aneurysms: a systematic review. Stroke 29:251–256
Schievink WI (1997) Intracranial aneurysms. N Engl J Med 336:28–40
Sonobe M, Yamazaki T, Yonekura M, Kikuchi H (2010) Small unruptured intracranial aneurysm verification study: SUAVe study, Japan. Stroke 41:1969–1977
Suzuki M, Yoneda H, Ishihara H, Shirao S, Nomura S, Koizumi H, Suehiro E, Goto H, Sadahiro H, Maruta Y, Inoue T, Oka F (2015) Adverse events after unruptured cerebral aneurysm treatment: a single-center experience with clipping/coil embolization combined units. J Stroke Cerebrovasc Dis 24:223–231
van Rooij WJ, Sluzewski M (2006) Procedural morbidity and mortality of elective coil treatment of unruptured intracranial aneurysms. AJNR Am J Neuroradiol 27:1678–1680
White PM, Wardlaw JM (2003) Unruptured intracranial aneurysms. J Neuroradiol 30:336–350
Yamashiro S, Nishi T, Koga K, Goto T, Kaji M, Muta D, Kuratsu J, Fujioka S (2007) Improvement of quality of life in patients surgically treated for asymptomatic unruptured intracranial aneurysms. J Neurol Neurosurg Psychiatry 78:497–500
Conflicts of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements) or nonfinancial interest (such as personal or professional relationships, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Comment
Whether patients with incidentally discovered intracranial aneurysm should be treated, regularly observed, or resume normal life with no further investigation is highly debated. Most of us balance many factors, using scores to support our decisions, but we all lack unbiased data because of the ethical impossibility of performing “a priori randomizations.” We therefore need to compare our results and benchmark as honestly as possible. Although the study reported herein is to some extend biased by its retrospective nature and by a significant fraction of patients being lost for follow-up, the figure reported should push us all to check our own results and report. A later literature metanalysis could then most probably improve our management. We fully support the presented concept of tailored treatment using the most appropriate technique to secure the aneurysm based on a multidisciplinary team. How good is your team? Compare your team performances to others! This is what this manuscript is about.
Philippe Bijlenga
Geneva, Switzerland
Rights and permissions
About this article
Cite this article
Song, J., Kim, BS. & Shin, Y.S. Treatment outcomes of unruptured intracranial aneurysm; experience of 1231 consecutive aneurysms. Acta Neurochir 157, 1303–1311 (2015). https://doi.org/10.1007/s00701-015-2460-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-015-2460-2