Abstract
Background
In order to reduce the consequences of narcotic-related side effects and provide effective analgesia after craniotomy, we conducted a randomized trial to compare the analgesic efficacy of preemptive scalp infiltrations with 1 % lidocaine and 0.5 % ropivacaine on the postoperative pain.
Methods
Sixty adult patients scheduled for craniotomy were enrolled. A solution contained 0.5 % ropivacaine and 1 % lidocaine (40 ml) was prepared. In group A, local anesthetic was injected throughout the entire thickness of the scalp before skin incision. In group B, it was injected before skin closure. Additional intravenous injection and patient-controlled analgesia with morphine was used to control postoperative pain if the verbal numerical rating scale > 4. Cumulative morphine consumption; numerical rating scale of pain at 1, 2, 4, 6, 8, 12, and 24 h; postoperative nausea, vomiting, and respiratory depression, were recorded for 24 h after the operation.
Results
Postoperative pain scores were lower in group A than in group B within the first 6 h after surgery. Mean time to demand for postoperative analgesic was statistically (p < 0.001) delayed in group A 300 (240, 360) min compared to group B 150 (105, 200) min. Ten patients in group A received morphine analgesia was half less than 21 patients in group B (p < 0.006). The median morphine consumption in 24 h after operation in group A 10.5 (8, 15) mg was less than that in group B 28 (22.5, 30.5) mg (p < 0.001).
Conclusions
Preemptive scalp infiltration with 0.5 % ropivacaine and 1 % lidocaine provides effective postoperative analgesia after craniotomy.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Recent studies have reported that 40–84 % patients suffered moderate to excruciating pain, where the maximum level occurred during the first 12 h after surgery [5, 31]. Under-treatment of postoperative pain after craniotomy may cause a series of adverse events, such as hypertension and postoperative intracerebral hemorrhage, which could negatively influence the final results [7, 31]. Traditionally, using narcotics can induce a moderate-to-high risk of postoperative nausea and vomiting (PONV) and consequences of analgesic-related side effects post craniotomy. Scalp infiltration with local anesthetic or regional anesthesia that is equally effective or superior to systemic drugs has been preferable in relieving postoperative pain after craniotomy [12]. Several clinical studies have shown that scalp infiltration with bupivacaine or ropivacaine decreased the incidence and severity of postoperative pain, but they usually apply local anesthetic before skin closure [4, 11, 21, 29].
Preemptive regional analgesia prior to surgical trauma theoretically achieves peripheral blockage of pain stimuli, which is more advantageous than treating pain after it occurs. Furthermore, it prevents the establishment of central hypersensitization by analgesic intervention [15, 18]. Pretraumatic versus posttraumatic infiltration with the purpose of pain prevention has not been addressed after craniotomy on adults. The aim of this study was to compare the analgesic efficacy of preincisional and postincisional scalp infiltrations with 1 % lidocaine and 0.5 % ropivacaine on postoperative pain after craniotomy.
Methods
Subject population
This study was approved by the Hospital Ethics Committee. Written informed consent was obtained from each patient. Sixty patients (age range, 18–60 years; weight, 56–98 kg) were scheduled for supratentorial craniotomy and classified as American Society of Anesthesiologists (ASA) physical status I-II were included. Patients were excluded if they had a history of ischemic heart disease, conduction disturbance, cognitive deficit, intellectual disability, or long-term use of certain medications (β-blockers, angiotensin-converting enzyme inhibitor, analgesics, sedatives, or tri-cyclic antidepressants); had impaired renal, hepatic, or pulmonary function; had a history of allergy to opiates or any other drug used in the study.
Anesthesia methods and surgery
During the preoperative visit, patients were taught how to indicate their postoperative pain levels based on a verbal numerical rating scale (NRS) ranging from 0 (no pain) to 10 (maximal pain). Also, patients were taught how to use the patient-controlled analgesia (PCA) system. Patients were randomly assigned by a computerized random-number generator list to one of two groups: group A (preemptive infiltration) and group B (before skin closure).
No premedication was administered. Intravenous and intra-arterial access was established and routine monitor (blood pressure, heart rate, electrocardiograms tracings, respiratory rate, and SpO2) was commenced before induction. General anesthesia was induced with Midazolam, 2 mg intravenous (IV), fentanyl, 2 μg/kg (IV), and propofol 1.5 mg/kg (IV), and tracheal intubation was facilitated with cis-atracurium 0.2 mg/kg (IV). After intubation, general anesthesia was maintained by propofol and remifentanil. The patients’ lungs were ventilated with 100 % oxygen to maintain normocapnia.
A local infiltration solution contained 20 ml of 2 % lidocaine with 20 ml of 1 % ropivacaine was prepared 5 ml of prepared solution was infiltrated at each pin site before skeletal fixation. In group A, the scalps were infiltrated with the remaining solution along the planned incision by a 22-gauge needle introduced to the skin at a 45°angle, penetrating deeply throughout the entire thickness of the scalp before skin incision. For group B, before skin closure, the solution was administered. Dexamethasone 10 mg IV, ondansetron 4 mg IV, and tramadol 100 mg IV (which was used to prevent the opioid- induce hyperalgesic of remifentanil) were administered before skin closure in both groups. The infusion of propofol and remifentanil was discontinued when the final suture was applied. The trachea was extubated after recovery of adequate spontaneous ventilation, and the patient was transferred to the post anesthetic care unit (PACU). The same group of neurological surgeons performed the surgery for all of the cases.
Data collection
Pain intensity was assessed for 24 h postoperatively and scored on NRS by anesthetic registrar. When the NRS reached 4 after extubation, morphine (2 mg) was titrated every 5 min until NRS decreased to < 4. Morphine titration was not given when RR was < 12/min or excessive drowsiness or sedation was observed. Then, PCA was started (1 mg of morphine as an IV bolus with a lockout interval of 5 min and a 4-h limit of 50 mg) after initial morphine titration. Patients were advised to push the analgesic demand button when they felt pain, and to repeat this until the pain was relieved. This PCA regimen was discontinued when it was no longer needed. All patients received paracetamol 1 g every 6 h. Non-steroidal anti-inflammatory drugs were not used.
The following were registered: NRS at 1, 2, 4, 6, 8, 12, and 24 h postoperatively; the patients on demand pain treatment; time to analgesic request; cumulative titration and PCA administered morphine consumption at 24 h postoperatively; respiratory depression, which was defined as a respiratory rate <10 breaths per minute or SpO2 was < 90 %. PONV was rated by patients as: 0, absent; 1, nausea not requiring treatment; 2, nausea requiring treatment; and 3, vomiting. Patients with nausea and vomiting were initially administered an IV bolus of 10 mg of metoclopramide, followed by 4 mg of ondansetron if metoclopramide was unsuccessful.
Statistical analysis
Sample size calculation was based on detection a difference in morphine consumption of at least 30 % between the two groups with α = 0.05, β = 0.2, and power = 80 %. All statistical analyses were performed using the SPSS version 13.0 statistical software (SPSS Inc., Chicago, IL, USA).Normally distributed variables were described using mean ± standard deviation (SD) and compared using unpaired two-tailed independent two-samples t test. Categorical variables were described using number (%) and compared using Pearson’s Chi-square test or Fisher’s exact test. Pain scores and abnormally distributed variables were described as median (25 %, 75 %) and compared by using the Mann–Whitney U test. p < 0.05 was considered statistically significant.
Results
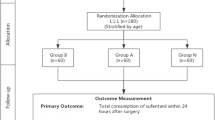
Sixty patients were recruited. Three patients’ surgeries were cancelled after randomization, five patients withdrew from the study after operation because they were admitted to ICU for ventilation, leaving 52 patients (25 in group A and 27 in group B; Fig. 1). Five patients had a frontal craniotomy, 13 temporal, seven fronto-temporal, 11 parietal-temporal, 12 parieto-occipital, and four posterior fossa craniectomy. Demographic characteristics did not differ between the two groups (Table 1).
Postoperative pain scores were significantly lower in group A than in group B within the first 6 h after surgery (Table 2). Mean time to postoperative analgesic request was significantly (p < 0.001) longer in group A 300 (240, 360) min compared to group B 150 (105, 200) min (p < 0.001). Thirty-one (60 %) patients received morphine titration and PCA in the PACU and 21 patients did not receive any morphine in the postoperative period. The number of supplementary analgesic requested (ten patients) in group A was about half the number requested (21 patients) in group B within 24 h after craniotomy; χ 2 = 7.695, p = 0.006). Furthermore, the median cumulative morphine consumption (PCA plus titration) was significantly less 10.5 (8, 15.0) mg in group A than 28 (22.5, 30.5) mg in group B within 24 h after surgery, p < 0.001.
No patients experienced respiratory inhibition. The PONV rate between the two groups was not different (in group A, the PONV rate for one patients was 1; in group B, the PONV rate for three patients was 1, for one patients was 2, and two patients vomited .χ 2 = 4.129, p = 0.248).
Discussion
During the 24 h after craniotomy, 60 % of patients experienced postoperative pain. This is in accordance with previous studies investigating postcraniotomy pain [5, 9]. This study shows that patients receiving preemptive scalp infiltration with 0.5 % ropivacaine and 1 % lidocaine for pain relief at the initial stage of craniotomy surgery consumed less morphine by PCA postoperatively and experienced better analgesia after surgery than before skin closure infiltration.
Effective pain control after craniotomy remains one of the greatest unmet challenges. Pain has a large impact upon patients and causes a state of discomfort that may directly influence recovery. However, analgesics must be administered judiciously after craniotomy, as anesthetic and opiate would increase the tendency for upper airway obstruction and respiratory depression. Scalp infiltration had been reported as an effective analgesia method. Biswas compared 20 patients received scalp infiltration with 25 ml of 0.25 % bupivacaine before incision and 21 patients received scalp infiltration with a similar volume of 0.9 % saline solution followed by 2 μg/kg of intravenous fentanyl before incision. They found a beneficial effect of bupivacaine scalp infiltration before craniotomy delayed the onset of the need for rescue analgesic [6]. Pakulski [25] reported that the infiltration of anesthesia along the projected line of skin incision in the head made it possible to administer lower doses of opiates before commencing surgery. Lawkoune [21] had found scalp infiltration with 0.375 % bupivacaine with epinephrine 1:200,000 or 0.75 % ropivacaine both could reduce the morphine consumption during the first two postoperative hours. Not all results were optimistic. Saringcarinkul [22] observed 50 patients who received wound infiltration before skin closure by either 0.5 % bupivacaine with adrenaline 1:400,000 or normal saline with adrenaline 1:400,000 then concluded surgical wound infiltration with 0.5 % bupivacaine with adrenaline decreased the incidence and severity of postoperative pain in patients undergoing supratentorial craniotomy, but only for the first hour after surgery. Batoz [4] observed 25 patients who received an infiltration of surgical site with 20 ml of 0.75 % ropivacaine at the end of the surgery. He found that there is limited interest in scalp infiltration with ropivacaine in the acute postoperative period. Different from them, in our article we improved the concentration and increased the volume of the local anesthetic agent, and added lidocaine. Ropivacaine is a new member of the long-acting amino amide class of local anesthetic agent and its lipophilicity associated with decreased potential for central nervous system toxicity and cardiotoxicity [19]. Furthermore, intradermally injected 0.25–0.75 % ropivacaine decreases local blood flows at the injection site, producing peripheral vasoconstriction effects [23]. Lidocaine has high tissue permeability and rapid diffuses from skin to adjacent tissue, especially exhibits a high affinity for nerves. We utilize the advantages of the rapid onset of lidocaine and the long duration of ropivacaine in our protocol, so the neurosurgeon did not need to wait for the onset of the local anesthetic. Considering the central nervous system toxicity and cardiotoxicity of bupivacaine and the untoward effects of accidental intravascular injection or systemic absorption of adrenaline in the neurosurgical patients, we did not use bupivacaine with epinephrine.
Studies have reported that preoperative analgesia, such as peripheral local anesthetic infiltration, is superior to postoperative administration. Furthermore, the analgesia effect persists beyond the presence of the analgesic drug in the biophase [2, 10, 13, 22, 31]. The conceivable mechanisms were as follows: First, the origin of most acute pain from surgical stimulation is the mechanical trauma to the local tissue and the subsequent acute inflammatory response [19]. Chemical mediators released by injury cause peripheral sensitization of primary sensory neurons and hyper-excitation of the spinal cord neuron, which causes low threshold A-β mechanoreceptors to begin transmitting painful sensations and results in central sensitization [18]. These sensitizations caused by operative tissue damage result in amplification of pain signals [8, 27, 32]. The local infiltration of anesthetic before surgical trauma aims to block nociceptive impulses from afferent C-fiber input to the dorsal horn, inhibit the development of central sensitization, and to decrease postsurgical pain from the surgical area. When a local anesthetic is applied before skin closure, peripheral and central sensitizations have already developed, so the postoperative pain relief is less pronounced. Clinical analgesia effect of pre-incision comparing with post-incision infiltration is pessimistic. The reasons for this are unsatisfactory afferent block, different intervention such as peripheral local anesthetic infiltration or nerve block, systemic NSAIDs or opioids used, and the variation in timing of drug dispensation [3, 20]. Kawamata et al. had a human experimental model to compare the analgesia effect between pre-incision and the post-incision. Human volunteers received local infiltration through the skin, fascia, and muscle in the forearm by a 4-mm incision. In the volunteer group, where the patients received local infiltration with lidocaine before incision, the acute, most intense pain was eliminated for up to 4 h after the incision, while those who received anesthetic after incision had significantly higher pain scores up to 4 h. They extrapolate that pre-incision with lidocaine reduces the excessive inputs from the injured peripheral nerves and suppressed the development of flare formation and the secondary peripheral and central sensitization. Pre-incision infiltration would temporarily stabilize the sensitized nerves in the injured area while the nerves were sensitized and the primary and secondary sensitization had fully developed. Pharmacokinetic studies of plasma levels of the agents after local anesthetic scalp infiltration indicate that systemic absorption occurs within minutes and in amounts of more than 50 % of the dose infiltrated due to the rich vascularity of the scalp. Procaine and lidocaine had been used as intravenous anesthetics in 19th century [1]. The presence of the synergism effect between the propofol and the local anesthetic is still unknown. We found that preoperative infiltration with 0.5 % ropivacaine and 1 % lidocaine reduced pain intensity during the first 6 h postoperatively and reduced morphine consumption during 24 h versus administered before skin closure. In addition, fewer patients required rescue analgesia (morphine) for pain control.
Local anesthetics have a known bactericidal and/or bacteriostatic effect, as well as a fungistatic effect [16]. Therefore, local anesthetic infusion could reduce the risk of infection. As evidenced by studies, local anesthetics serve not only as analgesic drugs but also as potential antimicrobial agents [17, 26, 28, 30]. Topical use of these anesthetic drugs may be useful in the management of cutaneous and vaginal candidosis. Kampe studied the effect of ropivacaine when mixed with sufentanil on the growth of the pathogens Staphylococcus aureus and Pseudomonas aeruginosa at room temperature. The combination of the local anesthetic and the opioid inhibited growth of P. aeruginosa significantly and multiplication of S. aureus [17]. Both bupivacaine and ropivacaine alone inhibited growth of Escherichia coli and Staphylococcus aureus [30]. The anesthetic molecule penetrates the membrane bilayer and accommodates in its hydrophobic interior. Membrane splitting results from insertion of several lipophilic molecules into the hydrophobic core of bacterial membranes. The mechanisms of the fungicidal action of the drugs are due to direct damage the cytoplasmic membrane and yeast metabolic impairment [26]. Johnson indicated the mechanism of action of antimicrobial activity of local anesthetics to a disruption of microbial cell membrane permeability, leading to leakage of cellular components and subsequent cell lysis. Rodrigues et al. suggested that local anesthetics inhibited fungal germ tube formation secondary to blockade of ionic channels [28].
Many studies have concluded that patients receiving ropivacaine infusions need lower amounts of opioids to control pain and have fewer opioid-related side effects [14, 24, 33]. In this study, we found no statistically significant difference regarding the PONV and respiratory depression between the two groups but the number of patients who used morphine analgesia was reduced in preemptive scalp infiltration group. No adverse reactions related to ropivacaine were observed in the study group. Complications associated with the use of local anesthesia, such as allergic reactions, local tissue, cardiovascular, central nervous system, and systemic toxicity, infection, changes in wound healing, or increased wound drainage were not observed in our study.
Conclusions
In conclusion, preemptive scalp infiltration with 0.5 % ropivacaine with 1 % lidocaine was significantly superior to before skin closure infiltration on postoperative analgesia after elective craniotomy.
References
Bao GX, Liu JJ, Zu TB (1987) Intravenous procaine is a useful addition to balanced anesthesia for thoracic surgery. J Cardiothorac Anesth 1:500–501
Barcznski M, Konturek A, Herman RM (2006) Superiority of preemptive analgesia with intraperitoneal instillation of bupivacaine before rather than after the creation of pneumoperitoneum for laparoscopic cholecystectomy: a randomized, double-blind, placebo-controlled study. Surg Endosc 20:1088–1093
Bashir MM, Shahzad MA, Yousaf MN (2014) Comparison of postoperative pain relief by intercostals block between pre-rib harvest and post-rib harvest groups. J Coll Physicians Surg Pak 24:43–46
Batoz H, Verdonck O, Pellerin C (2009) The analgesic properties of scalp infiltration with ropivacaine after intracranial tumoral resection. Anesth Analg 109:240–244
Benedittis DG, Lorenzetti A, Spagnoli D (1996) Postoperative pain in neurosurgery: a pilot study in brain surgery. Neurosurgery 38:466–470
Biswas BK, Bithal PK (2003) Preincision 0.25% bupivacaine scalp infiltration and postcraniotomy pain: a randomized double-blind, placebo-controlled study. J Neurosurg Anesthesiol 15:234–239
Bloomfield EL, Schubert A, Secic M (1998) The influence of scalp infiltration with bupivacaine on hemodynamics and postoperative pain in adult patients undergoing craniotomy. Anesth Analg 87:579–582
Coughlin SM, Karanicolas PJ, Emmerton-Coughlin HM (2010) Better late than never? Impact of local analgesia timing on postoperative pain in laparoscopic surgery: a systematic review and metaanalysis. Surg Endosc 24:3167–3176
Ersayli DT, Gurbet A, Bekar A (2006) Effects of perioperatively administered bupivacaine and bupivacaine-methylprednisolone on pain after lumbar discectomy. Spine 31:2221–2226
Gazoni FM, Pouratian N, Nemergut EC (2008) Effect of ropivacaine skull block on perioperative outcomes in patients with supratentorial brain tumors and comparison with remifentanil: a pilot study. J Neurosurg 109:44–49
Gray LC, Matta BF (2005) Acute and chronic pain following craniotomy: a review. Anaesthesia 60:693–704
Guilfoyle MR, Helmy A, Duane D (2013) Regional scalp block for postcraniotomy analgesia: a systematic review and meta-analysis. Anesth Analg 116:1093–1102
Gurbet A, Bekar A, Bilgin H (2008) Pre-emptive infiltration of levobupivacaine is superior to at-closure administration in lumbar laminectomy patients. Eur Spine J 17:1237–1241
Hansen MS, Brennum J, Moltke FB (2011) Pain treatment after craniotomy: where is the (procedure-specific) evidence? A qualitative systematic review. Eur J Anaesthesiol 28:821–829
Johnson SM, Saint John BE, Dine AP (2008) Local anesthetics as antimicrobial agents: a review. Surg Infect 9:205–213
Ju NY, Cui GX, Gao W (2013) Ropivacaine plus dexamethasone infiltration reduces postoperative pain after tonsillectomy and adenoidectomy. Int J Pediatr Otorhinolaryngol 77:1881–1885
Kampe S, Poetter C, Buzello S (2003) Ropivacaine 0.1% with sufentanil 1 microg/L inhibits in vitro growth of Pseudomonas aeruginosa and does not promote multiplication of Staphylococcus aureus. Anesth Analg 97:409–411
Katz J, Cohen L, Schmid R (2003) Postoperative morphine use and hyperalgesia are reduced by preoperative but not intraoperative epidural analgesia: implications for preemptive analgesia and the prevention of central sensitization. Anesthesiology 98:1449–1460
Kaufman E, Epstein JB, Gorsky M (2005) Preemptive analgesia and local anesthesia as a supplement to general anesthesia: a review. Anesth Prog 52:29–38
Kawamata M, Takahashi T, Kozuka Y (2002) Experimental incision-induced pain in human skin: effect of systemic lidocaine on flare formation and hyperalgesia. Pain 100:77–89
Lawkoune JD, Szekely B, Fermanian C (2005) Scalp infiltration with bupivacaine plus epinephrine or plain ropivacaine reduces postoperative pain after supratentorial craniotomy. J Neurosurg Anesthesiol 17:139–143
Lee IO, Kim SH, Kong MH (2001) Pain after laparoscopic cholecystectomy: the effect and timing of incisional and intraperitoneal bupivacaine. Can J Anaesth 48:545–550
Leone S, Di Cianni S, Casati A (2008) Pharmacology, toxicology, and clinical use of new long acting local anesthetics, ropivacaine, and levobupivacaine. Acta Biomed 79:92–105
Mordhorst C, Latz B, Kerz T (2010) Prospective assessment of postoperative pain after craniotomy. J Neurosurg Anesthesiol 22:202–206
Pakulski C, Nowicki R, Badowica B (2001) Effect of scalp infiltration with lidocaine on the circulatory response to craniotomy. Med Sci Monit 7:725–728
Pina-Vaz C, Rodrigues AG, Sansoenetty F (2000) Antifungal activity of local anesthetics against Candida species. Infect Dis Obstet Gynecol 8:124–137
Pogatzki-Zahn EM, Zahn PK (2006) From preemptive to preventive analgesia. Curr Opin Anaesthesiol 19:551–555
Rodrigues A, Pina-Vaz C, Mrdh PA (2000) Inhibition of germ tube formation by Candida albicans by local anesthetics: an effect related to ionic channel blockade. Curr Microbiol 40:145–148
Saringcarinkul A, Boonsri S (2008) Effect of scalp infiltration on postoperative pain relief in elective supratentorial craniotomy with 0.5% bupivacaine with adrenaline 1:400 000. J Med Assoc Thail 91:1518–1523
Tamanai-Shacoori Z, Shacorri V, Van Vo JM (2004) Sufentanil modifies the antibacterial activity of bupivacaine and ropivacaine. Can J Anaesth 51:911–914
Verchere E, Grenier B, Mesli A (2002) Postoperative pain management after supratentorial craniotomy. J Neurosurg Anesthesiol 14:96–101
Woolf CJ, Chong MS (1993) Preemptive analgesia: treating postoperative pain by preventing the establishment of sensitization. Anesth Analg 77:362–379
Yu H, Li ZY, Yu X (2013) Efficacy of postoperative continuous wound infiltration with local anesthesia after open hepatectomy. Zhonghua Yi Xue ZaZhi 93:2723–2726
Ethical standards
We state that all persons have given their informed consent prior to their inclusion in the study.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Song, J., Li, L., Yu, P. et al. Preemptive scalp infiltration with 0.5 % ropivacaine and 1 % lidocaine reduces postoperative pain after craniotomy. Acta Neurochir 157, 993–998 (2015). https://doi.org/10.1007/s00701-015-2394-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-015-2394-8