Abstract
Background
The dynamic relationship between pulse waveform of intracranial pressure (ICP) and transcranial Doppler (TCD) cerebral blood flow velocity (CBFV) may contain information about cerebrospinal compliance. This study investigated the possibility by focusing on the phase shift between fundamental harmonics of CBFV and ICP.
Methods
Thirty-seven normal pressure hydrocephalus patients (20 men, mean age 58) underwent the cerebrospinal fluid (CSF) infusion tests. The infusion was performed via pre-implanted Ommaya reservoir. The TCD FV was recorded in the middle cerebral artery. Resulting continuous ICP and pressure-volume (PV) signals were analyzed by ICM+ software.
Results
In initial stage of the CSF infusion, the phase shift was negative (median value = −11°, range = +60 to −117). There was significant inverse association of phase shift with brain elasticity (R = −0.51; p = 0.0009). In all tests, phase shift consistently decreased during gradual elevation of ICP (p = 0.00001). Magnitude of decrease in phase shift was inversely related to the peak-to-peak amplitude of ICP pulse waveform at a baseline (R = −0.51; p = 0.001).
Conclusions
Phase shift between fundamental harmonics of ICP and TCD waveforms decreases during elevation of ICP. This is caused by an increase of time delay between systolic peak of flow velocity wave and ICP pulse.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The pulse wave of intracranial pressure (ICP) is a response to pulsatile cerebral arterial blood inflow as the major input, cerebral venous blood outflow and compensatory movement of cerebrospinal fluid (CSF) within craniospinal fluid pools as minor inputs [1]. The ICP pulse waveform normally presents with three peaks: the P1, derived from propagation of arterial blood pressure (ABP); the P2, derived from arterial blood volume load; the P3, which is considered to be derived from the venous pulse [14].
The phase-contrast magnetic resonance (MR) imaging allows the detection of volume flow profiles of cerebral fluids (i.e. CSF and blood). With it, the sequence of events associated with a single-pulse fluids propagation into the brain can be monitored with impressive precision [2, 20]. The pulsatile cerebral arterial blood inflow induces various abnormal physiological response from the brain in pathological states. Thus, recent technological advancements such as phase-contrast MR imaging can be valuable in clinical assessment of cerebral hydrodynamics. However, such technology is not always readily available to all patients.
During CSF infusion tests, the physiologic signal being monitored is the CSF pressure, not the flow. On the other hand, transcranial Doppler ultrasonography (TCD) is able to monitor the profiles of arterial blood flow in main cerebral arteries. Though TCD itself allows the monitoring of only one aspect of cerebral hydrodynamics, the combination of flow velocity (FV) signal and the pressure signal from ICP monitoring can create unique opportunity to study important phenomena, such as cerebral autoregulation [10] or cerebral compartmental compliances [7].
A lot of interest was directed on the origin of ICP pulse waveform [7, 12, 21]. Nonetheless, the cerebral blood flow velocity (CBFV) has never been compared to the ICP pulse waveform, not to speak of in cases of changing mean ICP. The present study aims to address questions regarding phase shift between blood flow velocity and ICP pulse waveforms change during controlled elevation of mean ICP, by utilising the CSF infusion test.
Materials and methods
A total of 37 patients were admitted following clinical investigations to undergo a computerised CSF infusion test, a method for the accurate analysis of the traditional constant rate infusion of normal saline into the CSF space. The infusion tests were conducted to acquire the resistance to CSF outflow (Rout), which is known to be an effective predictor of response to shunting [5], though recent criticism challenges this point [22]. The patients were diagnosed as having normal pressure hydrocephalus (NPH); the infusion test is a routine clinical investigation for these patients, thus the approval from the local ethical committee was waived. The anonymised recordings of ICP and TCD blood flow velocity were retrospectively analysed as a part of a routine clinical audit.
Continuous ICP and blood flow velocity recordings
Infusion test allows the intraventricular infusion of normal saline into the CSF space, or subcutaneously positioned reservoir, connected to intraventricular catheter or shunt antechamber. Two hypodermic needles (25 gauge) were used to the infusion and the measurement of the pressure. One needle was connected to a pressure transducer via a stiff saline-filled tube, and the other one was to an infusion pump mounted on a purpose-built trolley, containing a pressure amplifier (Simonsen & Will, Sidcup, UK) and a personal computer. After 10 min of baseline measurements, the infusion of normal saline at a rate of 1.5 ml/min was infused until the resulting increase of ICP reached a steady plateau state, or when the ICP increased to 40 mmHg. Following the cessation of the saline infusion, ICP was continuously recorded until it decreased to baseline level.
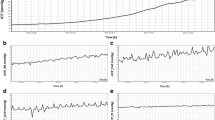
During the test, the CBFV waveform was also recorded using TCD at middle cerebral artery (Neuroguard; Medasonics, Fremont, CA, USA). A 2-MHz probe was fixed firmly on the skull using commercially available fixation system, and locked in stable position through the studies. Analogue output from the pressure monitors and the TCD unit (maximal frequency envelope) were processed via analogue-to-digital converter (DT 2814; Data Translation, Marlboro, USA) which fitted into IBM AT laptop computer (Amstrad ALT 386 SX; Amstrad, Brentwood, UK). Data were sampled, digitised and stored on the hard disk using software for waveform recording (WREC; W. Zabolotny, Warsaw University of Technology, Warsaw, Poland). Digital signals were then processed by a software specialised for assessment of CSF dynamics [19] (Fig. 1a). The CBFV and the ICP were continuously measured with 10-s windows.
a An example of continuous digital recording of ICP and CBFV during infusion study. Period of infusion of 1.5 ml/min is marked with a white field. ICP started to rise in response to infusion and reached a plateau which is used for calculation of the resistance to CSF outflow. The two peaks seen on ICP trend were vasogenic transients, associated with movement of the patient during the infusion study. b An example of shape and time delay between ICP and CBFV pulse waveform. These waves are from the period of infusion, when ICP was elevated. Note a delay of peak of ICP in comparison to the peak of CBFV wave
Derivation of indices
With continuous recording of ICP and CBFV, several indices that describe the cerebral hydrodynamics can be obtained. The Rout, which is indicative of CSF resorption capacity [8], can be calculated from a simple equation:
However, in many cases strong vasogenic waves or an excessive elevation of the pressure above the safe limit of 40 mmHg occurred, thus hindering the precise measurement of the final plateau pressure. The computerised infusion system and the ICM+ allows the approximation of the result, even in cases when the infusion was prematurely terminated [9]. The algorithm utilises a time series analysis for volume-pressure curve retrieval, the least mean square model fitting and an examination of the relationship between the pulse amplitude and the mean CSF pressure.
The model of cerebrospinal volume compensation investigated during the tests was described previously [17], and slightly modified later [18].
Apart from resting CSF pressure and the Rout, the pressure-volume curve was derived from the infusion tests and re-plotted on a logarithmic scale; the slope of this straight line gave the pressure-volume index (PVI) and the elastance coefficient E (defined as the inverse of PVI) [8]. The equations for deriving E and PVI are as follows;
Where Veff is the effective volume load, Pend the pressure at the end of infusion, Pb the baseline pressure before the infusion and P0 the reference pressure. The index describing the cerebrospinal compensatory reserve (RAP) [16] was also obtained as a correlation coefficient between changes in the pulse amplitude and mean CSF pressure.
The assessment of phase shift between CBFV and ICP by frequency analysis
The phase shift between CBFV and ICP pulse waves can be clearly visible (Fig. 1b). This is due to the relatively gradual slope of ICP pulse wave during the ascending phases, in comparison to the CBFV pulse wave or the time delay of the pulse wave peaks. Although the transmission from CBFV to ICP is undoubtedly non-linear, both waveforms can be processed by spectral analysis. Cross-spectrum CBFV and ICP signals show peaks at frequencies equivalent to the heart rate and its higher harmonics. The phase shift itself is chaotic in frequency bands where there is no power transmitted in both signals. However, at peaks of cross-spectrum, the phase shift is clearly regular and can be detected reliably (Fig. 2a). Repeating frequency analysis during the infusion test allows continuous analysis of the phase shift during the elevation of ICP observed within infusion period (Fig. 2b).
a Example cross-spectrum of CBFV and ICP signals. Peaks 1, 2, 3 and 4 show subsequent harmonics of the heart rate. In the lower panel there is a phase shift between CBFV (input) and ICP (output). It is chaotic between peaks of cross-spectrum, but close to the peaks it shows a consistent value. b Mean ICP, mean CBFV and phase shift, calculated as time varying values (10 s of averaging) during infusion study. Note that the phase shift, in this example decreases during elevation of ICP
Results
All subjects showed ventricular dilatation, with mean bi-caudate index = 0.35 (range = 0.23–0.67). Evidence of brain ischaemia was found in seven patients by MR (T1 and T2 weighted) or CT scans. The subjects were comprised of 17 women and 20 men. Mean age was 58 (range = 38–82). Common clinical symptoms of NPH, including gait disturbance, cognitive deterioration, memory loss or urinary incontinence were found in all subjects. Median values of baseline ICP, the Rout, elasticity and the pulse amplitude of ICP at baseline are given in Table 1.
In general, the baseline phase shift was negative, indicating that pulse of ICP at frequency of heart rate was lagging behind the CBFV. The median value of the phase shift angle was −11° (range = +60 to −117°). The baseline phase shift showed no significant correlations with mean ICP, Rout, or pulse amplitude of ICP. However, there was significant negative correlation between the phase shift angle and the brain elasticity (R = −0.51, p < 0.001) during the infusion test. Baseline phase shift angle also correlated with RAP (R = −0.38, p = 0.017). Both relationships indicates the lower phase shift angle is associated with less compliant cerebrospinal system (Fig. 3).
In all tests, the degree of phase shift consistently decreased during the gradual elevation of ICP due to the CSF infusion (p < 0.0001, Fig. 4a). The magnitude of decrease in phase shift was inversely correlated to the peak-to-peak amplitude of ICP pulse waveform (R = −0.51, p = 0.001, Fig. 4b).
Discussion
The concept of phase shift between neurophysiological signals is not new. There has been a number of studies that attempted assess cerebral autoregulation by investigating phase shift, mainly between TCD and ABP signals [4, 11]. While these previous studies provide valuable method for assessing cerebral autoregulation, there has been no study regarding phase shift between CBFV and ICP.
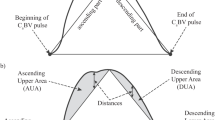
The interpretation of these findings would not be possible without the previous works on morphological analysis of ICP pulse waveform [13, 15]. According to the widely recognised three peaks of ICP theory, the first peak, P1, is a ‘percussion’ peak, associated with the transmission of ABP through elastic walls of cerebral arteries. Peaks P2 and P3 are associate with ‘volume transport’ of ABP and temporary rise of net cerebral volume [6]. At the baseline phase of the infusion test, the pressure-volume compensatory reserve is low. In this state the P1 dominates, or is equal to P2 and P3; the phase shift angle between ICP and CBFV is around zero, or even positive. With the infusion of normal saline, the compensatory reserve decreases, resulting in the domination of P2 over P1, and the decrease of phase shift between ICP and CBFV (Fig. 5).
As demonstrated in this study, the CSF infusion test is suitable for the manipulation of cerebral hydrodynamic components, and the findings of the presented study is in accordance with previously described sequence of cerebral hydrodynamics [3]. The arterial blood inflow causes a response in terms of visibly dominant P2 pulse peak, under the conditions of elevated brain elasticity (i.e. ‘stiffer’ brain). When the brain elasticity is low, the ‘percussion’ P1 peak dominates, causing the ICP pulse waveform to be more in phase or even precede the CBFV peak. During the infusion test, as compensatory reserve diminishes, phase shift further decreased.
At present, the results of the presented study do not carry a strong indication for practical application; that is, this study is designed and gave results as pure biophysics. Also, the study was conducted with a small number of subjects, who presented clinical symptoms of NPH; that is, patients with low elasticity were chosen. Further validation would be needed if the results of the study is to be applicable in wide variety of neurologically disturbed patients. Nonetheless, the results of this study suggest that the phase-shift analysis may prove to be a useful, readily available, indirect method for assessing brain elasticity. Considering the fact that both ICP and CBFV are often measured together, especially in situations when cerebral autoregulation or elasticity matters, the phase-shift analysis for ICP and CBFV signals is highly advised.
Conclusions
The phase-shift analysis between ICP and CBFV signals in NPH patients indicates the fluctuation of phase shift is significantly correlated with brain elasticity. Though it needs further validation in wider variety of neuropathological conditions, it showed potential to be a simple, convenient method for indirect assessment of brain elasticity.
References
Balédent O, Gondry-Jouet C, Meyer M-E, De Marco G, Le Gars D, Henry-Feugeas M-C, Idy-Peretti I (2004) Relationship between cerebrospinal fluid and blood dynamics in healthy volunteers and patients with communicating hydrocephalus. Invest Radiol 39:45–55
Balédent O, IDY-PERETTI I (2001) Cerebrospinal fluid dynamics and relation with blood flow: a magnetic resonance study with semiautomated cerebrospinal fluid segmentation. Invest Radiol 36:368–377
Baledent O, Fin L, Khuoy L, Ambarki K, Gauvin AC, Gondry-Jouet C, Meyer ME (2006) Brain hydrodynamics study by phase-contrast magnetic resonance imaging and transcranial color doppler. J Magn Reson Imaging 24:995–1004
Birch AA, Dirnhuber MJ, Hartley-Davies R, Iannotti F, Neil-Dwyer G (1995) Assessment of autoregulation by means of periodic changes in blood pressure. Stroke 26:834–837
Borgesen SE, Gjerris F (1982) The predictive value of conductance to outflow of CSF in normal pressure hydrocephalus. Brain 105:65–86
Cardoso ER, Rowan JO, Galbraith S (1983) Analysis of the cerebrospinal fluid pulse wave in intracranial pressure. J Neurosurg 59:817–821
Carrera E, Kim D-J, Castellani G, Zweifel C, Czosnyka Z, Kasprowicz M, Smielewski P, Pickard JD, Czosnyka M (2010) What shapes pulse amplitude of intracranial pressure? J Neurotraum 27:317–324
Czosnyka M, Czosnyka Z, Momjian S, Pickard JD (2004) Cerebrospinal fluid dynamics. Physiol Meas 25:R51–76
Czosnyka Z, Czosnyka M, Owler B, Momjian S, Kasprowicz M, Schmidt EA, Smielewski P, Pickard JD (2005) Clinical testing of CSF circulation in hydrocephalus. Acta Neurochir Suppl 95:247–251
Czosnyka ZH, Czosnyka M, Whitfield PC, Donovan T, Pickard JD (2002) Cerebral autoregulation among patients with symptoms of hydrocephalus. Neurosurgery 50:526–533
Diehl RR, Linden D, Lucke D, Berlit P (1995) Phase relationship between cerebral blood flow velocity and blood pressure A clinical test of autoregulation. Stroke 26:1801–1804
Eide P, Brean A (2006) Intracranial pulse pressure amplitude levels determined during preoperative assessment of subjects with possible idiopathic normal pressure hydrocephalus. Acta Neurochir (Wien) 148:1151–1156
Hu X, Glenn T, Scalzo F, Bergsneider M, Sarkiss C, Martin N, Vespa P (2010) Intracranial pressure pulse morphological features improved detection of decreased cerebral blood flow. Physiol Meas 31:679
Hu X, Subudhi AW, Xu P, Asgari S, Roach RC, Bergsneider M (2009) Inferring cerebrovascular changes from latencies of systemic and intracranial pulses: a model-based latency subtraction algorithm. J Cereb Blood Flow Metab 29:688–697
Kasprowicz M, Asgari S, Bergsneider M, Czosnyka M, Hamilton R, Hu X (2010) Pattern recognition of overnight intracranial pressure slow waves using morphological features of intracranial pressure pulse. J Neurosci Meth 190:310–318
Kim D-J, Czosnyka Z, Keong N, Radolovich DK, Smielewski P, Sutcliffe MP, Pickard JD, Czosnyka M (2009) Index of cerebrospinal compensatory reserve in hydrocephalus. Neurosurgery 64:494–502
Marmarou A, Shulman K, Rosende RM (1978) A nonlinear analysis of the cerebrospinal fluid system and intracranial pressure dynamics. J Neurosurg 48:332–344
Sliwka S (1980) A clinical system for the evaluation of selected dynamic properties of the intracranial system. Academy of Sciences, Warsaw (in Polish)
Smielewski P, Czosnyka Z, Kasprowicz M, Pickard JD, Czosnyka M (2012) ICM+: a versatile software for assessment of CSF dynamics. Acta Neurochir Suppl 114:75–79
Tain R-W, Alperin N (2009) Noninvasive intracranial compliance from MRI-based measurements of transcranial blood and CSF flows: indirect versus direct approach. IEEE Trans Biomed Eng 56:544–551
Wagshul ME, Kelly EJ, Yu HJ, Garlick B, Zimmerman T, Egnor MR (2009) Resonant and notch behavior in intracranial pressure dynamics: Laboratory investigation. J Neurosurg Pediatr 3:354–364
Wikkelso C, Hellstrom P, Klinge PM, Tans JT (2013) The European iNPH Multicentre Study on the predictive values of resistance to CSF outflow and the CSF Tap Test in patients with idiopathic normal pressure hydrocephalus. J Neurol Neurosur PS 84:562–568
Acknowledgments
This is European Community sponsored study, under the auspice of inter-regional cooperation between Amiens and Cambridge. D.J.K. and H.S.K. are sponsored by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2013R1A1A1004827). ICM+ software used in this study is licensed by the University of Cambridge (Cambridge Enterprise). M.C. and P.S. have a financial interest in a part of its licensing fee.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, DJ., Czosnyka, M., Kim, H. et al. Phase-shift between arterial flow and ICP pulse during infusion test. Acta Neurochir 157, 633–638 (2015). https://doi.org/10.1007/s00701-015-2353-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-015-2353-4