Abstract
Background
The postoperative biological behavior of nonfunctioning pituitary adenomas (NFPAs) is variable. Some residual NFPAs are stable long-term, others grow, and some recur despite complete removal. The usual histological markers of tumor aggressiveness are often similar between recurring, regrowing, and stable tumors, and therefore are not reliable as prognostic parameters. In this study, the clinical utility of proliferation indices (labeling index, Li) based on immunohistochemistry targeted at antigens Ki-67 and High-mobility group A1 (HMGA-1) for prediction of NFPA prognosis was investigated.
Methods
Fifty patients with NFPAs were investigated. In each patient, Ki-67 and HMGA-1 Li were evaluated. Based on postoperative magnetic resonance images, patients were classified as tumor-free (18 patients), or harboring a residual tumor (32 patients). The latter group was further subdivided into groups with stable tumor remnants (11 patients) or progressive tumor remnants (21 patients).
Results
The median follow-up period was 8 years. No significant relationship between HMGA-1 Li and residual tumor growth was found. Growing residual tumors showed a trend towards higher Ki-67 Li compared with stable ones (p = 0.104). All tumor remnants with Ki-67 Li above 2.2 % were growing. The relationship between residual tumor growth and Ki-67 Li exceeding the cutoff value of 2.2 % was significant (p = 0.01 in univariate, p = 0.044 in multivariate analysis).
Conclusions
The prognostic significance of the HMGA-1 antigen was not confirmed. In contrast, the Ki-67 Li provides useful and valuable information for the postoperative management of NFPAs. In residual adenomas with a Ki-67 Li above 2.2 %, regrowth should be expected, and these tumors may require shorter intervals of follow-up magnetic resonance imaging (MRI) and/or early adjuvant therapy. Future larger studies are needed to confirm the results of this study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The postoperative biological behavior of nonfunctioning pituitary adenomas (NFPAs) is variable. While complete tumor removal (CTR) represents a cure in the majority of patients, in some patients NFPAs recur despite CTR. Some residual NFPAs are stable in the long term, while others grow [17]. Considering the variability of NFPAs prognosis, there is a need for informative predictive factors to optimize postoperative management. However, the usual morphologic markers of tumor aggressiveness, including pleomorphism, nuclear atypia, increased cellularity, and mitotic activity, correlate poorly with the prognosis of pituitary adenomas [22]. Pituitary adenomas are not amenable to any form of histopathological grading, such as that available for astrocytic tumors, that could reliably distinguish aggressive variants from indolent ones [22].
A wide spectrum of NFPA biomarkers have been studied with regard to their predictive value for tumor recurrence/regrowth [49, 50]. However, a fully reliable predictor of tumor behavior has not been identified. Despite the fact that the antigen Ki-67 is routinely immunohistochemically assessed in pituitary adenomas, the prognostic significance of its expression is not generally accepted [5, 48]. The results of studies exploring the possible use of Ki-67 as a predictor of tumor recurrence/regrowth were inconsistent. High-mobility group A1 (HMGA-1) proteins were shown to play a significant role in the pathways that lead to pituitary tumor evolution [9, 10]. They were considered promising novel molecular markers of pituitary adenoma proliferation and invasiveness [59]. However, the potential association between HMGA-1 expression and tumor recurrence/regrowth has not yet been investigated. Thus, the aim of this study was to evaluate the predictive value of the expression of proliferation-associated antigens Ki-67 and HMGA-1 regarding postoperative regrowth and recurrence of NFPAs.
Patients and methods
During the years 2000–2006, 127 patients with newly diagnosed NFPAs were operated on in our department. All patients were followed up with magnetic resonance imaging (MRI) in our hospital during the first 2 years after surgery. After this, the follow-up of 62 patients residing in distant locations was performed by a local neurologist or oncologist. The remaining 65 patients continued to be followed up in our department.
The inclusion criteria were: 1) postoperative follow-up with MRI performed in our center; 2) availability of NFPA tissue specimens for new immunohistochemical analyses; 3) no postoperative radiotherapy, radiosurgery, or any other form of adjuvant treatment that could have changed the natural behavior of the residual tumor. Fifty patients (31 males, 19 females) were included in the study according to these criteria.
Surgical reports were used for evaluation of tumor consistency and bleeding tendency. The consistency was evaluated as soft if tumor removal with suction was possible. If the suction of the majority of tumor tissue was not possible, but resection was achievable with a curette, then consistency was evaluated as moderately firm. Consistency was considered firm if tumor resection with a curette was hard or not safely achievable. If bleeding from tumor tissue was unusually heavy, the tumor was evaluated as having a bleeding tendency. The bleeding tendency was not stated if the source of excessive bleeding was a normal structure (mucosa or cavernous sinus), and not the tumor tissue itself.
Adenomas were considered to be invasive if signs of dural, bone, and/or cavernous sinus invasion were noted during surgery. Only if the surgical report was inconclusive was preoperative MRI investigation used for evaluation of invasiveness. In these cases, tumor invasiveness into cavernous sinus was evaluated according the criteria defined by Knosp et al. [25] and Cottier et al. [4]. Preoperative MRI scans were also used for maximal tumor diameter measurement.
The extent of resection was evaluated according to the first postoperative MRI investigation. CTR was defined as the absence of the residual tumor. A tumor remnant was defined as a tissue mass with the same MRI characteristics as the tumor before the resection, localized exclusively in places where the tumor was identified preoperatively, which could not be explained as a normal structure or implanted material. In patients with a residual tumor, maximal residual tumor diameter was measured on the first postoperative MRI scans; the presence or absence of residual tumor growth was evaluated on the follow-up MRI scans. An unequivocal increase of tumor size in any dimension was evaluated as residual tumor growth. Recurrence was defined as tumor reappearance after previous CTR, confirmed on postoperative follow-up MRI scans.
Definition of patient subgroups
According to the postoperative MRI scans, patients were divided in two main subgroups: Patients with tumor remnants and tumor-free patients. The group of patients with a tumor remnant was further divided into two subgroups: patients with stable tumor remnants and patients with growing tumor remnants. The group of tumor-free patients was further divided into two subgroups: patients with recurrence and patients without recurrence (Fig. 1).
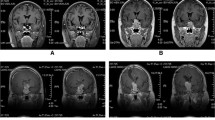
Follow-up MR scans a1 preoperative MR image revealing a tumor in a 51-year-old man from the tumor-free group. a2 First postoperative MR image demonstrating complete tumor removal. a3 MRI scan obtained 10 years after surgery shows no recurrence. b1 Preoperative MR image revealing a tumor in a 46-year-old woman from the tumor-free group. b2 First postoperative MR image demonstrating complete tumor removal. b3 MRI scan obtained 10 years after surgery shows a distinct tumor recurrence in the left part of the sella. c1 Preoperative MR image revealing a tumor in a 64-year-old man from the residual tumor group. c2 First postoperative MR image demonstrating a tumor remnant in the right cavernous sinus. c3 MRI scan obtained 11 years after surgery shows no regrowth of the residual tumor. d1 Preoperative MR image revealing a tumor in a 39-year-old man from the residual tumor group. d2 First postoperative MR image demonstrating a tumor remnant at the right cavernous sinus. d3 MRI scan obtained 8 years after surgery shows distinct regrowth of the residual tumor
In patients with a growing remnant or patients with tumor recurrence, the follow-up period was defined as the time between surgery and MRI, which served as an indication for the second therapeutic intervention. In patients with stable (non-growing) remnants and for patients after CTR without recurrence, the follow-up period was defined as the time between surgery and the last MRI scan.
To compare our results with previously published data, patients were experimentally combined into a group including patients with growing remnants and patients with recurrence; and into another group including patients with stable remnants and patients without recurrence after CTR.
Immunohistochemical studies
Tissue specimens of all patients were investigated. For adenoma tissue identification, standard hematoxylin and eosin-stained sections were used. For assessment of hormone expression, sections of formalin-fixed and paraffin-embedded tumor specimens were incubated with antibodies against pituitary hormones. Anti-adrenocorticotropin (MS-452-R7, Lab Vision Corporation, Fremont, CA, USA), anti-prolactin (MS-9083-R7, Lab Vision), and anti-human growth hormone (MS-1328-R7, Lab Vision) antibodies were used. Immunopositivity for cytoplasmic immunoreactivity was evaluated by two pathologists (J.B. and B.R.).
The presence of Ki-67 and HMGA-1 antigens within adenoma cell nuclei was detected immunohistochemically using the MIB-1 antibody (anti-Ki-67, RM9106-R7, Lab Vision) and HMGA-1 antibody (anti-HMGA-1, LS-B2037, LifeSpan Biosciences, Seattle, WA, USA). MIB-1 antibody and HMGA-1 antibody binding was apparent as nuclear staining. Tumor cell proliferation rate was assessed using the labeling index (Li) of Ki-67 and HMGA-1 (Fig. 2). The Li of both antigens was determined by counting the number of positive cells in a total of 1,000 tumor cells observed in the maximally stained region under high magnification (× 400). Photos of the observed fields were taken and all counted cells were marked manually (to count each positive or negative nucleus only once).
Light microscopy photographs of non-functioning pituitary adenoma tissue sections (original magnification × 400). a1 Immunostaining for Ki-67 in a tumor in a 56-year-old man from the stable residual tumor group. The Ki-67 Li was 1.2 %. a2 Immunostaining for Ki-67 in a tumor in a 46-year-old woman from the progressive residual tumor group. The Ki-67 Li was 3.1 %. b1 Immunostaining for HMGA-1 in a tumor in a 42-year-old man from the progressive residual tumor group. The HMGA-1 Li was 3.2 %. b2 Immunostaining for HMGA-1 in a tumor in a 50-year-old man from the stable residual tumor group. The HMGA-1 Li was 91.6 %
Detection of immunostaining was performed using diaminobenzidine chromogen (TA-060-HDX, Lab Vision). The pathologists evaluating the specimens were blinded to the clinical characteristics of the patient and biological behavior of the tumor.
Statistical analyses
Statistical analyses were conducted using IBM SPSS version 20.0 (IBM, Armonk, NY, USA). For the multivariate analysis identifying the determinants of variability of the observed parameters, the General Linear Model function was used. Univariate analysis was conducted using the non-parametric Mann–Whitney U-test and chi-square test. Probability-values <0.05 were considered statistically significant.
Results
The median follow-up period for the whole cohort was 8 years (range 2–11). The median follow-up period for the group of patients with a residual tumor and for the tumor-free group is presented in Table 1.
Factors associated with tumor invasiveness
Twenty-seven NFPAs (54 %) in the cohort were invasive. A significant relationship between invasiveness and maximal tumor diameter was found in univariate analysis (t = -3.136, p = 0.003), and in multivariate analysis (Table 2). Invasiveness was not associated with Ki-67 Li or HMGA-1 Li in univariate or multivariate analyses.
Factors determining complete tumor removal and residual tumor size
Postoperative MRI investigation revealed residual tumors in 32 patients (64 %), and CTR was achieved in 18 patients (36 %). The clinicopathological features of these patients are shown in Table 1. A significant positive association between tumor invasiveness and incomplete resection was found in univariate analysis (χ2 = 11.434; p = 0.001), and in multivariate analysis (Table 3).
Factors influencing residual tumor size were investigated using multivariate analysis (Table 3). Maximal preoperative tumor diameter was identified as a sole determinant of maximal residual tumor diameter (p < 0.001).
Factors associated with residual tumor growth and tumor recurrence
The clinicopathological features of patients with stable and growing remnants are shown in Table 4. A significant negative association between age and residual tumor growth was found in univariate analysis (χ2 = 44.5; p = 0.004). The relationship between HMGA-1 Li and residual tumor growth was not significant. Quantitative analysis of the association between Ki-67 Li and residual tumor growth showed a trend of growing remnants towards higher Ki-67 (U = 74; p = 0.104). The maximal Ki-67 Li of stable remnants was 2.2 %; all nine residual tumors with a Ki-67 Li above 2.2 % were growing (Fig. 3). When residual tumors were grouped into those with a Ki-67 Li ≤2.2 % and into those that exceeded this value, i.e. when the Ki-67 Li was used not as a quantitative variable, but exceeding the Ki-67 Li cutoff value of 2.2 % was used instead, the association between residual tumor growth and exceeding the cutoff value was significant in univariate analysis (χ2 = 6.559; p = 0.01). No cutoff value for the HMGA-1 Li could be identified (Fig. 3).
Upper diagram Scatter dot plot comparing Ki-67 Li values between growing tumor remnants and stable tumor remnants. All residual tumors with a Ki-67 Li > 2.2 % were growing. All stable residual tumors had a Ki-67 Li ≤ 2.2 %. Lower diagram Scatter dot plot comparing HMGA-1 Li values between growing tumor remnants and stable tumor remnants; no cutoff value could be identified
Factors associated with residual tumor growth were investigated in two multivariate analyses. In the first analysis, Ki-67 Li was used as a quantitative variable (Table 5). As in the univariate analysis, the first multivariate analysis showed only a trend of growing remnants towards higher Ki-67 Li (F = 3.451; p = 0.07). In the second multivariate analysis, exceeding the cutoff value of Ki-67 Li 2.2 % was used as a qualitative variable (Table 6). The significant association between residual tumor growth and exceeding the cutoff value found in the univariate analysis was confirmed (F = 4.673; p = 0.044). A significant association between age of patient and residual tumor growth was not confirmed by the multivariate analysis. Similarly, no association was found between residual tumor growth and gender, tumor size, hormonal immunophenotype, or invasiveness.
In the group of 18 tumor-free patients, only three recurrences were diagnosed (16.7 %), and NFPAs in 15 patients (83.3 %) did not recur during the follow-up period. Recurrences were diagnosed 7, 10, and 11 years after surgery. Two recurrences were subsequently treated by stereotactic radiosurgery, and one patient underwent re-operation. Owing to the low incidence of recurrences, identification of factors associated with tumor recurrence was not possible. In the group of 15 recurrence-free patients, five NFPAs (33 %) had a Ki-67 Li higher than 2.2 % (range 2.9–11.9 %).
When the cohort was experimentally divided into one group consisting of patients with a growing tumor remnant and patients with recurrence, and into another group consisting of patients with a stable tumor remnant and patients with no recurrence after CTR, no significant difference in HMGA-1 Li or Ki-67 Li was found between the two groups (in univariate and multivariate analysis). Similarly, no significant difference (in univariate and in multivariate analysis) was found when exceeding the cutoff value of Ki-67 Li 2.2 % was compared between the two groups.
Relationship between Ki-67 Li and HMGA-1 Li
In univariate analysis, a trend towards a significant association between Ki-67 Li and HMGA-1 Li was found (r = 0.244; p = 0.088). After adjustment for age and sex, a significant relationship between Ki-67 Li and HMGA-1 Li (F = 5.404; p = 0.029) was confirmed in multivariate analysis involving tumor size, invasiveness, consistency, bleeding tendency, and hormonal immunophenotype.
Discussion
Complete NFPA resection should be attempted in all cases when safely achievable as it has a clear impact on prognosis. While recurrence rates of NFPAs after CTR without adjuvant treatment were reported between 0 and 29 %, regrowth of residual NFPAs is much more common. Prior studies show regrowth rates in 38–95 % of cases without adjuvant treatment [6, 11, 17, 28, 31, 41, 53, 54, 56, 58, 60, 63]. Although these results were reported after various follow-up periods, it is clear that (despite occasional recurrences) CTR represents a cure in a substantial number of patients. Nevertheless, some residual NFPAs can be stable in the long term, and therefore, prophylactic postoperative radiotherapy is not justified in all cases of residual NFPAs [6, 22].
In addition to the variability of the biological behavior of NFPAs, the postoperative management of these tumors is complicated by the absence of a reliable prognostic parameter. Thus, continuous follow-up with MRI is necessary in all patients after NFPA resection, to detect tumor recurrence/regrowth before clinical signs of mass effect appear. However, because of the uncertain prognosis, determination of the optimal frequency of postoperative MRI controls is challenging.
The 2004 edition of the World Health Organization classification of pituitary adenomas included a new adenoma entity that should have borderline or uncertain behavior: an “atypical” adenoma [29]. This atypical variant is defined by invasive growth, Ki-67 Li greater than 3 %, excessive p53 immunoreactivity, and increased mitotic activity [29]. However, atypical adenomas meeting these criteria represent only a small proportion of all pituitary adenomas. In prior studies, their occurrence has been reported to be between 2.7 and 15 % [46, 51, 65, 67], which is far less than reported pituitary adenoma recurrence/regrowth rates. Thus, more sensitive prognostic parameters indicating probability of tumor recurrence/regrowth are needed. Such a factor would be helpful in optimizing the frequency of postoperative radiographic follow-up intervals, and could also provide crucial information for the timing of radiosurgery, radiotherapy, or re-operation [60]. In the present study, we analyzed the prognostic potential of the proliferation-associated antigens Ki-67 and HMGA-1.
Prognostic significance of the Ki-67 antigen
The expression of the nuclear protein Ki-67 is limited to the mitotic phase of the cell cycle [15]. The Li of Ki-67 (i.e. % of Ki-67 positive nuclei) correlates with growth potential in a variety of human tumor types and often provides valuable prognostic information [2]. Development of the monoclonal antibody MIB-1, which enables detection of the Ki-67 antigen in paraffin embedded tissue, contributed to the routine immunohistochemical assessment of Ki-67 Li in pituitary adenomas. However, the prognostic significance of the Ki-67 antigen in pituitary adenomas remains unclear [5, 48]
The relationship between pituitary adenoma Ki-67 Li and tumor invasiveness has been explored in multiple studies. However, the results are not consistent; while the majority of studies confirmed a significant association [24–26, 32–34, 40, 42, 52, 55, 61, 62, 68], others studies did not [1, 16, 19, 23, 27, 30, 39, 44, 51, 64]. The divergent results of previously published studies can be partially explained by the methodology of Ki-67 Li assessment [48, 61] (digital image analysis or manual cell counting, and different methods of tissue processing), but also by the methods of assessment of tumor invasiveness [48]. No significant association between pituitary adenoma Ki-67 Li and tumor invasiveness was found in our study. Invasiveness was evaluated on the basis of the relationships of the tumor to bony structures, dura, and the cavernous sinus, as seen through the operative microscope. The vast majority of patients were operated on using a microsurgical transsphenoidal approach, which often does not allow for inspection of the whole medial cavernous sinus wall. For these cases, we used MRI criteria of cavernous sinus invasion defined by Knosp et al. [25], and Cottier et al. [4]. Similar criteria for adenoma invasiveness evaluation have been used in previously published studies, where invasiveness was evaluated according to intraoperative findings and preoperative MRI [17, 19, 23, 31, 43, 51, 52, 60, 62], or according to preoperative MRI only [1, 14, 16, 30, 35, 39, 42, 68]. Our results can therefore be compared with data from relevant literature. However, using endoscopy for direct medial cavernous sinus wall visualization seems to be a more reliable method of evaluation of cavernous sinus invasion compared with indirect MRI criteria [36]. Indeed, correlation of MRI criteria [25] with the perioperative aspect of the medial wall of the cavernous sinus is currently being examined in an ongoing prospective study [36]. Another important fact regarding adenoma invasiveness evaluation is the existing evidence that the medial wall of the cavernous sinus is weaker compared with its superior and lateral walls, and that in some humans the medial cavernous sinus wall has small histological defects [66]. Therefore, the growth of the adenoma into the cavernous sinus does not necessarily mean that the tumor is actually invasive [66].
As expected, we found a strong association between invasiveness and incomplete tumor resection. However, tumor invasiveness was not associated with residual adenoma growth. Although a positive association between tumor invasiveness and potential to regrow or recur has been repeatedly reported [3, 17, 30, 43, 51, 60], several other reports did not find a significant association [7, 14, 31, 35, 45]. Considering the fact that invasive macroadenomas may remain indolent, it is questionable whether tumor invasiveness can be used as a reliable postoperative prognostic marker [48]. Thus, to predict postoperative tumor behavior, it may be that the relationship between Ki-67 Li and recurrence/regrowth is more important than the relationship between Ki-67 Li and invasiveness.
Several authors have studied the possible use of the Ki-67 Li as a predictor of tumor recurrence/regrowth, but the results were inconsistent. While some authors reported the prognostic significance of Ki-67 [1, 12, 14, 35, 38, 39, 43, 60], others found no significant association between Ki-67 Li and postoperative tumor behavior [7, 18, 21, 30, 57]. However, in several reports, the potential of Ki-67 to predict postoperative tumor behavior (i.e. potential to recur or regrow) was evaluated in patients after complete and incomplete resection together [7, 12, 14, 18, 21, 39, 57]. Considering the fact that true recurrence is relatively rare in patients after CTR and that regrowth of residual NFPAs is much more common [60], it is possible that some results were influenced by Ki-67 evaluation in patients with persistent disease (i.e. visible residual tumors) together with patients after CTR, most of whom were potentially “cured”. Authors who evaluated the prognostic significance of Ki-67 Li in patients after incomplete resection only (or separately from patients after CTR) found either a positive association between residual tumor growth and Ki-67 Li [1, 35, 60], or at least a trend towards the association in univariate analyses [30]. Righi et al. [43], who evaluated the prognostic value of Ki-67 Li >3 % in patients after complete and incomplete resection, but used incomplete resection as a variable in multivariate Cox regression analysis, found that Ki-67 Li >3 % was a strong predictor of pituitary adenoma recurrence/progression. Similarly, Nakabayashi et al. [38] evaluated the prognostic value of Ki-67 Li in patients after complete and incomplete resection, but used incomplete resection as a variable in multivariate regression analysis. They found Ki-67 Li to be a significant predictor of pituitary adenoma recurrence/progression. Interestingly, among authors who evaluated the relationship between pituitary adenoma growth velocity (or tumor volume doubling time) and Ki-67 Li, all [3, 8, 19, 20, 23, 37] but one [47] group found a positive correlation.
Despite the fact that we found only a trend of growing tumor remnants towards higher Ki-67 Li, we were able to identify a Ki-67 Li cutoff value of 2.2 %. The relationship between residual tumor growth and exceeding the cutoff value was significant both in univariate and multivariate analyses. More importantly, the cutoff value of 2.2 % found in our cohort is very close to the cutoff value of 2 % found by Widhalm et al. [60]. Thus, despite the fact that the Ki-67 Li cannot reliably predict prognosis in all patients, exceeding the cutoff value allows us to identify a subset of patients in whom further growth should be expected. In our cohort, this subgroup formed 43 % of all growing remnants.
In patients harboring residual NFPAs with a Ki-67 Li over 2.2 %, more frequent MRI controls should be considered during the postoperative period, because the probability that the residual tumor will grow is very high. If such a remnant is resectable, we found that re-operation and resection of the residual tumor is justified, as the subsequent growth of the tumor remnant can potentially make delayed surgery more complicated. In unresectable residual tumors with a Ki-67 Li over 2.2 % localized close to the optic apparatus, an early postoperative stereotactic radiosurgery should be considered. Expected regrowth of such tumor remnants could potentially complicate stereotactic radiosurgery, by growing too close to visual structures. However, we must emphasize that residual NFPAs with a Ki-67 Li below 2.2 % can also grow, and regular follow-up MRI scans are, so far, irreplaceable.
To compare our results with data published by authors who used the term “recurrence” both for the re-appearance of a completely resected tumor as well as for the growth of a tumor remnant, we experimentally subdivided our cohort into two groups. One group included patients with recurrence and patients with a growing remnant, and the other group included patients with a stable remnant and patients with no recurrence after CTR. No significant difference in the Ki-67 Li was found between the two groups. This was also the case with exceeding the cutoff Ki-67 Li value of 2.2 % between these two groups. Accordingly, it can be hypothesized that one of the reasons for the lack of a significant relationship between Ki-67 Li and “recurrence” in some previous studies could be the absence of differentiation between tumors after CTR and those after incomplete removal.
Prognostic significance of the HMGA-1 antigen
HMGA-1 proteins are non-histone chromosomal proteins that alter chromatin structure, and thereby regulate the transcription of several genes by either enhancing or suppressing the action of transcription factors [13]. This protein family is implicated, through different mechanisms, in both benign and malignant tumors [13]. As cell cycle regulators, HMGA proteins play significant roles in pathways that lead to pituitary tumor evolution in humans and in experimental animal models [9, 10]. Wang et al. [59] found HMGA-1 expression was significantly higher in invasive adenomas or macroadenomas compared with non-invasive adenomas or microadenomas. Additionally, HMGA-1 showed the highest expression in the most aggressive pituitary adenomas. A significant correlation between HMGA-1 expression and the MIB-1 Li was also found. The authors concluded that HMGA-1 may be a novel molecular marker of tumor proliferation and invasiveness. However, the potential association between HMGA-1 Li and tumor recurrence/regrowth was not investigated.
In agreement with Wang et al. [59], we found a significant correlation between the HMGA-1 Li and Ki-67 Li in our cohort. However, no significant association was found between the HMGA-1 Li and NFPA invasiveness. In addition, no significant relationship between the HMGA-1 Li and tumor regrowth was found. Thus, according to our findings, HMGA-1 is not a reliable prognostic marker for NFPAs.
Association between age and residual tumor growth
A significant relationship between age of the patient and residual adenoma growth velocity was found by Tanaka et al. [54]. A significant association between age and residual adenoma growth was found by Matsuyama, where patients in the progression group were significantly younger [35]. In contrast, no significant association was found by Soto-Ares et al. [53] or Widhalm et al. [60]. In our cohort, younger patients had a greater tendency for residual tumor growth. However, a significant negative association between age and residual tumor growth was found only in univariate analysis, and not in multivariate analysis. Other factors related to age may explain the indirect dependent association between age and postoperative tumor growth.
Factors associated with tumor recurrence
In the group of 15 recurrence-free patients, five NFPAs (33 %) had a Ki-67 Li higher than 2.2 %. According to data published by Widhalm et al. [60] and according to our own findings, if CTR could not be achieved in these patients, then the residual tumor would most likely grow and the chance of achieving a stable disease (without adjuvant treatment) would be very small.
Despite the fact that, owing to the low incidence of recurrences, identification of determining factors was not possible, the low recurrence rate itself is an important finding. This result, as well as the absence of recurrences in a number of NFPAs with Ki-67 Li over 2.2 %, emphasizes the importance of CTR, which should always be attempted when safely achievable.
Conclusions
The prognostic significance of the HMGA-1 antigen was not confirmed in this study. In contrast, the Ki-67 Li provided valuable prognostic information in a substantial proportion of patients with residual NFPAs, and in residual tumors with a Ki-67 Li above 2.2 % further regrowth should be expected. These tumors may require shorter intervals of follow-up MRI and/or early adjuvant therapy. Future larger studies are needed to confirm these results. However, regular MRI controls are so far irreplaceable, as residual NFPAs with a Ki-67 Li below 2.2 % can still grow. Investigation of the prognostic value of other biomarkers is essential to identify a factor that can predict recurrence/regrowth of NFPAs reliably in all patients.
References
Abe T, Sanno N, Osamura YR, Matsumoto K (1997) Proliferative potential in pituitary adenomas: measurement by monoclonal antibody MIB-1. Acta Neurochir 139:613–618
Brown DC, Gatter KC (1990) Monoclonal antibody Ki-67: its use in histopathology. Histopathology 17:489–503
Comtois R, Beauregard H, Somma M, Serri O, Aris-Jilwan N, Hardy J (1991) The clinical and endocrine outcome to trans-sphenoidal microsurgery of nonsecreting pituitary adenomas. Cancer 68:860–866
Cottier JP, Destrieux C, Brunereau L, Bertrand P, Moreau L, Jan M, Herbreteau D (2000) Cavernous sinus invasion by pituitary adenoma: MR imaging. Radiology 215:463–469
de Aguiar PH, Aires R, Laws ER, Isolan GR, Logullo A, Patil C, Katznelson L (2010) Labeling index in pituitary adenomas evaluated by means of MIB-1: is there a prognostic role? A critical review. Neurol Res 32:1060–1071
Dekkers OM, Pereira AM, Roelfsema F, Voormolen JH, Neelis KJ, Schroijen MA, Smit JW, Romijn JA (2006) Observation alone after transsphenoidal surgery for nonfunctioning pituitary macroadenoma. J Clin Endocrinol Metab 91:1796–1801
Dubois S, Guyétant S, Menei P, Rodien P, Illouz F, Vielle B, Rohmer V (2007) Relevance of Ki-67 and prognostic factors for recurrence/progression of gonadotropic adenomas after first surgery. Eur J Endocrinol 157:141–147
Ekramullah SM, Saitoh Y, Arita N, Ohnishi T, Hayakawa T (1996) The correlation of Ki-67 staining indices with tumour doubling times in regrowing non-functioning pituitary adenomas. Acta Neurochir 138:1449–1455
Fedele M, Pentimalli F, Baldassarre G, Battista S, Klein-Szanto AJ, Kenyon L, Visone R, De Martino I, Ciarmiello A, Arra C, Viglietto G, Croce CM, Fusco A (2005) Transgenic mice overexpressing the wild-type form of the HMGA1 gene develop mixed growth hormone/prolactin cell pituitary adenomas and natural killer cell lymphomas. Oncogene 24:3427–3435
Fedele M, Fusco A (2010) Role of the high mobility group A proteins in the regulation of pituitary cell cycle. J Mol Endocrinol 44:309–318
Ferrante E, Ferraroni M, Castrignanò T, Menicatti L, Anagni M, Reimondo G, Del Monte P, Bernasconi D, Loli P, Faustini-Fustini M, Borretta G, Terzolo M, Losa M, Morabito A, Spada A, Beck-Peccoz P, Lania AG (2006) Non-functioning pituitary adenoma database: a useful resource to improve the clinical management of pituitary tumors. Eur J Endocrinol 155:823–829
Filippella M, Galland F, Kujas M, Young J, Faggiano A, Lombardi G, Colao A, Meduri G, Chanson P (2006) Pituitary tumour transforming gene (PTTG) expression correlates with the proliferative activity and recurrence status of pituitary adenomas: a clinical and immunohistochemical study. Clin Endocrinol 65:536–543
Fusco A, Fedele M (2007) Roles of HMGA proteins in cancer. Nat Rev Cancer 7:899–910
Gejman R, Swearingen B, Hedley-Whyte ET (2008) Role of Ki-67 proliferation index and p53 expression in predicting progression of pituitary adenomas. Hum Pathol 39:758–766
Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H (1984) Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol 133:1710–1715
Gözü H, Bilgiç B, Hazneci J, Sargın H, Erkal F, Sargın M, Sönmez B, Orbay E, Şeker M, Bozbuğa M, Bayındır Ç (2005) Is Ki-67 Index a useful labeling marker for invasion of pituitary adenomas? Turk Jem 4:107–113
Greenman Y, Ouaknine G, Veshchev I, Reider-Groswasser II, Segev Y, Stern N (2003) Postoperative surveillance of clinically nonfunctioning pituitary macroadenomas: markers of tumour quiescence and regrowth. Clin Endocrinol 58:763–769
Hentschel SJ, McCutcheon IE, Moore W, Durity FA (2003) P53 and MIB-1 immunohistochemistry as predictors of the clinical behavior of nonfunctioning pituitary adenomas. Can J Neurol Sci 30:215–219
Honegger J, Prettin C, Feuerhake F, Petrick M, Schulte-Mönting J, Reincke M (2003) Expression of Ki-67 antigen in nonfunctioning pituitary adenomas: correlation with growth velocity and invasiveness. J Neurosurg 99:674–679
Hsu CY, Guo WY, Chien CP, Ho DM (2010) MIB-1 labeling index correlated with magnetic resonance imaging detected tumor volume doubling time in pituitary adenoma. Eur J Endocrinol 162:1027–1033
Jaffrain-Rea ML, Di Stefano D, Minniti G, Esposito V, Bultrini A, Ferretti E, Santoro A, Faticanti Scucchi L, Gulino A, Cantore G (2002) A critical reappraisal of MIB-1 labelling index significance in a large series of pituitary tumours: secreting versus non-secreting adenomas. Endocr Relat Cancer 9:103–113
Jane JA, Thapar K, Laws ER (2011) Pituitary Tumors: Functioning and Nonfunctioning. In: Winn RH (ed) Youmans Neurological Surgery. Elsevier Saunders, pp 1476–1510
Kawamoto H, Uozumi T, Kawamoto K, Arita K, Yano T, Hirohata T (1995) Analysis of the growth rate and cavernous sinus invasion of pituitary adenomas. Acta Neurochir 136:37–43
Knosp E, Kitz K, Perneczky A (1989) Proliferation activity in pituitary adenomas: measurement by monoclonal antibody Ki-67. Neurosurgery 25:927–930
Knosp E, Steiner E, Kitz K, Matula C (1993) Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings. Neurosurgery 33:610–617
Landolt AM, Shibata T, Kleihues P (1987) Growth rate of human pituitary adenomas. J Neurosurg 67:803–806
Lath R, Chacko G, Chandy MJ (2001) Determination of Ki-67 labeling index in pituitary adenomas using MIB-1 monoclonal antibody. Neurol India 49:144–147
Lillehei KO, Kirschman DL, Kleinschmidt-DeMasters BK, Ridgway EC (1998) Reassessment of the role of radiation therapy in the treatment of endocrine-inactive pituitary macroadenomas. Neurosurgery 43:432–438
Lloyd RV, Kovcs K, Young WF Jr, Farrell WE, Asa SL, Trouillas J, Kontogeorgos G, Sano T, Scheithauer BW, Horvath E (2004) Tumours of the Pituitary Gland. In: DeLellis RA, Lloyd RV, Heitz PU (eds) Pathology and Genetics: Tumours of Endocrine Organs (World Health Organization Classification of Tumours). IARC Press, Lyon, France, pp 10–47
Losa M, Franzin A, Mangili F, Terreni MR, Barzaghi R, Veglia F, Mortini P, Giovanelli M (2000) Proliferation index of nonfunctioning pituitary adenomas: correlations with clinical characteristics and long-term follow-up results. Neurosurgery 47:1313–1318
Losa M, Mortini P, Barzaghi R, Ribotto P, Terreni MR, Marzoli SB, Pieralli S, Giovanelli M (2008) Early results of surgery in patients with nonfunctioning pituitary adenoma and analysis of the risk of tumor recurrence. J Neurosurg 108:525–532
Mahta A, Haghpanah V, Lashkari A, Heshmat R, Larijani B, Tavangar SM (2007) Non-functioning pituitary adenoma: immunohistochemical analysis of 85 cases. Folia Neuropathol 45:72–77
Mastronardi L, Guiducci A, Spera C, Puzzilli F, Liberati F, Maira G (1999) Ki-67 labelling index and invasiveness among anterior pituitary adenomas: analysis of 103 cases using the MIB-1 monoclonal antibody. J Clin Pathol 52:107–111
Mastronardi L, Guiducci A, Puzzilli F (2001) Lack of correlation between Ki-67 labelling index and tumor size of anterior pituitary adenomas. BMC Cancer 1:12
Matsuyama J (2012) Ki-67 expression for predicting progression of postoperative residual pituitary adenomas: correlations with clinical variables. Neurol Med Chir (Tokyo) 52:563–569
Messerer M, De Battista JC, Raverot G, Kassis S, Dubourg J, Lapras V, Trouillas J, Perrin G, Jouanneau E (2011) Evidence of improved surgical outcome following endoscopy for nonfunctioning pituitary adenoma removal. Neurosurg Focus 30:E11
Mizoue T, Kawamoto H, Arita K, Kurisu K, Tominaga A, Uozumi T (1997) MIB1 immunopositivity is associated with rapid regrowth of pituitary adenomas. Acta Neurochir 139:426–431
Nakabayashi H, Sunada I, Hara M (2001) Immunohistochemical analyses of cell cycle-related proteins, apoptosis, and proliferation in pituitary adenomas. J Histochem Cytochem 49:1193–1194
Paek KI, Kim SH, Song SH, Choi SW, Koh HS, Youm JY, Kim Y (2005) Clinical significance of Ki-67 labeling index in pituitary macroadenoma. J Korean Med Sci 20:489–494
Pan LX, Chen ZP, Liu YS, Zhao JH (2005) Magnetic resonance imaging and biological markers in pituitary adenomas with invasion of the cavernous sinus space. J Neurooncol 74:71–76
Picozzi P, Losa M, Mortini P, Valle MA, Franzin A, Attuati L, Ferrari da Passano C, Giovanelli M (2005) Radiosurgery and the prevention of regrowth of incompletely removed nonfunctioning pituitary adenomas. J Neurosurg 102(Suppl):71–74
Pizarro CB, Oliveira MC, Coutinho LB, Ferreira NP (2004) Measurement of Ki-67 antigen in 159 pituitary adenomas using the MIB-1 monoclonal antibody. Braz J Med Biol Res 37:235–243
Righi A, Agati P, Sisto A, Frank G, Faustini-Fustini M, Agati R, Mazzatenta D, Farnedi A, Menetti F, Marucci G, Foschini MP (2012) A classification tree approach for pituitary adenomas. Hum Pathol 43:1627–1637
Rishi A, Sharma MC, Sarkar C, Jain D, Singh M, Mahapatra AK, Mehta VS, Das TK (2010) A clinicopathological and immunohistochemical study of clinically non-functioning pituitary adenomas: a single institutional experience. Neurol India 58:418–423
Roelfsema F, Biermasz NR, Pereira AM (2012) Clinical factors involved in the recurrence of pituitary adenomas after surgical remission: a structured review and meta-analysis. Pituitary 15:71–83
Saeger W, Lüdecke DK, Buchfelder M, Fahlbusch R, Quabbe HJ, Petersenn S (2007) Pathohistological classification of pituitary tumors: 10 years of experience with the German Pituitary Tumor Registry. Eur J Endocrinol 156:203–216
Saeger W, Lüdecke B, Lüdecke DK (2008) Clinical tumor growth and comparison with proliferation markers in non-functioning (inactive) pituitary adenomas. Exp Clin Endocrinol Diabetes 116:80–85
Salehi F, Agur A, Scheithauer BW, Kovacs K, Lloyd RV, Cusimano M (2009) Ki-67 in pituitary neoplasms: a review–part I. Neurosurgery 65:429–437
Salehi F, Agur A, Scheithauer BW, Kovacs K, Lloyd RV, Cusimano M (2010) Biomarkers of pituitary neoplasms: a review (Part II). Neurosurgery 67:1790–1798
Sav A, Rotondo F, Syro LV, Scheithauer BW, Kovacs K (2012) Biomarkers of pituitary neoplasms. Anticancer Res 32:4639–4654
Scheithauer BW, Gaffey TA, Lloyd RV, Sebo TJ, Kovacs KT, Horvath E, Yapicier O, Young WF Jr, Meyer FB, Kuroki T, Riehle DL, Laws ER Jr (2006) Pathobiology of pituitary adenomas and carcinomas. Neurosurgery 59:341–353
Schreiber S, Saeger W, Lüdecke DK (1999) Proliferation markers in different types of clinically non-secreting pituitary adenomas. Pituitary 1:213–220
Soto-Ares G, Cortet-Rudelli C, Assaker R, Boulinguez A, Dubest C, Dewailly D, Pruvo JP (2002) MRI protocol technique in the optimal therapeutic strategy of non-functioning pituitary adenomas. Eur J Endocrinol 146:179–186
Tanaka Y, Hongo K, Tada T, Sakai K, Kakizawa Y, Kobayashi S (2003) Growth pattern and rate in residual nonfunctioning pituitary adenomas: correlations among tumor volume doubling time, patient age, and MIB-1 index. J Neurosurg 98:359–365
Thapar K, Kovacs K, Scheithauer BW, Stefaneanu L, Horvath E, Pernicone PJ, Murray D, Laws ER Jr (1996) Proliferative activity and invasiveness among pituitary adenomas and carcinomas: an analysis using the MIB-1 antibody. Neurosurgery 38:99–106
Turner HE, Stratton IM, Byrne JV, Adams CB, Wass JA (1999) Audit of selected patients with nonfunctioning pituitary adenomas treated without irradiation—a follow-up study. Clin Endocrinol 51:281–284
Turner HE, Nagy Z, Gatter KC, Esiri MM, Wass JA, Harris AL (2000) Proliferation, bcl-2 expression and angiogenesis in pituitary adenomas: relationship to tumour behaviour. Br J Cancer 82:1441–1445
van den Bergh AC, van den Berg G, Schoorl MA, Sluiter WJ, van der Vliet AM, Hoving EW, Szabó BG, Langendijk JA, Wolffenbuttel BH, Dullaart RP (2007) Immediate postoperative radiotherapy in residual nonfunctioning pituitary adenoma: beneficial effect on local control without additional negative impact on pituitary function and life expectancy. Int J Radiat Oncol Biol Phys 67:863–869
Wang EL, Qian ZR, Rahman MM, Yoshimoto K, Yamada S, Kudo E, Sano T (2010) Increased expression of HMGA1 correlates with tumour invasiveness and proliferation in human pituitary adenomas. Histopathology 56:501–509
Widhalm G, Wolfsberger S, Preusser M, Fischer I, Woehrer A, Wunderer J, Hainfellner JA, Knosp E (2009) Residual nonfunctioning pituitary adenomas: prognostic value of MIB-1 labeling index for tumor progression. J Neurosurg 111:563–571
Wolfsberger S, Kitz K, Wunderer J, Czech T, Boecher-Schwarz HG, Hainfellner JA, Knosp E (2004) Multiregional sampling reveals a homogenous distribution of Ki-67 proliferation rate in pituitary adenomas. Acta Neurochir 146:1323–1327
Wolfsberger S, Wunderer J, Zachenhofer I, Czech T, Böcher-Schwarz HG, Hainfellner J, Knosp E (2004) Expression of cell proliferation markers in pituitary adenomas–correlation and clinical relevance of MIB-1 and anti-topoisomerase-IIalpha. Acta Neurochir 146:831–839
Woollons AC, Hunn MK, Rajapakse YR, Toomath R, Hamilton DA, Conaglen JV, Balakrishnan V (2000) Non-functioning pituitary adenomas: indications for postoperative radiotherapy. Clin Endocrinol 53:713–717
Yamada S, Ohyama K, Taguchi M, Takeshita A, Morita K, Takano K, Sano T (2007) A study of the correlation between morphological findings and biological activities in clinically nonfunctioning pituitary adenomas. Neurosurgery 61:580–584
Yildirim AE, Divanlioglu D, Nacar OA, Dursun E, Sahinoglu M, Unal T, Belen AD (2013) Incidence, hormonal distribution and postoperative follow up of atypical pituitary adenomas. Turk Neurosurg 23:226–231
Yokoyama S, Hirano H, Moroki K, Goto M, Imamura S, Kuratsu JI (2001) Are nonfunctioning pituitary adenomas extending into the cavernous sinus aggressive and/or invasive? Neurosurgery 49:857–862
Zada G, Woodmansee WW, Ramkissoon S, Amadio J, Nose V, Laws ER Jr (2011) Atypical pituitary adenomas: incidence, clinical characteristics, and implications. J Neurosurg 114:336–344
Zhao D, Tomono Y, Nose T (1999) Expression of P27kip1 and Ki-67 in pituitary adenomas: an investigation of marker of adenoma invasiveness. Acta Neurochir 141:187–192
Acknowledgments
This study was supported by a grant from the Scientific Grant Agency of the Ministry of Education of the Slovak Republic and the Slovak Academy of Sciences (VEGA) number 1/1166/10.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Comments
We all search for a histological marker to warn us of the faster growing non-functioning pituitary adenomas after we have resected them. Sadly HMGA1 is not it.
Despite its slight unpredictability, Ki67 Labelling Index remains the best marker. When it is high, then close review of residuals or radiotherapy are indicated. The study does show that complete resection is the best way to protect our patients, and that residuals are likely to regrow, albeit not always.
Michael Powell
London, UK
Rights and permissions
About this article
Cite this article
Šteňo, A., Bocko, J., Rychlý, B. et al. Nonfunctioning pituitary adenomas: association of Ki-67 and HMGA-1 labeling indices with residual tumor growth. Acta Neurochir 156, 451–461 (2014). https://doi.org/10.1007/s00701-014-1993-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-014-1993-0