Abstract
Background
Intraoperative high-field magnetic resonance imaging (iMRI) is used as an immediate intraoperative quality control, evaluating the extent of tumor removal during the surgical procedure and allowing us to extend resections in those cases where tumor remnants are documented. The aim of the study was to analyze the typical localization of residual tumor remnants, detected by iMRI during transsphenoidal surgery of pituitary adenomas.
Methods
We reviewed a series of 72 patients. All patients presented with macroadenomas with or without suprasellar extension. After high-field MRI investigation, we divided the series preoperatively into totally resectable (TR) and non-totally resectable (NTR) tumors. Tumor remnants were documented by iMRI, obtained directly after tumor removal, as well as by intraoperative surgical inspection of the sellar content.
Results
In the TR group, we observed 23 cases suspicious for tumor remnants, located anteriorly, laterally, posteriorly, and suprasellar under descending folds of the diaphragm. Continuing surgery, upon a “second inspection”, tumor resection could be completed in all cases.
Conclusions
Incomplete removal of resectable pituitary adenomas could be avoided in a higher number of cases with the knowledge of the location of the typical remnant tumors. In those cases where it is not possible to achieve a complete resection of adenoma, further treatment can be planned at an earlier stage, without any need to wait for the conventional postoperative MRI scan performed 2 to 3 months after surgery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The development of intraoperative magnetic resonance imaging started in the mid-1990s. In the last half decade, it has been used in transsphenoidal surgery, in addition to other advanced technologies already introduced into the operating room, such as neuronavigation [5, 8, 11, 19, 24] and endoscopy [2, 9, 25]. Intraoperative magnetic resonance imaging [1, 4, 6, 12, 17, 18, 24] is used as an immediate intraoperative quality control, evaluating the extent of tumor removal during the surgical procedure. This allows us to extend resections in those cases where tumor remnants are documented [1, 4, 6, 12, 17, 18]. In one-third of macroadenomas, both low-field and especially high-field 1.5 T intraoperative MRI scanners are able to document adenoma remnants as small as 3–4 mm for “high-field scanners”, which are hidden to the surgeon’s eye in folds of the descending diaphragm sella. The evolution from low-field to high-field MR scanners led to a renaissance of the idea of intraoperative imaging in transsphenoidal surgery for resection control and high quality intraoperative images. High-field MRI has a clear advantage in image resolution compared with the intraoperative low-field and mid-field MR system (0.12–0.5 T) [14, 21].
About 30 to 50 % of totally resectable pituitary adenomas achieved total removal, which could not be obtained at the primary procedure without iMRI.
Patients with not only large tumors, but also smaller adenomas, especially those extending against and into the cavernous sinus, can profit from iMRI control. Patients with not only hormonally inactive, but also larger hormonally active tumors, benefit from iMRI by an increased rate of complete tumor removal, as well as a higher degree of endocrine normalization and “near normalization” [7]. In non-functioning tumors, there are no hormonal markers available that would allow for an early prognosis of outcome.
In the great majority of cases, there are excellent intraoperative high-resolution MRI images, which permit comprehensive evaluation of the relevant anatomy.
The conventional postoperative MRI scan performed 2–3 months after surgery (without artifact) can be avoided.
Subjects and methods
Patient population
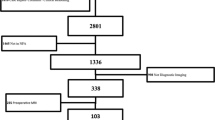
Using intraoperative high-field magnetic resonance imaging control (iMRI), between February 2007 and February 2011, a consecutive series of 72 patients (26 females and 46 males between the ages of 10 and 84 years), who presented with 72 pituitary macroadenomas, underwent transsphenoidal surgery at the International Neuroscience Institute, Hannover, Germany.
The criteria for selecting patients included pituitary macroadenoma with or without suprasellar extension. All patients had preoperative high-field MRI contrast-enhanced scans focused on the sellar region to determine extent of tumor growth and invasion of adjacent structures.
After high-field brain MRI investigation, we distinguished preoperatively between totally resectable (TR) and non-totally resectable (NTR) tumors. Non-total resection was defined as invasion of the lateral part of the cavernous sinus, asymmetrical suprasellar extension, extensive retrosellar and subfrontal development, as well as generally invasive pituitary adenomas. Smaller extensions of these tumors outside the sella, including focally invasive tumors into the cavernous sinus, were regarded as resectable.
In tumors with parasellar extension, we differentiated between: a) displacement of the cavernous sinus; b) focal invasion with protrusion through the middle wall of the cavernous sinus; or c) general invasiveness of the total cavernous sinus. Tumors located in the lateral part of the cavernous sinus, lateral to the carotid artery, were regarded as not totally resectable.
A comprehensive preoperative and postoperative endocrinological and ophthalmological evaluation was also routinely carried out. Endocrinological findings were documented as partial pituitary functions (hypogonadism, hypothyroidism, hypocortisolism and diabetes insipidus).
Operating room setup
In 2006/2007, we installed the first “open” 1.5 T MRI for therapeutic purposes at the International Neuroscience Institute (INI) (Hannover, Germany). The “INI Brain Suite”, our current system, is an operating theater with an high-field Magnetom Espree scanner (Siemens AG Medical Solutions, Hannover, Germany), with a superconductive 1.5 T magnet with a length of 160 cm and an inner bore diameter of 70 cm, equipped with a gradient system having a field strength of up to 40 mT/m (effective 69 mT/M) and an effective slew rate of up to 200 T/m/s effective. A rotatable surgical table (Trumpf, Saalfeld, Germany) is adapted to the scanner to allow for a special surgical MRI tabletop. This surgical table can be locked into various positions. The principal surgical position is at 160 degrees, with the patient’s head at the 5 G line (distance of 4 m to the center of the scanner). As soon as the rotating mechanism has been locked, the height of the table, the angle of tilt and the lateral tilt can be modified. The table movements are controlled remotely. Only the rotation about the table axis to turn the table into the axis of the scanner is performed manually, for safety reasons.
MRI-compatible ventilation (Aestiva 5/MRI, General Electric, Hannover, Germany) and MRI-compatible monitoring are available for control of anesthesia and for wireless 2.4 GHz data transfer from the radiofrequency-shielded cabin. The perfusions and infusion pumps are shielded for MRI compatibility.
Our endoscopic equipment includes a 0° and 30°, 4 mm rigid endoscope Hopkin II (Karl Storz, Tuttingen, Germany), a full high definition (HD) camera IMAGE1TM H3-Z and a wide view HD screen. The use of endoscope in our “BrainSuite” operating room, with 1.5 T MRI, performing surgery at the 5 Gauss-line, is similar to the normal, routinely used operating theater without iMRI.
In the majority of cases, microscopic tumor removal is performed with the ceiling-mounted Pentero C Multivision microscope (Zeiss, Oberkochen, Germany). The holding device is placed in the middle line within the 5 G line; the microscope itself can have a flexible position in connection with the preferred position of the operating field (we tested this MRI compatible ceiling mounted Zeiss Pentero system for the first time).
Navigation is indicated either in tumors with encased arteries or loss of anatomical landmarks, as occurs in re-operations. We use the BrainLab integrated VectorVision Sky Navigation System (BrianLab, Heimstetten, Germany) with a ceiling-mounted infrared camera and touch screen display. The patient is fixed in a special ceramic head-holding system, and registration is done automatically through an integrated special coil system. A fiber optic connection ensures MRI-compatible integration into the radiofrequency (RF) room. The camera used to monitor the position of the microscope and other instruments is also ceiling-mounted, as well as the touch screen, which is used to operate the navigation system. Two 50-inch flat-screen monitors mounted on the left wall of the BrainSuite are available for viewing the images from the microscope and the MRI console, as well as various software applications. The microscope and endoscope video are recorded using Medimage software (Vepro, Pfungstadt, Germany) in parallel in the MRI control room.
The instrument table and various rotating stools (Trumpf) are fully MRI compatible.
The procedures for emergency magnet quenching and for monitoring of oxygen levels in the operating room are the same as those in standard clinical MR imaging installations.
Transsphenoidal Surgery
Head fixation is not required, and imaging is performed using an adapted standard U-shaped large flexible coil draped near the head. The surgeon, who prefers Cushing’s positioning for transsphenoidal surgery, is standing behind the patient’s head. A schematic outline of the operating room has been published previously [10, 20, 22].
The whole transsphenoidal procedure is identical to that performed in regular operating rooms. The sublabial and unilateral paraseptal approaches to the sphenoidal sinus are used less frequently. We generally prefer the direct endonasal transsphenoidal approach with endoscope assistance. The entrance into the sphenoid sinus is guided by endoscopic visualization, as well as identification of the sellar floor and the carotid arteries. Furthermore, the endoscope is very helpful during resection of tumor remnants in the cavernous sinus and in folds of the descending diaphragm, supplementing microsurgical tumor removal.
Less frequently, we have performed a purely endoscopic resection of an adenoma, although purely endoscopic surgery is frequently used during the removal of other parasellar tumors, such as chordomas centered in the clivus.
Porcelain-coated drills are used, especially to avoid metal artifacts during imaging, to open the sphenoid sinus and to remove the sellar floor. Transsphenoidal surgery is routinely accompanied by endonasal endoscopic inspection using 0° and 30°, 4-mm rigid endoscopes to visualize the surgical site, which is projected on a ceiling-mounted monitor or through the eyepiece of the microscope. Selective adenomectomy is achieved in all patients. In general, regular operating micro-instruments are used during the transsphenoidal surgery at the 5 G line. In contrast to earlier years when we used an MRI-compatible nasal speculum for intraoperative imaging, the direct endoscopic assisted endonasal approach allows us to examine the sella without the speculum, by installing a simple piece of cotton as a placeholder in the nasal cavity. If necessary, especially in cases with defects of the diaphragm sella and cerebrospinal fluid (CFS) leaks, the sellar floor was covered at the end of surgery with fascia and/or subcutaneous fat tissue, which was obtained from the right thigh positioned within the 5 G perimeter, during the same surgical session. For safety reasons, we used fully MRI-compatible instruments to obtain the fascia. In the case of intraoperative cerebrospinal fluid leakage, temporary lumbar drainage was utilized. In direct transnasal transsphenoidal surgery, no nasal packing is necessary.
Intraoperative MRI
Before surgery, all patients gave their informed consent for intraoperative MRI investigation in order to evaluate the extent of adenoma removal.
Intraoperative high-field MRI was performed in all 72 cases; 25 cases required a second iMRI, and one case needed a third iMRI.
Tumor visualization with the high-field iop 1.5 Tesla MRI Espree scanner intra OR under anesthesia is very good, and often better when compared to the preoperative high-field MRI (1.5 Tesla).
The timing of intraoperative imaging is decided by the neurosurgeon. Intraoperative imaging is performed either when the surgeon has the impression of complete tumor removal or, in the case of incomplete tumor removal, when the surgeon thinks no further removal at this stage of surgery is possible via the transsphenoidal approach. Just before intraoperative imaging, the opened sellar floor is covered with a flat piece of bone wax (Fig. 1) for better delineation of the sellae outlines in the intraoperative images, minimizing artifacts caused by blood from the sphenoid sinus or the nasal cavity. The surgical site is then covered with a sterile drape, and the MRI table is rotated 160° into the scanner. The time between the decision for intraoperative MRI and the actual start of imaging is approximately 2 minutes.
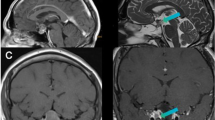
Patient LW, a 61-year-old man. (a–b) Preoperative: Intrasellar and left parasellar (1/3) and suprasellar (2/3) hormonally inactive pituitary adenoma. Chiasma syndrome. (c–d) Intraoperative-1: Primarily complete selective adenomectomy through a direct transnasal transsphenoidal microscopic, endoscopic-assisted approach. W = wax plate. (e–f) Postoperative 5-year control: No tumor recurrence. Pituitary stalk and dorso-medial gland. No pituitary insufficiency preoperatively and postoperatively. Normal visual acuity and visual fields
After the patient is moved into the center of the scanner, certain circuits are switched off, including the fluorescent lamps and the operating microscope. The imaging starts with a localizer sequence (field of view [FOV], 280 mm; repeat time [RT], 20 ms; echo time [TE], 50 ms; scan time, 9 s). High-resolution T2-weighted turbo-spin echo sequences with an in-plane resolution of 0.6 × 0.4 mm are obtained (slice thickness, 3 mm; FOV, 230 mm; RT, 4000 ms; TE, 97 ms; scan time, 6 min 6 s, at three acquisitions). Since high-resolution T2-weighted turbo-spin echo sequences proved to be superior in identification of tumor remnants and additional T1-weighted imaging did not provide any further information, T1-weighted imaging was omitted from the intraoperative imaging protocol in most of the patients.
If the intraoperative imaging depicts some remaining tumor (which was not previously detected by endoscopic inspection) that appears to be accessible for further resection, surgery is continued.
After further resection, a repeated intraoperative MRI scan is performed before closure. Scanning at the identical preoperative and intraoperative image slice positions allows a good comparison of preoperative and intraoperative images in a side-by-side display. Hereby fibrin glue, applied for hemostasis, can be identified and differentiated from tumor remnants. Furthermore, the use of porcelain-coated drills prevents extensive drilling artifacts, which would otherwise obscure image interpretation.
The identical imaging protocol is applied for preoperative, as well as postoperative imaging after 3 months.
Results
Using the techniques described above, we operated on 72 pituitary adenomas (40 with non-functional adenomas, 18 with acromegaly, ten with prolactinomas, and four with adrenocorticotropic hormone-producing tumors) during a 4-year period.
All the cases were studied preoperatively with high-field brain MRI images (1.5 and/or 3 Tesla). In 13 of 72 patients (18 %) in which tumors had encased carotid arteries and/or the sphenoid sinus was only poorly pneumatized, we used the integrated navigation system for the surgical transsphenoidal approach and intraoperative guidance for tumor removal.
A total of 35 of 72 patients (49 %) presented unilateral or bilateral cavernous sinus invasion on the preoperative MRI. Therefore, intended complete tumor resection (Target TR) was considered to be possible in only 49 of 72 patients (68 %) and a non-total tumor resection (Target NTR) in 23 patients of 72 patients (32 %) (Table 1).
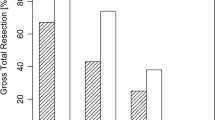
We achieved a total tumor resection in about 74 % of our patients (53/72). We obtained our first target resection (TR I and NTR I) in 64 % of our cases (46/72), as documented by first intraoperative MRI control (Figs. 1, 2 and 3). We were also able to improve our target, obtaining total tumor removal (TR) in four patients who were preoperatively assigned to the NTR group (Fig. 4). After the second intraoperative MRI control, we achieved our target in 22 cases for the total tumor removal group (Figs. 5 and 6), and in three cases for the NTR group. In one case, we needed a third intraoperative MRI control to achieve the last total tumor resection in the TR group.
Patient SW, a 63-year-old man. (a–b) Preoperative: Intrasellar and mainly asymmetric right suprasellar and left parasellar, mixed solid and cystic, GH-producing pituitary adenoma. (c–d) Intraoperative-1: Primarily complete selective adenomectomy with asymmetric complete descent of diaphragm sella. Compressed pituitary gland is located right dorso-lateral. Optimal optic chiasma decompression. (e–f) Postoperative 1-year control: Complete tumor removal, with preservation of the pituitary gland and no deterioration of partial preoperative pituitary insufficiency. No completely normalized GH and IGF-1 levels (medical treatment)
Patient GY, a 54-year-old man. (a–b) Preoperative: Hormonally inactive invasive recurrence pituitary adenoma, mainly intrasellar and right parasellar development infiltrating the cavernous sinus too far lateral, as well as suprasellar with compression of optic chiasma. Non-totally resectable adenoma. (c–d) Intraoperative-1: Complete resection of intrasellar and suprasellar tumor parts. Normalization of chiasma syndrome. Descent and preservation of the pituitary gland located in the left and middle part of the sella. Non-resectable firm tumor part in the cavernous sinus, mainly lateral of the carotid artery. (e–f) Postoperative 3-month control: Tumor remnant in the cavernous sinus confirmed. Preservation of decompressed and displaced pituitary gland. No improvement of the complete preoperative anterior pituitary (ap) insufficiency
Patient NM, a 26-year-old woman. (a-b) Preoperative: Intrasellar (1/3) and right parasellar (2/3) GH-producing pituitary adenoma with invasion of cavernous sinus—questionable complete removability. (c–d) Intraoperative-1: Primarily complete selective adenomectomy through a direct transnasal transsphenoidal microscopic, endoscopic-assisted approach. (e–f) Postoperative 6-month control: Complete tumor removal, with preservation of the pituitary gland in the middle lateral left position. Normalization of preoperative hypogonadismus (pregnancy). Preoperatively elevated GH level normalized, IGF-1 mildly elevated (medical treatment)
Patient FM, a 44-year-old man. (a-b) Preoperative: Hormonally inactive recurrence pituitary adenoma: mainly intrasellar and bilateral parasellar development with displacement of cavernous sinus, as well as suprasellar with compression of optic chiasma. (c–d) Intraoperative-1: Left anterior parasellar remnant close to the medial wall oft he cavernous sinus; meanwhile, re-expansion of decompressed cavernous sinus on both sides. (e–f) Intraoperative-2: Complete selective adenomectomy (hyper-signal and hypo-signal in the tumor cavity = blood and fluid). (g–h) Postoperative 3-month control:, Totally tumor removal with preservation of the pituitary gland in the middle lateral left position. Normalization of the preoperative insufficient adrenals function, as well as of the preoperative visual field deficit
Patient NA, a 46-year-old man. (a–b) Preoperative: Intrasellar and suprasellar hormonally inactive pituitary adenoma, with compression of the optic chiasma. Chiasma syndrome. (c–d) Intraoperative-1: small tumour remnant in the fold of the diaphragm, anterior to the pituitary stalk. (e–f) Intraoperative-2: Complete selective adenomectomy. Normalization of the chiasma syndrome and of the adrenal function. Descent and preservation of the pituitary gland located in the left and middle part of the sella. (g-h) Postoperative 6-month control: Total tumor removal with preservation of the pituitary gland in the middle lateral left position
In the Target TR group, we achieved a total tumor resection in 100 % (49/49) of cases. Within the Target TR group, we achieved total tumor resection as documented by the first iMRI in 53 % (26/49) of cases. The remaining 47 % (23/49) of patients required further tumor resection after the first iMRI before total tumor resection was achieved.
Based on residual tumor detection by the first iMRI, further surgery was required in a total of 26 of the 72 patients (36 %) (Table 1).
In the Target TR group, we observed 23 patients with suspicious tumor remnants: located lateral, close to the medial wall of the cavernous sinus (n = 3), suprasellar, close attachment to the pituitary stalk (n = 7), anteriorly, under descending folds of the diaphragm sellae (n = 7) and also posteriorly (n = 6) (Figs. 7, 8, 9 and 10). In one case, no tumor remnant was found during the “second inspection”, but only blood that was therefore valuated as artifact.
High-resolution T2-weighted turbo-spin echo sequences in coronal and sagittal views compared with schematic drawings of the typical suprasellar localization of remnant tumor. One of seven cases where the diaphragm did not descend totally while fixed to the pituitary stalk, and a small remnant tumor is exactly localized in the fold of the diaphragm and to the pituitary stalk
Schematic drawing of the typical localization oftumor remnants in transsphenoidal pituitary adenoma surgery. (a) Laterally, close to the medial wall of cavernous sinus (coronal view); (b–d) suprasellar, in the fold of the diaphragm and close attachment to the pituitary stalk (coronal and sagittal views); (c) anteriorly, under descending folds of the diaphragm sellae and posteriorly (sagittal view) as well
In the Target NTR group, 13 % (3/23) of cases with intended non-complete tumor resection, an additional tumor resection was carried out in order to achieve maximal subtotal tumor removal. In 17 % (4/23) of cases in whom incomplete tumor resection was initially planned, total resectability was achieved. Based on the intraoperative finding, no extensive invasion, but rather displacement of the cavernous sinus, was found.
The 26 above-mentioned patients, who received additional tumor resection after an iMRI control, benefitted from the use of iMRI.
Implementation of iMRI led to an increased operative time of either 20 or 40 min, depending on whether one or two MRI scans were acquired.
In seven cases, the intraoperative interpretation of iMRI was equivocal for tumor remnants smaller than 3–4 mm. It was difficult to distinguish between very small tumor remnants and perioperative changes and artifacts produced by blood and fibrin glue. However, when tumor remnants were larger than 3 mm, the sensitivity of the iMRI was 100 % in the suprasellar, intrasellar, and lateral parasellar regions.
Discussion
Utility of iMRI in pituitary surgery has been proven by an increasing number of surgeons demanding the necessity of such imaging for guidance and control in challenging cases [23, 25].
For more than 10 years, neurosurgeons around the world have been acquiring experience with iMRI theaters, starting with low-field MRI.
High-field MR (1.5 tesla) and ultra high-field MR (3 tesla) iop scanners are already becoming available from most manufacturers for clinical applications. Compared with low-field (0.15-and 0.2-tesla) and mid-field (0.4 tesla) [14], conventional high-field MR units offer the advantage of a higher signal-to-noise ratio, which allows higher spatial resolution, and thus the images provide details approaching those of a histological specimen [20, 21].
Nowadays, several different industrial companies offer low-field (0.15 Tesla—Medtronic, 0.2 Tesla—Siemens), mid-field (0.3 Tesla—Hitachi, 0.5 Tesla—General Electric), high-field (1.5 Tesla—General Electric, Philips, Siemens) and ultra-high-field (3 Tesla—General Electric, Philips, Siemens) scanners.
In the previous literature improvement rates for total resections are described in up to 30–40 % of the cases [13, 14, 16, 25]. Among these publications, higher resection rates are reported in series using high-field MRI, in contrast to lower resection rates in series performing low-field iMRI [1, 14].
Since 1996, the senior author (RF) started using the 0.2 Tesla system (Magnetom Open, Siemens) in Erlangen, and then in 2002, began using the 1.5 Tesla system (Sonata, Siemens), in the same department.
Despite radical total removal including tumor remnants close to the displaced pituitary gland, our goal was for total or maximal tumor resection with preservation of pituitary function. We did not detect a difference of pituitary insufficiency in this series compared with our previous results without intraoperative MRI, in spite of the major surgical aggressivity applied in order to achieve total tumor removal after iMRI visualization of the tumor remnant.
Lower recurrent rates are seen in cases where MRI documented total tumor removed. After total tumor resection, we have observed a recurrence rate of 4 % and 5 %, respectively, at 5 and 10 years. This is significant when we compare the non-total tumor removal group with a recurrence rate of 40 % and 52 %, respectively, at 5 and 10 years.
Tumor dimension smaller than 3 mm cannot be detected, which explains thatsuch small tumor remnants could be missed, despite normal intraoperative MRI, . In such cases, the preoperative hormone excess in acromegaly and prolactinomas, for example, will not be totally eliminated.
The lateral tumor’s extension into the parasellar spaces, which occurs in 30 to 45 % of pituitary adenomas, is the most common limiting factor in the resection of these processes, especially in the case of extensive cavernous sinus invasion. The invasive parasellar growth of sellar lesions through the medial border of the cavernous sinus is important for surgical planning, but visualization of the fine structure of the medial cavernous sinus border is often unsatisfactory with standard MRI imaging. Therefore, prediction of cavernous sinus invasion based on MR images may rely on indirect signs, such as encasement of the intra-cavernous internal carotid artery (ICA), replacement of the medial cavernous sinus compartment by tumor tissue, or tumor growth beyond a tangential line joining the intra-cavernous and supra-clinoid segments of the ICA. Sometimes, definitive decisions can only be performed intraoperatively by direct visualization of anatomical situs, to be confirmed by intraoperative MRI.
Large sella tumors may flatten the normal pituitary gland so that it is difficult to recognize on preoperative conventional MR images. The preoperative knowledge of its position in relation to the lesion may be crucial for preserving its integrity and function.
Due to its higher resolution, 3-Tesla MR imaging may allow the delineation of parasellar anatomy in such detail that the medial cavernous sinus border may be visible, which is particularly important for surgery for laterally invasive sellar lesions. 3-Tesla MR imaging also provides optimal imaging during intraoperative navigation [20]. However there is no convincing data available demonstrating that intraoperatively, the 3 Tesla MRI is superior to iop 1.5 tesla MRI in imaging of pituitary lesions. This could be solved by the introduction of useful head coils and addressing the current problem of geometric distortion.
The intraoperative assessment of the completeness of tumor resection is often very difficult. Intraoperative surgical assessment is based on direct visualization as well as the surgeon’s eyes, and experience is the standard. Due to the relatively small size of the operative field, the extensive recesses of the sella are often invisible, even with modern endoscopic techniques and even for expert neurosurgeons.
In all our discussed cases, we used the endoscope, routinely and schematically, at the beginning of surgery for identifying the approach into the sphenoid sinus, and furthermore to identify the middle line of the sella area, and later on at the end of microsurgical tumor removal, to detect potential tumor remnants in those cases with parasellar tumor development into the cavernous sinus. Despite using the endoscope to look “around the corner” at the end of tumor removal, a thin remnant within a fold of the descending diaphragm sellae could not be detected endoscopically, but only using the iMRI (Fig. 6).
The application of all this technology increases the operating time by only a minimal amount. Except for the interruption of surgery for scanning, iMRI offers the chance for improved cure rates. The learning curve of the operating team is such that more frequent use of iMRI clearly improves scanning time and reduces unnecessary delays. Techniques for positioning and optimizing image quality also arise with increased experience.
The ability of iMRI to assess the extent of resection of pituitary macroadenomas also depends on the ability to obtain technically adequate images for interpretation during an operation, making it more or less easy to differentiate residual tumor from the adjacent anatomic structures (e.g., optic chiasm, infundibulum and normal gland) and from artifacts caused by surgery. We prefer high-resolution T2-weighted turbo-spin echo sequences for obtaining rapid information about large remnants, and then continue with surgery without having to perform multiple sequences such as T1.
Application of a piece of bone wax at the position of the removed sellar floor followed by saline irrigation, which remains in the cavity during imaging, prevents problems with image interpretation like blood mimicking tumor remnants (Fig. 1). Other practices to define the tumor cavity by implantation of Gelfoam are being discussed [3, 10].
In our experience, there is a surgical learning process when using iMRI for pituitary surgery. We were able to improve our results from 82 % as previously published [22] to 100 % in our current series of resectable tumors in which complete tumor removal seemed to be possible via the transsphenoidal approach. The primary difference between our previously published results and current results are the 47 % (23/49) of the patients who obtained total tumor resection only after visualizing tumor remnants with iMRI. In the previous study, 24 % of the patients achieved total tumor resection only after visualization of tumor remnants with iMRI (Fig. 12). These patients were particularly benefitted, because they would not have achieved complete tumor resection during their surgery without using the iMRI.
The reason for leaving typical tumor remnants could be that the surgeon tried to avoid anterior CSF leaks, lateral bleeding from the cavernous sinus, and posteriorly did not take time for an adequate drilling of the sella floor into the clivus. In large extended intrasellar and suprasellar adenomas, a typical small tumor remnant is localized in the folds of the descending diaphragm sella and hidden to the surgeon’s eye (Fig. 11).
We regard iMRI as important, even for experienced pituitary neurosurgeons, to identify tumor remnants in these sensitive areas. This naturally suggests that less experienced neurosurgeons would benefit even more from the use of iMRI in order to avoid typical remnants in transsphenoidal microscopic and endoscopic pituitary adenoma surgery (Fig. 12).
From our point of view and personal experience, early postoperative MRI outside the operating room (OR) directly after finishing surgery, or one hour later, can be helpful in selected cases, when fortunately—and in general surprisingly—no early artifacts are visible. Many times, however, artifact (blood, plastic material, etc.) is visible and impairs sufficient image interpretation [15]. It is our belief that even early postoperative MRI outside the OR is not an equivalent alternative to iMRI. The iMRI allows for questionable artifact to be immediately explored with the head and anatomy in the same position. Only images performed 2–3 months after surgery provide definitively reliable information about the extent of resection. Comparing the result of intraoperative imaging and scanning after 3 months, the rate of false-positives was very low at 6 %, and there were no false-negative findings. The main disadvantage to the delayed MRI, however, is the missed opportunity for continuing tumor resection afforded by iMRI.
The utility of iMRI is also based on many ergonomic and financial considerations. The cost/benefit ratio includes construction costs and shielding of the OR, the available investment budget to purchase the iMRI system, and the requirements to use the scanner either strictly for intraoperative scans or also for diagnostic purposes [26]. A shared-resource magnetic resonance operating suite that facilitates performance of both neurosurgical and diagnostic procedures in a single unit greatly improves the cost-benefit ratio [19].
Conclusion
The knowledge of the typical location of residual tumor was fundamental to achieve total tumor resection.
In our series, the utility of intraoperative high-field magnetic resonance imaging MRI scanning in pituitary tumors has been well documented. IMRI images were particularly beneficial to patients where total tumor resection was the target of surgery. We could definitely achieve our target, total tumor resection, for all resectable adenomas after localizing the remnants and continuing the operative procedures in one step. Even in non-totally resectable tumors, mainly with cavernous sinus invasion, utmost removal of the mass could be obtained by stepwise maneuvers.
In our experience, iMRI has several advantages to early or late postoperative MRI. IMRI allows for immediate exploration of any questionable artifact commonly seen on early postoperative MRI. We have found it comparable in accuracy with late postoperative MRI, but it provides an opportunity for immediate tumor resection when needed.
In the case of known residual tumor, planning for further tumor management can already be initiated during or after surgery, via surveillance, transcranial resection, and treatment with antiproliferative substances or radiotherapy, respectively. Immediate intraoperative quality control predicts the result of the early control after 2 to 3 months.
Whatever future developments and improvements may occur in iMRI imaging for pituitary surgery, there are sufficient tools available today to significantly improve traditional surgical results.
References
Arita K, Kurisu K, Tominaga A, Kawamoto H, Iida K, Mizoue T, Pant B, Uozumi T (1998) Trans-sellar color Doppler ultrasonography during transspenoidal surgery. Neurosurgery 42:81–86
Black PM, Moriarty T, Alexander E 3rd, Stieg P, Woodard EJ, Gleason PL, Martin CH, Kikinis R, Schwartz RB, Jolesz FA (1997) Development and implecementation of intraoperative magnetic resonance imaging and its neurosurgical applications. Neurosurgery 41:831–845
Bohinski RJ, Warnick RE, Gaskill-Shipley MF, Zuccarello M, van Loveren HR, Kormos DW, Tew JM Jr (2001) Intraoperative magnetic resonance imaging to determine the extent of resection of pituitary macroadenomas during transsphenoidal microsurgery. Neurosurgery 49:1133–1144
Cappabianca P, Cavallo LM, Colao A, de Divitis E (2002) Surgical complications associated with the endoscopic endonasal transsphenoidal approach for pituitary adenomas. J Neurosurg 97:293–298
Dina TS, Feaster SH, Laws ER, Davis DO (1993) MR of the pituitary gland postsurgery. Serial MR studies following transsphenoidal resection. AJNR Am J Neuroradiol 14:763–769
Doppman JL, Ram Z, Shawker TH, Oldfield EH (1994) Intraoperative US of the pituitary gland. Work in progress. Radiology 192:111–115
Dort JC, Sutherland GR (2001) Intraoperative magnetic resonance imaging for skull base surgery. Laryngoscope 111(9):1570–1575
Elias WJ, Chadduck JB, Alden TD, Laws ER (1999) Frameless stereotaxy for transsphenoidal surgery. Neurosurgery 45:271–277
Fahlbusch R, Ganslandt O, Buchfelder M, Schott W, Nimsky C (2001) Intraoperative magnetic resonance imaging during transsphenoidal surgery. J Neurosurg 95:381–390
Fahlbusch R, Keller B, Ganslandt O, Kreutzer J, Nimsky C (2005) Transsphenoidal surgery in acromegaly investigated by intraoperative high-field magnetic resonance imaging. Eur J Endocrinol 153(2):239–248
Gerlach R, du Mesnil de Rochemont R, Gasser T, Marquardt G, Reusch J, Imoehl L, Seifert V (2008) Feasibility of Polestar N20, an Ultra-Low-Field intraoperative magnetic resonance imaging system in resection control of pituitary macroadenomas: lessons learned from the first 40 cases. Neurosurgery 63:272–285
Hardy J, Wigser SM (1965) Trans-sphenoidal surgery of pituitary fossa tumors with televised radiofluoroscopic control. J Neurosurg 23:612–619
Jane JA Jr, Thapar K, Alden TD, Laws ER Jr (2001) Fluoroscopic framless stereotaxy for transsphenoidal surgery. Neurosurgery 48:1302–1308
Jho HD, Alfieri A (2001) Endoscopic endonasal pituitary surgery: evolution of surgical technique and equipment in 150 operations. Minim Invasive Neurosurg 44:1–12
Kilic T, Ekinci G, Seker A, Elmaci I, Erzen C, Pamir MN (2001) Determining optimal MRI follow-up after transsphenoidal surgery for pituitary adenoma: scan at 24 hours postsurgery provides reliable information. Acta Neurochir (Wien) 143(11):1103–1126
Knosp E, Steiner E, Kitz K, Matula C (1993) Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings. Neurosurgery 33:610–618
Lasio G, Ferroli P, Felisati G, Broggi G (2001) Image-guided endoscopic transnasal removal of recurrent pituitary adenoms. Neurosurgery 48:1302–1308
Martin CH, Schwartz R, Jolesz F, Black PM (1999) Transsphenoidal resection of pituitary adenomas in an intraoperative MRI unit. Pituitary 2:155–162
McPherson CM, Bohinski RJ, Dagnew E, Warnick RE, Tew JM (2003) Tumor resection in a shared-resource magnetic resonance operating room: experience at the University of Cincinnati. Acta Neurochir Suppl 85:39–44
Nimsky C, Ganslandt O, von Keller B, Romstöck J, Fahlbusch R (2004) Intraoperative high-field-strenght MR imaging: implementation and experience in 200 patients. Radiology 233:67–78
Nimsky C, Ganslandt O, Fahlbusch R (2005) Comparing 0.2 Tesla with 1.5 Tesla intraoperative magnetic resonance imaging: analysis of setup, workflow and efficiency. Acad Radiol 12:1065–1079
Nimsky C, von Keller B, Ganslandt O, Fahlbusch R (2006) Intraoperative high-field magnetic resonance imaging in transsphenoidal surgery of hormonally inactive pituitary macroadenomas. Neurosurgery 59(1):105–114
Pergolizzi RS Jr, Nabavi A, Schwartz RB, Hsu L, Wong TZ, Martin C, Black PM, Jolesz FA (2001) Intra-operative MR guidance during trans-sphenoidal pituitary resection: preliminary results. J Magn Reson Imaging 13:136–141
Schulder M, Sernas T, Carmel PW (2003) Cranial surgery and navigation with a compact intraoperative MRI system. Acta Neurochir Suppl 85:79–86
Schwartz TH, Stieg PE, Anand VK (2006) Endoscopic transsphenoidal pituitary surgery with intraoperative magnetic resonance imaging. Neurosurgery 58(1 Suppl):ONS44–ONS51
Wolfsberger S, Ba-Ssalamah A, Pinker K, Mlynarik V, Czech T, Knosp E, Trattnig S (2004) Application of three-tesla magnetic resonance imaging for diagnosis and surgery of sellar lesions. J Neurosurg 100(2):278–286
Conflict of Interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Comment
The authors have reviewed a series of 72 patients with pituitary macroadenomas resected with imaging control in a high-field intraoperative MRI. This is a contemporary series, and can be compared to Professor Fahlbusch's previous publications on the advantages of MRI in determining extent of removal. The authors noted ability to remove 100% of tumors in which total resection was the preoperative goal. They also noted increase total removal of some tumors in which preoperative imaging did not indicate such a removal was possible. An important issue for the contemporary pituitary surgeon is gleaned from this report—as many neurosurgeons may believe that residuals may be readily identified with an angled endoscope, this experience has demonstrated that despite using the endoscope (to look around the corners), thin remnants within a fold of the descending diaphragm sellae could not be detected endoscopically but only using the iMRI. Such information is important, and in this regard, the authors have demonstrated the unique value of iMRI for the removal of larger adenomas.
WT Couldwell
Utah, USA
Many experienced pituitary surgeons noted the initial results of the Erlangen group with iMRI, and whilst acknowledging them as real pioneers in this field, were not really convinced by their reports. Those lucky enough to be able to use iMRI in their own units, myself included, found the significant time penalty (approximately more than doubling OR time in my own unit) unacceptable and the information predictable. We knew there would be tumor residuals in the cases where we used it, but these would be in places we could not get to, so many felt the initial Erlangen experience 'over egged' the case.
This report redresses this criticism, and strongly makes the case for regular use of iMRI in the majority of transsphenoidal procedures for macroadenomas. I have little doubt that if one makes the commitment, then scan time, etc., will make less inroads, and I will be interested to read in future, if others will more readily adopt iMRI following this report.
Michael Powell
London,UK
Rights and permissions
About this article
Cite this article
Paterno′, V., Fahlbusch, R. High-Field iMRI in transsphenoidal pituitary adenoma surgery with special respect to typical localization of residual tumor. Acta Neurochir 156, 463–474 (2014). https://doi.org/10.1007/s00701-013-1978-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-013-1978-4