Abstract
Background
Surgeons undertaking transsphenoidal surgery in patients with acromegaly confront multiple unique challenges secondary to the anatomic alterations caused by growth hormone–secreting tumors. The senior author has noted a fusiform dilatation of the cavernous carotid artery in many acromegalic patients. The authors aim to quantify this dilatation and correlate it with potential contributing factors.
Methods
Clinical and radiographic data were retrospectively assessed in acromegalic patients undergoing transsphenoidal surgery from 2000 through 2011. Randomly selected patients with nonsecreting pituitary adenomas were used as the control cohort. Demographic information, comorbidities, and preoperative growth hormone and insulin-like growth factor-1 levels were recorded. Magnetic resonance (MR) imaging variables included tumor size, diameters of the petrous, cavernous, and supraclinoid segments of the carotid artery, and extent and location of cavernous sinus invasion. Independent correlations between acromegaly and each variable were assessed with multivariate regression analysis.
Results
Forty randomly selected patients with growth hormone–secreting adenomas who underwent surgery and had MR imaging with thin coronal slices of the pituitary region were enlisted in our study cohort. The mean age was 45.7 years. Forty-two males (52.5 %) were included in the study. Mean carotid artery diameter measurements for acromegalic and control patients, respectively, were 4.2 vs. 3.8 mm (petrous carotid), 5.0 vs. 4.0 mm (cavernous carotid), and 3.3 vs. 2.9 mm (supraclinoid carotid). Multivariate analysis showed only age and cavernous carotid diameter were statistically significant independent variables (p = 0.02, p < 0.001, respectively). Age, tumor size, growth-hormone or insulin-like growth factor-1 levels, and cavernous sinus invasion did not correlate with cavernous carotid artery diameter.
Conclusions
In patients with acromegaly, there is a fusiform dilatation of the cavernous carotid artery that must be considered when planning transsphenoidal surgery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The first reliable medical account of acromegaly in 1567 by Dr. Johannes Wierus described a middle-aged woman who progressively slowed and developed coarse facial features secondary to presumed growth hormone (GH) hypersecretion [16]. Since then, tremendous strides have been made in the understanding of the clinical histopathology, diagnosis, and treatment of acromegaly. Nevertheless, acromegaly secondary to a functional pituitary adenoma, if left untreated, remains a potentially life-threatening illness secondary to hypertension, cardiac hypertrophy, diabetes, and other serious comorbidities [5, 16, 20].
Transsphenoidal surgery is the primary surgical approach for treatment of pituitary adenomas, but surgeons undertaking this procedure in patients with acromegaly face structural alterations caused by elevated GH secretion. Many documented alterations affect not only the surgical portion of the case but the preoperative, anesthetic, and postoperative portions. Navigation through the surgical corridor is hindered by bony overgrowth of the structures encountered, soft-tissue edema, and mucosal hypertrophy. Access to the sellar floor is hindered by thickened sphenoid sinus mucosa and significant variation in sinus dimensions, which can combine to hinder the freedom of surgical instrument manipulation [8, 13, 15, 19]. Once the sella floor has been breached, the primary cause for concern becomes avoiding cavernous carotid artery injury. Carotid variations in acromegalic patients have been noted, including increased tortuosity, prominence, course asymmetry, as well as decreased intercarotid distance [4, 7, 13, 19].
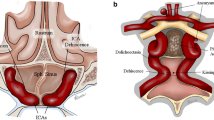
The senior author has observed a fusiform dilatation of the cavernous portion of the acromegalic carotid artery in many patients with acromegaly (Fig. 1), which has prompted discussion when planning transsphenoidal surgery. In this study, we quantified the cavernous carotid dimensions in patients with GH-secreting pituitary tumors and compared them with the dimensions in patients harboring pituitary tumors that do not secrete GH. We also evaluated potential contributing factors for correlation.
a Artist’s illustration depicting fusiform dilatation of the cavernous portion of the internal carotid artery in acromegaly. Blue illustrates the normal caliber of the internal carotid artery, and pink illustrates the presumed dilated caliber in an acromegalic patient. Dilatation primarily occurs in the cavernous segment. b and c Consecutive MR images illustrating the fusiform dilatation of the cavernous carotid artery (small arrow) by the tumor (large arrow)
Methods
Patient selection
This retrospective cohort study consisted of two groups of patients with pituitary adenomas. Patients were identified via an institutional review board-approved retrospective data review spanning the years 2000 to 2011. All patients who underwent transsphenoidal craniotomy for resection of pituitary tumors at the University of Utah hospital were identified. The study cohort included 40 randomly selected patients with histologically confirmed GH-secreting pituitary adenomas. Patients were excluded from this study if specific 1.5-T or 3-T magnetic resonance (MR) imaging with thin cuts through the sellar region were not readily available. The control cohort included the same number of randomly selected patients with histologically confirmed nonsecreting pituitary adenomas for which adequate MRI imaging was readily available.
The chart analysis elucidated pertinent clinical information including demographic statistics, anamnestic data, preoperative GH and insulin-like growth factor-1 (IGF-1) levels, radiographic measurements of tumor burden and internal carotid diameter, and pathologic findings from surgery. Recorded anamnestic data included a previous history of hypertension, type 2 diabetes mellitus, or smoking. This study was carried out in compliance with the requirements of the Health Insurance Portability and Accountability Act (HIPAA).
Radiographic evaluation
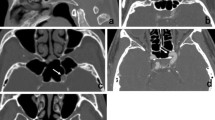
Measurements of tumor size, cavernous sinus invasion, and internal carotid artery diameter were obtained on post-contrast T1-weighted images using the picture archiving and communication system measurement tool. Tumor size was measured as an area taking the longest longitudinal and craniocaudal axes in the coronal plane. Diameters of the anterior vertical segment of the petrous segment (Fig. 2a), the best-visualized cross-sectional view of the cavernous segment (Fig. 2b), and the supraclinoid segment (Fig. 2c) of the carotid artery just proximal to the bifurcation were recorded. The vessel diameters were measured perpendicular to the long axis for both the right and left internal carotid arteries. As the artery diameters for each side were found to be similar, the mean artery diameter was calculated. All measurements were taken in succession by both an attending neurosurgeon (RBC) and an attending neuroradiologist (KLS). The presence of cavernous sinus invasion and the sidedness of tumors in patients with GH-secreting tumors were also noted.
Statistical analysis
Patients were stratified according to the primary outcome of histologically confirmed GH-secreting pituitary adenomas (acromegaly) or non-secreting pituitary adenomas. For univariate analysis, categorical variables were compared to the outcome using chi-square analyses, and continuous variables were compared to the outcome using t-tests. Statistically significant variables (p < 0.05) on univariate analyses were evaluated using multivariate logistic regression modeling to identify independent predictors of acromegaly. A probability value of <0.05 was also considered statistically significant with the multivariate analysis.
Pearson coefficients were used to measure the correlation between age, tumor area, GH levels or cavernous sinus invasion, and the mean cavernous carotid diameter, as all of these continuous variables had a parametric distribution. IGF-1 levels were not normally distributed; therefore, the Spearman coefficient was used to compare with the mean cavernous carotid diameter.
To measure agreement in the carotid artery diameter measurements between the two independent raters (RBC and KLS), interclass correlation coefficients were employed. Pearson correlation coefficients were also used to confirm this agreement. The statistical software package SAS (version 9.2; SAS Institute Inc.) was used for statistical analysis.
Results
Patient characteristics
We included 40 randomly selected acromegalic patients treated at our institution from 2000 to 2011. All patients had histologically confirmed GH-secreting tumors and MR imaging with thin-section imaging of the sellar region. Similarly, we randomly selected 40 patients with non-secreting adenomas (also from our institution) as our control group. Baseline demographic data are presented in Table 1 for both acromegalic and nonsecreting adenoma patients. The mean age for all patients was 45.7 years. A total of 42 males (52.5 %) and 38 females (47.5 %) were included.
Anamnestic data
Key comorbidity information for both cohorts is presented in Table 1. Both cohorts had similar low rates of comorbidities. Overall, 23.8 % of patients had hypertension, 11.3 % had type 2 diabetes mellitus, and 10.0 % reported smoking. The low rates of hypertension and type 2 diabetes correlated with health data reports from Utah in 2012 (http://healthyamericans.org/states/?stateid=UT#section=1,year=2011,code=undefined).
Radiographic measurements
Table 1 highlights tumor measurements and diameters of the internal carotid artery. The mean tumor area for acromegalic patients was 234.6 mm2, whereas the mean tumor area in nonsecreting adenoma patients was 275.8 mm2. The mean diameter for the internal carotid artery of acromegalic patients was significantly larger than that of patients with nonsecreting adenomas at all three locations evaluated (p < 0.001) (Fig. 3).
Factors associated with acromegaly
Table 2 summarizes the univariate and multivariate analysis for categorical and continuous variables associated with acromegaly. Age was noted to be associated with acromegaly in our patient cohort in that patients with acromegaly were noted to be younger at the time of treatment (p = 0.01). No such association was noted for patient sex, history of hypertension, diabetes, or smoking. Tumor size was not associated with acromegaly. A significant association was noted between acromegaly and the cavernous, petrous, and supraclinoid carotid diameters on univariate analysis (p < 0.001). We then took all the significant variables and used stepwise multivariate regression analysis to develop a model predicting acromegaly (Table 2). Only age (p = 0.02) and the cavernous carotid artery diameter (p < 0.001) retained an independent statistical significance in predicting acromegaly, whereas both the petrous carotid and supraclinoid carotid measurements failed to retain statistical significance. This finding, therefore, demonstrates that the acromegalic population maintains a significant dilatation of the cavernous carotid segment compared with non-secreting control patients. The model was highly discriminatory in our study population (c = 0.88).
Factors associated with cavernous carotid diameter
Table 3 summarizes a subgroup analysis of variables in acromegalics that might affect cavernous carotid diameter. Neither age (Pearson coefficient of 0.13, p = 0.41) nor tumor size (Pearson coefficient of −0.07, p = 0.71) was found to be associated with cavernous carotid artery diameter. Both followed a parametric distribution.
Growth hormone levels were available in 29 patients with acromegaly (Table 1). The levels did not follow a parametric distribution, with a median value of 21.3 ng/ml and an interquartile range of 39.2 ng/ml. IGF-1 levels followed a parametric distribution in the 32 acromegalic patients for whom they were available, with a mean value of 784.0 ng/ml and a standard deviation of 288 ng/ml. Neither GH levels (Spearman correlation coefficient of 0.28, p = 0.13) nor IGF-1 levels (Pearson correlation coefficient of 0.19, p = 0.3) were found to correlate with cavernous carotid diameter.
Cavernous sinus invasion was confirmed in eight patients with acromegaly (right = 5, left = 3). None of the patients in our series had bilateral cavernous sinus invasion. Sinus invasion did not correlate with mean cavernous carotid diameter (p = 0.12).
Reproducibility and reliability of measurements
Interclass correlation coefficients demonstrated substantial agreement between the mean cavernous carotid diameter measurements performed by the attending neurosurgeon and attending neuroradiologist (ICC = 0.79 [95 % CI 0.69–0.86]). The Pearson coefficient confirmed this agreement (r = 0.84, p < 0.001). This agreement was confirmed when evaluating the raw single-sided data as well, namely the right cavernous carotid diameter (ICC = 0.73 [95 % CI 0.61–0.82]; r = 0.76, p < 0.001). Similar values were noted for all of the individual segment measurements.
Discussion
Untreated acromegaly caused by a GH-secreting pituitary adenoma can be life threatening because of a myriad of somatic alterations and multiple serious comorbidities [1, 5, 16, 19, 20]. As prompt diagnosis and treatment can lead to a mortality risk reduction to levels similar to those of the general population, considerable emphasis has been placed on the timely normalization of GH levels, reduction of tumor bulk, correction of endocrinopathies, and prevention of recurrence [9].
Transsphenoidal surgery in acromegalic patients
Although transsphenoidal surgery remains the primary surgical approach for treatment of pituitary adenomas, numerous anatomic alterations caused by acromegaly have been noted in structures encountered during the transsphenoidal approach [2]. Edema of the airway structures, preexisting cardiopulmonary conditions, and subsequent venous congestion increase the risk of anesthetic complications and require special preoperative planning [3, 11, 19]. Mucosal edema, hypertrophic nasal turbinates, and bony overgrowth of the nasal and sphenoid structures distort the anticipated anatomy and constrict the natural working corridor of standard transsphenoidal surgery [13, 15]. The risk of carotid artery injury in transsphenoidal surgery because of variations in the typical carotid artery course remains the most feared complication. Ebner et al. [4] used helical computed tomography imaging indirectly to show that the intercarotid distance was approximately 1.64 cm in acromegalics and 1.90 cm in nonacromegalics. Increased tortuosity, asymmetric courses, and marked prominence of the carotid artery have also been noted in patients with long-standing acromegaly [6, 13, 19]. Occasionally, the arteries on each side can breach the cavernous walls and enter the intrasellar space, a phenomenon known as “kissing carotids.” Manara et al. [10] retrospectively reviewed 152 consecutive neuroimaging studies on acromegalic patients and found 17.3 % harbored intracranial aneurysms. This differed markedly from the estimates of 0.4 % to 6 % in the general population [10, 18]. These factors all need to be considered when planning transsphenoidal surgery on acromegalic patients.
Carotid artery diameter in acromegalic patients
We directly measured, via modern cross-sectional imaging, the diameter of the carotid artery adjacent to GH-secreting tumors. Patients with acromegaly had significantly larger cavernous segments of the internal carotid artery than patients with nonfunctioning pituitary adenomas (5 mm vs. 4 mm, p < 0.001). This dilatation appeared mainly contained within the cavernous segment, as confirmed by multivariate regression analysis. Measurements were found to be reproducible as we found substantial agreement between investigators. Age retains statistical significance after the multivariate regression analysis as acromegalic patients were diagnosed and treated at a younger age. Operative intervention for nonfunctional adenomas is typically offered only when the visual defects, mass effect on the optic chiasm, hypopituitarism caused by macroadenoma, and serially documented enlargement of the mass are present, leading to a later start of treatment [14].
Interestingly, when we attempted to elucidate what factors account for this cavernous carotid dilatation in acromegalics, age, tumor size, GH levels, IGF-1 levels, and cavernous sinus invasion were not found to correlate with vessel diameter.
Implications of carotid artery enlargement in acromegalic patients
The observations from this study have implications for the surgical management of patients with acromegaly. It has been noted by other authors that the intercarotid distance is smaller in acromegalic patients than in other patients harboring nonfunctional adenomas [5]. This observation may in part be explained by general enlargement of the artery as noted in the present study. Based on our results and previously reported findings regarding the anatomical changes to the surgical corridor and vascular changes seen in acromegalic individuals [8, 13, 15, 19], these patients may be more susceptible to carotid injury during the transsphenoidal approach. In this regard, Laws [7] reported a 0.78 % rate of carotid artery injury in a series of 3,061 transsphenoidal surgeries for pituitary disease. Specifically, studies examining rates of carotid artery injury in acromegalic patients are nonexistent.
The observation of carotid fusiform enlargement in patients with acromegaly also may have broader implications regarding the genesis of intracranial aneurysms. When cerebral angiography was commonly used in the workup of pituitary adenomas, there was significant debate regarding whether intracranial systemic aneurysms were more prevalent in patients with acromegaly [17]. In support of this is the recent study of Manara et al. [10], which has documented an increased prevalence of intracranial saccular aneurysms in patients with acromegaly. Additional studies have hinted at the direct effects of excess GH and IGF-1 in acromegalic individuals leading to cerebral vascular wall thickening and subsequent aneurysm development [12]. This is a specific question that is being addressed in our own research. Further studies are warranted to assess the effect of GH or IGF-1 on the arterial wall as potential etiologic factors for both fusiform dilatation and aneurysmal development.
Conclusion
We report the first study to use modern cross-sectional imaging technology to quantify the disproportionate fusiform dilatation of the cavernous carotid artery in acromegalic patients. The cause for this observation is unknown at this time but prompts additional consideration when planning transsphenoidal surgery in these patients to prevent carotid artery injury. This observation suggests further study is needed in assessing the effect of hormonal factors in the genesis of intracranial aneurysms.
References
Colao A, Ferone D, Marzullo P, Lombardi G (2004) Systemic complications of acromegaly: epidemiology, pathogenesis, and management. Endocr Rev 25:102–152
Couldwell WT (2004) Transsphenoidal and transcranial surgery for pituitary adenomas. J Neurooncol 69:237–256
Dougherty TB, Cronau LH Jr (1998) Anesthetic implications for surgical patients with endocrine tumors. Int Anesthesiol Clin 36:31–44
Ebner FH, Kuerschner V, Dietz K, Bueltmann E, Naegele T, Honegger J (2009) Reduced intercarotid artery distance in acromegaly: pathophysiologic considerations and implications for transsphenoidal surgery. Surg Neurol 72:456–460, discussion 460
Harris AG (1996) Acromegaly and its managment. Lippincott-Raven Publishers, Philadephia
Laws ER (2008) Surgery for acromegaly: evolution of the techniques and outcomes. Rev Endocr Metab Disord 9:67–70
Laws ER Jr (1999) Vascular complications of transsphenoidal surgery. Pituitary 2:163–170
Laws ER Jr, Piepgras DG, Randall RV, Abboud CF (1979) Neurosurgical management of acromegaly. Results in 82 patients treated between 1972 and 1977. J Neurosurg 50:454–461
Liu JK, Weiss MH, Couldwell WT (2003) Surgical approaches to pituitary tumors. Neurosurg Clin N Am 14:93–107
Manara R, Maffei P, Citton V, Rizzati S, Bommarito G, Ermani M, Albano I, Della Puppa A, Carollo C, Pavesi G, Scanarini M, Ceccato F, Sicolo N, Mantero F, Scaroni C, Martini C (2011) Increased rate of intracranial saccular aneurysms in acromegaly: an MR angiography study and review of the literature. J Clin Endocrinol Metab 96:1292–1300
Nemergut EC, Dumont AS, Barry UT, Laws ER (2005) Perioperative management of patients undergoing transsphenoidal pituitary surgery. Anesth Analg 101:1170–1181
Oshino S, Nishino A, Suzuki T, Arita H, Tateishi A, Matsumoto K, Shimokawa T, Kinoshita M, Yoshimine T, Saitoh Y (2012) Prevalence of cerebral aneurysm in patients with acromegaly. Pituitary. doi:10.1007/s11102-012-0404-x
Saeki N, Iuchi T, Higuchi Y, Uchino Y, Murai H, Isono S, Yasuda T, Minagawa M, Yamaura A, Sunami K (2000) Bone CT evaluation of nasal cavity of acromegalics—its morphological and surgical implication in comparison to non-acromegalics. Endocr J 47(Suppl):S65–S68
Sivakumar W, Chamoun R, Nguyen V, Couldwell WT (2011) Incidental pituitary adenomas. Neurosurg Focus 31(6):E18
Skinner DW, Richards SH (1988) Acromegaly—the mucosal changes within the nose and paranasal sinuses. J Laryngol Otol 102:1107–1110
van der Lely AJ, Beckers A, Daly AF, Lamberts SW, Clemmons DR (2005) Acromegaly—pathology, diagnosis, and treatment. Taylor & Francis Group, Boca Raton
Weir B (1992) Pituitary tumors and aneurysms: case report and review of the literature. Neurosurgery 30:585–591
White PM, Wardlaw JM (2003) Unruptured intracranial aneurysms. J Neuroradiol 30:336–350
Zada G, Cavallo LM, Esposito F, Fernandez-Jimenez JC, Tasiou A, De Angelis M, Cafiero T, Cappabianca P, Laws ER (2010) Transsphenoidal surgery in patients with acromegaly: operative strategies for overcoming technically challenging anatomical variations. Neurosurg Focus 29(4):E8
Zada G, Sivakumar W, Fishback D, Singer PA, Weiss MH (2010) Significance of postoperative fluid diuresis in patients undergoing transsphenoidal surgery for growth hormone-secreting pituitary adenomas. J Neurosurg 112:744–749
Acknowledgments
The authors thank Kristin Kraus, M.Sc., for her steadfast assistance in the preparation of this article and Jennie Swensen, M.A., for her medical illustration.
Conflicts of interest
None.
Author contribution
Conception and design: Couldwell, Chamoun, Sivakumar. Acquisition of data: Chamoun, Salzman, Sivakumar. Analysis and interpretation of data: Couldwell, Riva-Cambrin, Chamoun, Sivakumar. Drafting the article: Sivakumar. Critically revising the article: all authors. Reviewed submitted version of the manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Couldwell. Statistical analysis: Riva-Cambrin, Sivakumar. Study supervision: Couldwell.
Author information
Authors and Affiliations
Corresponding author
Additional information
Comment
This is an interesting paper, but of course without a conclusion as to why these tumours should cause dilatation. I was frankly surprised that neither hypertension nor, particularly, diabetes was more prevalent as our acromegalics are definitely more often diabetic and, I thought, hypertensive. Perhaps this is a phenomenon of healthy Utah.
I would be interested if this same study could be done on your Cushing's patients, which I understand is in preparation. Perhaps this will give the answer.
The message—surgeon beware the carotid in acromegalics—is a useful reminder.
Michael Powell
London, UK
Rights and permissions
About this article
Cite this article
Sivakumar, W., Chamoun, R.B., Riva-Cambrin, J. et al. Fusiform dilatation of the cavernous carotid artery in acromegalic patients. Acta Neurochir 155, 1077–1083 (2013). https://doi.org/10.1007/s00701-013-1691-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-013-1691-3