Abstract
Background
Wnt proteins are bifunctional axon guidance molecules, several of which appear to mediate guidance of corticospinal tract axons along the spinal cord. Here, we studied increasing effect on regeneration by Wnt-containing alginate scaffolds on spinal cord injury (SCI).
Methods
A total of 32 rats were injured at the T7–8 level with an NYU impactor. According to transplantation materials, rats were classified into four groups: a Wnt3a-secreting fibroblast transplantation group (Wnt group, n = 8), a Wnt3a-secreting fibroblast with alginate transplantation group (Wnt + alginate group, n = 8), an alginate transplantation group (alginate group, n = 8), and a contusion-only group (sham group, n = 8). Behavioral tests were performed on the first, second, and third days after injury, and then weekly for 8 weeks. Five of the eight rats from each group were selected for manganese-enhanced magnetic resonance imaging (ME-MRI). Two rats from each group were examined for GAP43 and MAP2 expression using monoclonal and polyclonal primary antibodies, respectively.
Results
Seven weeks after transplantation (8 weeks after SCI), Wnt + alginate group rats achieved an average Basso–Beattie–Bresnahan locomotor score of 19.0, which was significantly higher than that of other groups. ME-MRI at 8 weeks after SCI revealed significantly higher relative signal intensities in the Wnt + alginate group. Gap43 and Map2 immunostaining, showed strong positive in the Wnt + alginate group.
Conclusion
The Wnt + alginate complex exerted significantly enhanced recovery in a rat SCI model compared to alginate or Wnt3a alone. These results suggest that alginate scaffolds facilitate the regeneration of axon working with Wnt3a protein that promotes regeneration of the injured spinal cord.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wnt proteins are important regulators of the proliferation, self-renewal, specification, and differentiation of neural precursors in the CNS. Accumulating evidence suggests that Wnt proteins also play important roles in axon development during the formation of the nervous system, acting as bifunctional axon-guidance molecules. Notably, several Wnt proteins appear to mediate guidance of corticospinal tract axons along the spinal cord. Among them, Wnt-1 and Wnt-3a control spinal cord dorsal interneuron specification. Different Wnt proteins distinctly regulate the proliferation and neurogenesis of developing midbrain and cortical neural precursors [12, 13, 17, 20, 26].
Our previous report has shown that transplanted Wnt3a-secreting fibroblasts promote axonal regeneration and functional improvement after spinal cord injury (SCI) [28]. However, the efficacy of directly injected Wnt-expressing fibroblasts is limited by leakage of Wnt protein through the injection tract, prompting us to test co-injection of Wnt-expressing fibroblasts with supporting materials as scaffolds. In an animal study, an alginate scaffold used as a bioartificial material showed a significant beneficial effect on axonal regrowth into the cord caudal to the alginate gap. Regenerating axons elongated into white as well as grey matter after traversing through alginate [29]. So, we hypothesized the role of alginate as a scaffold to prevent the leakage of Wnt-expressing fibroblasts thus result in much stronger regenerative effect on injured spinal cord. Here, we describe the enhancing effect by alginate as a scaffold on regeneration with the treatment of Wnt-secreting fibroblasts in a rat SCI model.
Materials and methods
Animal preparation
Animal experiments were approved by the Institutional Animal Care and Use Committee of Asan Institute for Life Sciences, Seoul, Republic of Korea. A total of 32 female Wistar rats weighing 230–280 g at the time of injury were used. Before injury, all rats were housed two per cage under simulated daylight conditions with alternating 12-h light–dark cycles, and had free access to food and water. All rats were anesthetized with an intraperitoneal injection of a mixture of Zoletil (35 mg/kg) and Rompun (5 mg/kg). The vertebral column was exposed between T7 and T8, and a total laminectomy was performed at T7 without incision of the dura. A moderate spinal cord contusion injury was induced by dropping a 10-g rod from a height of 25 mm using an NYU impactor (New York University weight-drop device). After the procedure, the skin was primarily closed, and rats were given a prophylactic subcutaneous injection of cefazolin (10 mg/kg). For pain management, all animals were given a subcutaneous injection of ketoprofen (5 mg/kg) once daily for 3 days. After injury, all rats were housed individually in the same pre-injury environment. Bladders were emptied manually once daily until spontaneous control of urination returned.
Alginate preparation
Alginate (ProNova MVG; ProNova Polymers, Norway) was formed from a 1 % (w/w) solution of alginate polymer. To prepare solution, the 1 % alginate solutions were combined with 1 × 106 Wnt3a-secreting fibroblasts. And then cell mixture combined 0.5 % calcium sulfate using a syringe for polymerization.
Cell preparation and transplantation
All cell lines were provided by the Department of Life Sciences, The University of Seoul, Seoul, Republic of Korea. Wnt3a-secreting fibroblasts were established as previously described [15, 32].
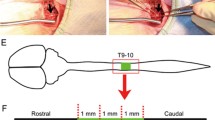
One week after injury, rats were divided into four groups: (1) a contusion-only group (sham group, n = 8); (2) a Wnt3a-secreting fibroblast transplantation group (Wnt group, n = 8); (3) an alginate transplantation group (alginate group, n = 8); and (4) a Wnt3a-secreting fibroblast with alginate transplantation group (Wnt + alginate group, n = 8). For cell transplantation, animals were anesthetized and the injured cord site was re-exposed. In the Wnt group, 15 μL of an aqueous solution containing 1 × 106 Wnt3a-secreting fibroblasts was injected. In the Wnt + alginate group, 15 μL of an aqueous solution containing 1 × 106 Wnt3a-secreting fibroblasts, 1 % alginate, and 0.5 % CaSO4 was injected. In the alginate group, 15 μL of an aqueous solution containing 1 % alginate was injected. The solution was delivered at a rate of 0.5 μL/min via a 20-μm diameter, 15-μL Hamilton syringe (Hamilton Company, NV, USA) inserted into the center of the contusion site at a puncture rate of 1 mm/min and to a depth of 1 mm from the dorsal dura without opening the dura [28]. After injection, the needle was left in place for 3 min and then withdrawn at a rate of 1 mm/min. The surgical site was then re-closed.
Behavioral tests and magnetic resonance imaging
Open-field testing procedures were performed for all groups as previously described. Before the test, each rat was adapted to the open field (90 × 90 cm, 10 cm wall height). When a rat had demonstrated acclimatization by walking continuously in the open field, its locomotor performance was recorded with a video camera for 10 min. From the video, an examiner, blinded to group-identifying information, quantitatively assessed the locomotor performance of each rat according to the Basso–Beattie–Bresnahan (BBB) locomotor rating scale [1]. Behavioral tests were performed on the first, second, and third days after injury, and then weekly for 8 weeks. This BBB score measurement was done by two different examiners. Data processing and statistical analyses of BBB scores in all three groups were analyzed by one-way ANOVA followed by a post hoc Tukey–Kramer multiple comparison test. Interobserver reliability was calculated with interclass correlation coefficient (ICC).
Five of the eight rats in each group were randomly selected for manganese-enhanced magnetic resonance imaging (ME-MRI) with the same methodology as the authors described previously [31]. The rats underwent ME-MRI twice, once before transplantation and again 7 weeks after transplantation (8 weeks after SCI). For enhancement, the contrast agent manganese was injected into the lateral ventricle 48 h before the MRI scan. Under general anesthesia, the bregma of the rat was exposed, and the animal was mounted on a stereotaxic frame (myNeuroLab, Richmond, IL, USA). Then, 6 μL of an aqueous 0.17 M MnCl2 solution was slowly (1 μL/min) injected unilaterally into the lateral ventricle (1 mm caudal, 1.5 mm lateral, and 3.5 mm deep from the bregma) using a Hamilton syringe and a Nano-injector. After the injection, the needle was slowly withdrawn to prevent regurgitation, and the skin was primarily closed. After MnCl2 solution injection, all MRI scans were performed using a clinical 4.7-T Bruker Biospin imager (Bruker Medical Systems, Karlsruhe, Germany) with the following parameters: TR (repetition time), 300.0 ms; TE (echo time), 12.0 ms; partition thickness, 0.94 mm; field of view, 12.0 × 4.0 cm; and matrix size, 256 × 256. During MRI, all rats were anesthetized and artificially ventilated through a nose cover with a mixture of O2 and N2O (1:2) and 5 % isoflurane. Relative signal intensities (RSIs) were then measured using a 4.7-T Bruker Biospin imager and associated analysis software (Bruker Medical Systems, Karlsruhe, Germany), and compared among groups. To evaluate the recovery of neuronal connections by ME-MRI, we compared the RSIs of spinal cords caudal to the lesion to those of ventricles. To establish a reference value (100 %), we calculated the mean signal intensity in a 2 × 2-mm square in a uniformly enhanced region of the ventricle. Mean signals in spinal cords in same-sized regions caudal to the lesion were calculated, and expressed relative to the reference ventricle value as a percent. The RSIs before and 7 weeks after transplantation (8 weeks after SCI) were compared for each group using Student's t-test, and differences among groups were analyzed by one-way ANOVAs followed by post hoc Tukey–Kramer multiple comparison tests.
Immunohistochemical staining
For immunofluorescence analysis, 7 weeks after transplantation (8 weeks after SCI), all rats were perfused with phosphate-buffered saline (PBS) and 4 % paraformaldehyde (PFA). The spinal cords were extracted and fixed with 4 % PFA for 6 h followed by incubation with 30 % sucrose in PBS overnight. Serial longitudinal sections of the spinal cord (5-μm thick) were obtained using a cryotome (Microm, Germany) and mounted on poly-L-lysine-coated Superfrost-Plus slides (Matsunami, Japan). The sections were washed three times with Tris-buffered saline (TBS), then blocked with TBS containing 5 % bovine serum albumin and 0.1 % Triton X-100 for 1 h at room temperature. After incubating overnight, sections were washed three times with TBS and then incubated with a fluorescent-conjugated secondary antibody (1:1,000; Invitrogen, CA, USA). A monoclonal antibody against Gap43 (1:200; Abcam, UK) and an antibody that recognizes Map2 (1:200; Abcam, UK) were used as primary antibodies. Sections were observed under a fluorescence microscope (Carl Zeiss Meditec, Germany). Appropriate controls, including omission of either primary or secondary antibodies, were included to confirm that the primary antibodies produced specific staining.
Comparing of existing cell numbers with or without alginate
The difference in existence of Wnt3a-secreting fibroblasts injected into rat spinal cords between Wnt and Wnt + alginate groups in the immediate post-injection stage was demonstrated by injecting labeled Wnt3a-secreting fibroblasts into one rat in each group. Labeled cells were prepared by seeding Wnt3a-secreting fibroblasts (3 × 105 cells) onto 6-well plates, and after allowing cells to grow for 24 h, adding Cell Stalker (Biterials, Korea) at a final concentration of 0.2 mg/ml. After centrifugation, 15 μL of an aqueous solution containing 1 × 106 labeled Wnt3a-secreting fibroblasts were injected as described above. Twenty-four hours after injection, two rats with labeled Wnt3a-secreting fibroblasts were sacrificed, and spinal cord sections were prepared, stained and observed under a fluorescence microscope as described above.
Results
Seven weeks after transplantation (8 weeks after SCI), rats in the Wnt + alginate group achieved an average BBB score of 19.0 ± 0.46, which was significantly higher than the BBB scores of rats in the sham (14.3 ± 0.45), Wnt (17.6 ± 0.26), and alginate (14.8 ± 0.53) groups (P < 0.05; Fig. 1), indicating gradual partial recovery of locomotor function. Therefore, neurological improvement by transplantation of Wnt3a-secreting fibroblasts was significantly increased with alginate after SCI in rats. Overall ICC was 0.56 (95 % confidence interval: 0.507–0.614). Each ICCs at 7, 14, 21, 28, 35, 42, 49, and 56 days were 0.51, 0.86. 0.70, 0.90, 0.84, 0.93, 0. 94, and 0.96.
To confirm axonal regeneration using ME-MRI, we first examined all rats by ME-MRI prior to transplantation (1 weeks after injury), at which time the RSIs of the four groups were not significantly different. RSIs of spinal cords at the middle T9 body level were compared to those of the brain stem on ME-MRI before transplantation and 7 weeks after transplantation in the sham group, Wnt group, alginate group, and Wnt + alginate group. As shown in Fig. 2, the second ME-MRI performed 7 weeks after transplantation (8 weeks after SCI) revealed a significantly higher RSI in the Wnt + alginate group (60.3 % ± 1.2 %) compared to the sham (30.0 % ± 1.6 %), Wnt (33.3 ± 1.8 %), and alginate (34.4 % ± 1.6 %) groups. Because manganese is transported distally through the axon, a higher RSI is indicative of greater axonal regeneration. RSIs in the Wnt + alginate group 7 weeks after transplantation (60.3 % ± 1.2 %) were significantly increased compared to pre-transplantation values (33.4 % ± 1.7 %; P < 0.05), and also greater than those of the other groups (P < 0.05), which showed no significant difference in RSIs between pre- and post-transplantation (P = 0.725, 0.15, and 0.13 for sham, Wnt, and alginate groups, respectively). This analysis, which compares values in a distal part of the contusion to that in the brain, indicates increased connectivity through axonal regeneration in the Wnt group (Fig. 3).
Gap43, a small acidic membrane protein expressed in the growth cones of axons, is one of the best known axonal regeneration markers. Confocal microscopic analyses showed that Gap43 immunoreactivity in the injured spinal cord was greater in Wnt + alginate group rats than in those in sham, Wnt, and alginate groups at 5 weeks after transplantation (6 weeks after SCI) (Fig. 4). In addition, anti-Map2 staining at this time was also highest in the Wnt + alginate group, indicating that an environment favorable for axonal regeneration was created in the Wnt + alginate group (Fig. 5).
Cell-labeling test showed larger numbers of labeled cells in the Wnt + alginate group compared to the Wnt group, indicating that the addition of alginate to Wnt3a-secreting fibroblasts allowed more cells to exist in the injured spinal cord (Fig. 6).
Discussion
We have reported that transplanted Wnt3a-secreting fibroblasts promote axonal regeneration and functional improvement after SCI [28], consistent with previous reports on the recovering-promoting action of Wnt in spinal cord injury. Wnt proteins signal through three main pathways: the canonical Wnt/β-catenin pathway, the planar cell polarity pathway, and the calcium pathway [19]. In the canonical pathway, Wnt proteins inhibit glycogen synthase kinase (GSK)-3β, resulting in β-catenin cytoplasmic stabilization, translocation to the nucleus, and transcription of T-cell factor (TCF)/Lef target genes. It is well documented that β-catenin controls the expansion of neural precursors through TCF activity [6, 11, 33]. Thus, β-catenin stabilization results in an enlarged brain and spinal cord, whereas loss of β-catenin reduces brain size and increases neuronal differentiation [11, 31]. In agreement with these findings, the dorsal neural tube is reduced in size in Wnt1/Wnt3a mutant mice [14]. In contrast, β-catenin stabilization increases neuronal differentiation of embryonic stem cells and neural precursors [12, 24]. Therefore, activation of the Wnt/β-catenin signaling pathway regulates proliferation and neuronal differentiation of neural precursors and could be considered a therapeutic target for the treatment of neurodegenerative diseases and nerve injury [7, 9, 12].
Wnt3a, which was used in our previous study, is expressed at dorsal areas of the developing spinal cord, and acts through the canonical pathway to promote spinal cord neural precursor development, and stimulate neurite outgrowth [18, 25].
In addition to showing that exogenous Wnt3a administration substantially improves locomotor function and increases neuronal population, a previous study demonstrated a continuous Wnt protein-secreting effect of fibroblasts are similar [28].
Scaffolds have been previously shown to exert recovery-promoting effects at spinal cord injury sites [5, 30]. Notably, one study suggested that alginate contributed to reducing the barrier composed of connective tissues and served as a scaffold for the outgrowth of regenerating axons and elongation of astrocytic processes [16]. Our results using Wnt3a-secreting fibroblast with alginate are promising in the context of recovery of injured spinal cords. Three mechanisms may contribute to the beneficial effects of alginate scaffolds on the efficacy of Wnt-expressing fibroblasts: (1) prevention of leakage of Wnt protein and secreting fibroblasts, (2) creation of a biocompatible environment within the cavity, and (3) serving as a bridge in the cavity. An anti-leakage effect was shown in our cell labeling comparison between Wnt and Wnt + alginate groups. We believe that the biocompatibility and bridging effects can be explained by the properties of the alginate polymer (Fig. 7). So, we hypothesized the role of alginate as a scaffold to prevent the leakage of Wnt-expressing fibroblasts thus result in much stronger regenerative effect on injured spinal cord.
Schematic representation of the role of alginate as a scaffold. Alginate polymer entrap the Wnt3a-secreting fibroblasts in the injured spinal cord cavity to prevent the leakage of the fibroblast. Three mechanisms may contribute to the beneficial effects of alginate scaffolds on the efficacy of Wnt-expressing fibroblasts: (1) prevention of leakage of Wnt protein and secreting fibroblasts, (2) creation of a biocompatible environment within the cavity, and (3) serving as a bridge in the cavity
We showed significant improvement in BBB scores and RSIs in the Wnt + alginate group. In previous study we observed BBB score for 6 weeks, but there wasn’t plateau at the peak BBB score [49]. So, we decided to observe the BBB score for 2 weeks more to show the steady state at the peak BBB score. Therefore, neurological improvement by transplantation of Wnt3a-secreting fibroblasts was significantly increased with alginate after SCI in rats. Although overall interobserver reliability (ICC: 0.56) seems to be relatively lower, those values at each stages except 7 days showed higher reliability. The reason for lower value of overall interobserver reliability seems to be related with high statistical sensitivity in lower BBB scores before 7 days.
ME-MRI revealed definite enhancement below the injury level in the Wnt + alginate group. The transportation of manganese via only live fibers in ME-MRI was demonstrated in previous studies [3, 27, 28]. Our results showed a statistically significant RSI difference and gross enhancement in the Wnt + Alginate group. However, unlike our previous report [28], we found little change in ME-MRI and no significant RSI improvement in the Wnt group despite of significant BBB improvement compared to sham and alginate groups. We believe that this apparent discrepancy is related to differences in the intensity of the injury. In the previous report, a severe spinal cord contusion injury was induced by dropping 10-g rod from a height of 50 mm using an NYU impactor [28], whereas, in the present study, we dropped a 10-g rod from a height of 25 mm using the same device. The less injury in our model might produce less change in ME-MRI, accounting for the absence of significant RSI improvement in the Wnt group.
The neuronal protein, Gap43, has been implicated in synaptic membrane reorganization and synaptic function [2, 23]. In addition, GAP43 is known to be selectively enriched in presynaptic axonal terminals and growth cones [2, 4, 23], maximally expressed in developing and regenerating neurons, and is involved in neurite outgrowth and growth cone navigation [2, 4, 8]. It also plays an important role in synaptic plasticity by regulating neurotransmitter release, endocytosis, and long-term potentiation [21]. Studies have indicated that Gap43 interacts with several signaling molecules, including calmodulin and protein kinase C [2, 8, 22]. Although the cytoskeleton protein Map2 is mainly localized in dendrites and dendritic spines [2, 23], it can also be present with Gap43 in axons and axonal terminals in the neuronal soma and axonal base in immature or developing neurons [10]. Our immunostaining results showed that rats in the Wnt + alginate group exhibited a prominent staining in these two proteins compared to Wnt group. It suggests alginate as a scaffold might enhance axonal regeneration induced by Wnt protein.
Conclusion
Treatment with a Wnt + alginate complex significantly improved neurological recovery and produced more neuronal regeneration markers in a rat SCI model compared to treatment with either agent alone, and more Wnt3a-secreting cells found to exist in injured spinal cord when treated together. These results suggest that alginate scaffolds facilitate environment for exogenous therapeutic cells in the injured spinal cord.
References
Basso DM, Beattie MS, Bresnahan JC (1995) A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 12(1):1–21
Benowitz LI, Routtenberg A (1997) GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosci 20(2):84–91
Bilgen M, Dancause N, Al-Hafez B, He YY, Malone TM (2005) Manganese-enhanced MRI of rat spinal cord injury. Magn Reson Imaging 23(7):829–832
Burry RW, Lah JJ, Hayes DM (1992) GAP-43 distribution is correlated with development of growth cones and presynaptic terminals. J Neurocytol 21(6):413–425
Chen BK, Knight AM, de Ruiter GC, Spinner RJ, Yaszemski MJ, Currier BL, Windebank AJ (2009) Axon regeneration through scaffold into distal spinal cord after transection. J Neurotrauma 26(10):1759–1771
Chenn A, Walsh CA (2002) Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science 297(5580):365–369
David MD, Canti C, Herreros J (2010) Wnt-3a and Wnt-3 differently stimulate proliferation and neurogenesis of spinal neural precursors and promote neurite outgrowth by canonical signaling. J Neurosci Res 88(14):3011–3023
Frey D, Laux T, Xu L, Schneider C, Caroni P (2000) Shared and unique roles of CAP23 and GAP43 in actin regulation, neurite outgrowth, and anatomical plasticity. J Cell Biol 149(7):1443–1454
Fuchs E, Tumbar T, Guasch G (2004) Socializing with the neighbors: stem cells and their niche. Cell 116(6):769–778
Goslin K, Schreyer DJ, Skene JH, Banker G (1990) Changes in the distribution of GAP-43 during the development of neuronal polarity. J Neurosci 10(2):588–602
Gulacsi AA, Anderson SA (2008) Beta-catenin-mediated Wnt signaling regulates neurogenesis in the ventral telencephalon. Nat Neurosci 11(12):1383–1391
Hirabayashi Y, Itoh Y, Tabata H, Nakajima K, Akiyama T, Masuyama N, Gotoh Y (2004) The Wnt/beta-catenin pathway directs neuronal differentiation of cortical neural precursor cells. Development 131(12):2791–2801
Huelsken J, Behrens J (2002) The Wnt signalling pathway. J Cell Sci 115(Pt 21):3977–3978
Ikeya M, Lee SM, Johnson JE, McMahon AP, Takada S (1997) Wnt signalling required for expansion of neural crest and CNS progenitors. Nature 389(6654):966–970
Im J, Kim H, Kim S, Jho EH (2007) Wnt/beta-catenin signaling regulates expression of PRDC, an antagonist of the BMP-4 signaling pathway. Biochem Biophys Res Commun 354(1):296–301
Kataoka K, Suzuki Y, Kitada M, Hashimoto T, Chou H, Bai H, Ohta M, Wu S, Suzuki K, Ide C (2004) Alginate enhances elongation of early regenerating axons in spinal cord of young rats. Tissue Eng 10(3–4):493–504
Lie DC, Colamarino SA, Song HJ, Desire L, Mira H, Consiglio A, Lein ES, Jessberger S, Lansford H, Dearie AR, Gage FH (2005) Wnt signalling regulates adult hippocampal neurogenesis. Nature 437(7063):1370–1375
Megason SG, McMahon AP (2002) A mitogen gradient of dorsal midline Wnts organizes growth in the CNS. Development 129(9):2087–2098
Michaelidis TM, Lie DC (2008) Wnt signaling and neural stem cells: caught in the Wnt web. Cell Tissue Res 331(1):193–210
Murashov AK, Pak ES, Hendricks WA, Owensby JP, Sierpinski PL, Tatko LM, Fletcher PL (2005) Directed differentiation of embryonic stem cells into dorsal interneurons. FASEB J 19(2):252–254
Namgung U, Routtenberg A (2000) Transcriptional and post-transcriptional regulation of a brain growth protein: regional differentiation and regeneration induction of GAP-43. Eur J Neurosci 12(9):3124–3136
Neve RL, Coopersmith R, McPhie DL, Santeufemio C, Pratt KG, Murphy CJ, Lynn SD (1998) The neuronal growth-associated protein GAP-43 interacts with rabaptin-5 and participates in endocytosis. J Neurosci 18(19):7757–7767
Oestreicher AB, De Graan PN, Gispen WH, Verhaagen J, Schrama LH (1997) B-50, the growth associated protein-43: modulation of cell morphology and communication in the nervous system. Prog Neurobiol 53(6):627–686
Otero JJ, Fu W, Kan L, Cuadra AE, Kessler JA (2004) Beta-catenin signaling is required for neural differentiation of embryonic stem cells. Development 131(15):3545–3557
Parr BA, Shea MJ, Vassileva G, McMahon AP (1993) Mouse Wnt genes exhibit discrete domains of expression in the early embryonic CNS and limb buds. Development 119(1):247–261
Sousa KM, Villaescusa JC, Cajanek L, Ondr JK, Castelo-Branco G, Hofstra W, Bryja V, Palmberg C, Bergman T, Wainwright B, Lang RA, Arenas E (2010) Wnt2 regulates progenitor proliferation in the developing ventral midbrain. J Biol Chem 285(10):7246–7253
Stieltjes B, Klussmann S, Bock M, Umathum R, Mangalathu J, Letellier E, Rittgen W, Edler L, Krammer PH, Kauczor HU, Martin-Villalba A, Essig M (2006) Manganese-enhanced magnetic resonance imaging for in vivo assessment of damage and functional improvement following spinal cord injury in mice. Magn Reson Med 55(5):1124–1131
Suh HI, Min J, Choi KH, Kim SW, Kim KS, Jeon SR (2011) Axonal regeneration effects of Wnt3a-secreting fibroblast transplantation in spinal cord-injured rats. Acta Neurochir (Wien) 153(5):1003–1010
Suzuki Y, Kitaura M, Wu S, Kataoka K, Suzuki K, Endo K, Nishimura Y, Ide C (2002) Electrophysiological and horseradish peroxidase-tracing studies of nerve regeneration through alginate-filled gap in adult rat spinal cord. Neurosci Lett 318(3):121–124
Wang M, Zhai P, Chen X, Schreyer DJ, Sun X, Cui F (2011) Bioengineered scaffolds for spinal cord repair. Tissue Eng Part B Rev 17(3):177–194
Woodhead GJ, Mutch CA, Olson EC, Chenn A (2006) Cell-autonomous beta-catenin signaling regulates cortical precursor proliferation. J Neurosci 26(48):12620–12630
Yun S, Rim Y, Jho EH (2007) Induced expression of the transcription of tropomodulin 1 by Wnt5a and characterization of the tropomodulin 1 promoter. Biochem Biophys Res Commun 363(3):727–732
Zechner D, Fujita Y, Hulsken J, Muller T, Walther I, Taketo MM, Crenshaw EB, Birchmeier W, Birchmeier C (2003) Beta-catenin signals regulate cell growth and the balance between progenitor cell expansion and differentiation in the nervous system. Dev Biol 258(2):406–418
Acknowledgment
This work was supported by an Asan Life Science Institute Grant (12-176) from the Asan Medical Center, Seoul, Korea, and by the Pioneer Research Center Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (2012-0000447).
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jin Hoon Park and Joongkee Min contributed equally to this work.
Rights and permissions
About this article
Cite this article
Park, J.H., Min, J., Baek, S.R. et al. Enhanced neuroregenerative effects by scaffold for the treatment of a rat spinal cord injury with Wnt3a-secreting fibroblasts. Acta Neurochir 155, 809–816 (2013). https://doi.org/10.1007/s00701-013-1663-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-013-1663-7