Abstract
Objective
In glioma surgery, the extent of resection (EOR) is one important predictor of progression-free survival. In 2006, fluorescence-guided surgery using 5-aminolevulinic acid (5-ALA) was shown to improve the EOR in malignant gliomas. However, the use of 5-ALA is complex and causes certain side effects. Sodium fluorescein (FL) is a fluorescent dye that is used for angiography in ophthalmic surgery. FL accumulates in areas of the disturbed blood-brain barrier and can be visualized under a 560-nm wavelength fluorescent light source (YELLOW 560 nm, Carl Zeiss Meditec, Oberkochen, Germany). Here, we present the first experiences with low-dose FL and YELLOW 560 nm in 35 patients with malignant brain tumors.
Patients and method
A total of 200 mg of FL (3–4 mg/kg bodyweight) was administered in 35 patients during craniotomy as an off-label use between May and August 2012. We retrospectively analyzed the histology, pre-treatment, clinical parameters pre- and postoperatively and occurrence of any adverse effects. The feasibility and efficacy (‘helpful,’ ‘not helpful’) of FL under YELLOW 560 nm (demarcation of the tumor margin) was assessed by the responsible neurosurgeon (n = 5) for each surgical procedure.
Results
Twenty-six patients had gliomas (1 WHO grade I, 3 WHO grade II, 5 WHO grade III, 17 WHO grade IV), 5 patients had cerebral metastases, 2 had non-malignant astrogliosis and 2 had post-radiation necrosis. The fluorescence signal was detected in all patients immediately after the FL administration. FL application was classified as ‘helpful’ in 28 patients, implying improved visualization of the tumor margins. The intensity of the fluorescence signal seemed to be correlated to the histology and was strongly dependent on the pre-treatment status. We did not record any allergic reactions or any other adverse effects.
Conclusion
The use of FL for the resection of brain tumors is safe and feasible. Presumably, the visualization of the tumor margin depends on the histopathology and on the pre-treatment status. A randomized evaluation of FL under the YELLOW 560 nm filter is planned to prospectively analyze the extent of resection in patients with malignant brain tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The extent of resection (EOR) is a significant predictor of survival [15, 18, 24] and recurrence [2] in patients with malignant glioma. Currently, concepts are being developed to ensure the best EOR without surgery-related new neurological deficits.
Fluorescence-guided surgery for malignant glioma has been a matter of great interest [5–7, 12, 22] especially since Stummer et al. presented the promising results of the ‘ALA trial’ [25, 26]. The ALA study confirmed a significantly higher EOR in the 5-aminolevulinic acid (ALA) fluorescence-enhanced group compared to the control group (65 % vs. 36 %). Furthermore, the 6-month progression-free survival was significantly improved in the ALA group (41 % vs. 21 %). Consequently, 5-ALA has been established as an important clinical tool for safe and extensive resection in glioma patients. However, the drug has to be administered orally 3 h before induction of anesthesia to allow the substance to circulate and to be taken up by the tumor cells. If a case is started without 5-ALA application and the neurosurgeon fails to detect the tumor borders, fluorescence guidance is not an option so far.
In contrast, fluorochrom sodium fluorescein (‘FL,’ C20H12O5) is a fluorescent dye with a molecular weight of 332.32 g·mol−1 that can be used for immediate improved visualization of brain tumor tissue. The substance has been widely used in ophthalmic surgery [17] and is virtually free of side effects.
Normally, circulating FL is excluded from normal brain tissue by the blood-brain barrier (BBB). In neovascularized tumors with pathologic endothelial function, FL accumulates in the vascularized tumor tissue and is easily visualized under yellow-filtered light (560 nm) [10, 11, 13]. The group of Kuroiwa et al. was the first to generate surgical experiences using FL for improved resection of brain tumors [10–12], and in 2003, Shinoda et al. presented a study of 32 patients with malignant glioma assigned to FL-guided resection [22]. Other groups performed FL-guided surgery in metastatic brain tumors [14] and skull base tumors [1]. Taken together, the use of FL led to improved tumor visualization and to improved EOR. However, the detection of tumor tissue under conventional white light illumination required high doses of FL, since a specialized surgical microscope equipped with a fluorescence detection system was not available.
This retrospective analysis reports the first experiences with fluorescence-guided surgery for malignant brain tumors using a newly developed surgical microscope (PENTERO 900, Carl Zeiss Meditec, Germany) equipped with a YELLOW 560 nm filter allowing highly sensitive and specific detection after intraoperative administration of a low dose (200 mg) of FL (Natrium-Fluorescein®, Alcon, Germany). The major goal of this study was to analyze the feasibility of this approach.
Patients and method
Thirty-five patients (mean age 59 years; range 18 to 84 years) with brain tumors were treated with FL-guided resection between May and August 2012 in our neurosurgical department. The demographics, anatomical localization and pathological diagnoses of the population are presented in Table 1.
The administration of FL was planned preoperatively in all patients suitable for its use. Only patients with cerebral lesions suspicious for malignant brain tumors were included. We excluded all patients with severe liver and renal dysfunction or with known hyper-reactivity against any contrast agent.
Treatment protocol
In all cases, 2 ml (200 mg = approximately 3–4 mg/kg bodyweight) of FL 10 % was injected intravenously via the central venous line after the bone flap had been removed immediately prior to the durotomy. Under white light, no fluorescent effect was detected. The fluorescent dye was visualized under the YELLOW 560 nm filter, mounted to the Pentero 900 surgical microscope (Carl Zeiss Meditec, Oberkochen, Germany). The microscope allows immediate switching of the light filters. Every surgical procedure was recorded and stored digitally.
The fluorescence, depicting the vital tumor margins, was evaluated by the responsible neurosurgeon (n = 5) of each surgical procedure (see Classification of efficacy). The improved identification of the tumor and its margin under filtered light was then classified into ‘helpful’ and ‘not helpful’ (Table 1).
After tumor removal, the final inspection and the wound closure were performed under white light. All patients were transferred to the intensive care unit for postoperative monitoring for at least 24 h, and all patients were treated according to our standardized postoperative treatment protocol. Post-surgical MRI was obtained within 24 h. Suture material was removed on day 10.
After histological examination of the tissue, patients were treated according to the recommendations of our interdisciplinary neuro-oncology board. Clinical follow-up was carried out by our outpatient clinic.
We reviewed the charts for diagnoses, intra- and postoperative complications, and for the clinical course.
In all patients recruited, FL was administered in terms of an off-label use. All patients signed the informed consent for the off-label use. The retrospective analysis was approved by our local ethics committee.
Results
General use
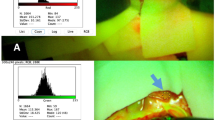
After administration of 2 ml (200 mg) of FL intravenously, the fluorescent dye was seen in all patients immediately under the Pentero 900 surgical microscope, equipped with the 560-nm wavelength fluorescence light filter, whereas under white light, no fluorescence was visible (Fig. 1a). In all cases, the tissue fluorescence was brightly visible 20–30 min after administration of FL (Fig. 1b) and lasted for the time of the procedure (mean operation time 216 min, range 102 to 453 min). During the procedure, the YELLOW 560 nm filter was easily switched on and off by pressing a button on the handle of the microscope.
The resection of the tumor was frequently obtained using the Cavitron Ultrasonic Surgical Aspirator (CUSA). The CUSA was increasingly used under the YELLOW filter, allowing precise tumor removal during the application, without switching from white light to filtered light.
Classification of efficacy
In 28 cases, the fluorescence staining of the tumor under YELLOW 560 nm filtered light was classified as ‘helpful,’ meaning that the identification of the tumor mass and of the tumor margin was significantly better than under white light. The unaffected brain tissue was not enhanced under fluorescence light. A ‘helpful’ effect of FL was found in all patients with initial glioblastoma (n = 12), recurrent glioblastoma (n = 5) and metastasis (n = 5). Additionally, FL was classified ‘helpful’ in two patients with glioma WHO grade III (n = 5), two patients with glioma WHO grade II (n = 3) and in both patients who histologically showed necrosis without vital tumor tissue. For details, see Table 1.
In seven cases (3 gliomas WHO grade III, 1 glioma WHO grade II, 1 glioma WHO grade I, 2 astroglioses), the fluorescence did not facilitate the resection as either the tumor tissue did not enhance or the margin of the process was not definable. For details, see Table 1.
Grade of fluorescent staining in glioma
Our impression was that there was a significant relationship between the contrast enhancement of the tumors in MRI and the dye uptake. The more impressive the contrast enhancement, the more intensive was the intraoperative presentation of sodium fluorescein. The peritumoral edema seemed not to be enhanced by the fluorescent dye. The relationship between radiographic contrast enhancement and surgical presentation will be addressed by a prospective trial.
In WHO grade III and IV glioma (glioblastoma) that presented without any previous treatment, the fluorescent effect of the vital tumor tissue was the most obvious.
In patients presenting with high-grade glioma with a central necrosis, the fluorescent effect was pronounced in the vital tumor margin, but was absent in the necrotic parts. In recurrent tumors, the visible effects were rather heterogeneous in some cases, but still allowed a clear delineation of the tumor margins (except in patient 15).
The fluorescent effect was less obvious in low-grade glioma (WHO grade I, II; n = 4). In two cases, a clear distinction between the tumor margin, infiltration zone and edematous brain tissue was not possible.
Thus, the intensity of the fluorescence signal seemed to depend on the histopathology and depended strongly on the pre-treatment status (precedent surgical procedure = ‘recurrency,’ radiation, chemotherapy); see Table 1.
Extent of resection (EOR)
The preliminary analysis of the volumetric data of our heterogeneous cohort revealed that gross total resection (GTR) > 95 % of the contrast-enhancing tumor was achieved in 80 % of the patients. However, a detailed analysis of the EOR is the major subject of the planned prospective study.
Case illustration
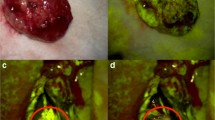
Figure 2a–d illustrates the surgical course of a 57-year-old female patient with a recurrent left frontal glioblastoma. The preoperative MR revealed the recurrent glioblastoma (initial resection 18 months before) after combined adjuvant radiation and chemotherapy with temozolamide. Figure 2a shows the surgical view under white light; Fig. 2b depicts the surgical view under the YELLOW 560 nm filter, clearly visualizing the fluorescent dye within the vital tumor. After careful resection of the fluorescent tissue (Fig. 2c), the postoperative MRI revealed complete removal of the contrast-enhanced area (Fig. 2d).
Grade of fluorescent manifestation in any other entities
In all patients with metastatic tumors, excellent visualization of the lesions was achieved (Fig. 3a, b).
In the two patients with (radio)-necrosis, the application of FL was useful for identification of the lesion, but not for resection control.
False-positive effect
FL seemed to accumulate in scar and in devitalized cerebral tissue, especially in the border zone of tumors that had been pre-treated surgically and by radiation. In a solitary cystic metastasis, the cyst fluid showed an intense fluorescent signal (Fig. 3a), an effect that was also seen in cystic gliomas, the fluorescent effect was pronounced similarly.
Adverse reaction and surgical morbidity
We also did not encounter any local or systemic side effects such as drops in blood pressure or any allergic reactions in our patients.
In all patients, the urine turned yellow for at least 6 h, but none of the patients experienced discoloration of the skin or the sclera.
We registered no re-bleeding. The rate of wound healing problems (n = 2) and cerebrospinal fluid (CSF) leaks (n = 1) did not differ significantly from those of the standard surgical procedure (Table 1). The mean follow-up time was 6 weeks.
Discussion
In glioma surgery, complete resection (100 %) and gross-total resection (> 95 %) are superior to partial resection combined with adjuvant therapy [19, 23]. The recurrence rate and the progression-free survival are significantly improved [15]. The main goal is to detect the intraoperative detection of the tumor border zone and to distinguish cerebral tissue with tumor infiltration from merely edematous brain.
Different modalities provide promising results: intraoperative MRI-guided resection [8, 9, 21], neuronavigation [28], sonography [4] and fluorescence-guided resection using 5-aminolevulinic acid (ALA) [26]. Neuronavigation and intraoperative ultrasound are widely used in neurosurgical operating rooms all around the world; however, the beneficial effect of these modalities is considerably surpassed by intraoperative MRI and ALA. Although the benefit of ALA and MRI-guided resection is unrivalled, the application of these sophisticated modalities is time-consuming and expensive. The cost of one application of ALA is approximately 38 times as high as one application of FL (approximately 950 € and 25 €, respectively), while the upgrade of the microscope is equally expensive (approximately 39,000 € for the ALA module and for the YELLOW module, respectively).
Furthermore, some authors describe ‘bleaching’ of the ALA-fluorescent dye, obscuring the tumor margin [22]. The main difference between ALA and FL is the etiology of enhancement: while ALA is metabolized in the tumor tissues [25, 26], FL accumulates in areas with disrupted BBB [10, 13], thus allowing the almost immediate detection of tumor tissue following the administration of the compound.
In 1998–1999, Kuroiwa et al. presented their idea of a surgical microscope, equipped with different light filters allowing fluorescence-guided resection using FL [10–12]. Ichioka et al. studied the effect of Kuroiwa’s microscope on human serum albumin-conjugated fluorescein in rodents, successfully improving the contrast in brightness after administration of sodium FL. However, due to technical difficulties, this prototype never reached the level of clinical availability. Five years later, the group of Kremer investigated the labeling of malignant brain tumors with 5-aminofluorescein-labeled albumin in 13 glioma patients [7]. For fluorescence activation of tumor tissue, a laser beam at 488 nm from a 10-W argon laser source was applied at a distance of 10 to 30 cm. The association between fluorescence and histopathology was in concordance in nearly 84 %; the positive predictive value for a fluorescent sample to resemble tumor was 97 %. However, the drug was administered 96–12 h before the surgical procedure.
Other groups investigated the application of FL under white light in brain tumor surgery. Shinoda et al. presented a series of 32 patients with glioblastoma for whom resection was guided by high-dose FL (20 mg/kg bodyweight) [22]. They achieved gross total removal (GTR) in the FL group of 84.4 % compared to 30.1 % in the non-FL group. Surprisingly, a beneficial outcome was not obtained. In 2008, the group of Koc prospectively examined FL-guided resection (20 mg/kg bodyweight) under xenon white light in 47 patients with glioblastoma, revealing GTR in the FL group of 83 % compared to 55 % in the non-FL group. Similarly, the survival time did not vary significantly between the two groups (44 vs. 42 weeks).
Finally, in 2010, Okuda et al. presented a clinical study of FL-guided resection (20 mg/kg bodyweight) under white light in 38 patients with brain metastases [14]. In the FL group, they achieved a local control rate of 80 % without whole brain radiation therapy (WBRT), stressing that conventional surgery control rates range between 54 and 64 % [16, 20]. Additionally, the effective use of FL in six patients with skull base tumors was recently investigated by Da Silva et al. [1].
In our population, we encountered no adverse effects or allergic reactions as gastrointestinal distress, syncope and vomiting. In general, adverse effects due to the use of FL in neurosurgery are quite rare. Two reports can be extracted from the literature dealing with serious anaphylactic intraoperative hypotension with mild bradycardia [3, 27]. In both cases, FL was administered at 20 mg/kg bodyweight.
To our knowledge, this is the first report focusing on the application of FL under a modern surgical microscope equipped with the YELLOW 560 nm filter in brain tumor surgery. Our report aims to communicate the feasibility of this method as a potentially useful tool in brain tumor surgery. We therefore deliberately collected data from a consecutive series of heterogeneous patients to provide a more general overview of the clinical application of this technique.
-
1.
Using FL under YELLOW 560 nm improved visualization of the tumor tissue compared to white light in the majority of the analyzed patients.
-
2.
We could show that an intraoperative low-dose administration of FL (200 mg, = approximately 3–4 mg/kg bodyweight) is sufficient for visualization of tumor tissue under the YELLOW 560 nm filter. The effect of FL was achieved immediately and lasted for the time of the surgical procedure without loss of intensity or bleaching.
-
3.
The handling of the YELLOW tool and switching between YELLOW and white light was easy, and it did not prolong the operative procedure. The use of the CUSA was unproblematic under fluorescence guidance.
-
4.
We encountered no adverse effects or other allergic reactions. The rate of surgery-related complications was not increased.
However, the accumulation of FL in regions with a disrupted BBB seemed to depend on histopathology and/or pre-treatment with surgery, radiation or chemotherapy. There was an indication that FL accumulated in CSF and cyst fluid. We are in the process of planning and conducting a prospective, randomized trial to validate the beneficial use of FL in glioma surgery.
Conclusion
The use of the fluorescent dye sodium fluorescein for the resection of brain tumors is feasible. However, the grade of visualization of the tumor margin and the infiltration area depends on the histopathology and on the pre-treatment status of the tumor.
Based on these encouraging first results, a prospective, randomized study is planned to evaluate the effect of sodium fluorescein under the YELLOW 560 nm filter on the extent of resection and on the progression-free survival in patients with gliomas.
References
da Silva CE, da Silva JL, da Silva VD (2010) Use of sodium fluorescein in skull base tumors. Surg Neurol Int 1:70
De Bonis P, Anile C, Pompucci A, Fiorentino A, Balducci M, Chiesa S, Lauriola L, Maira G, Mangiola A (2012) The influence of surgery on recurrence pattern of glioblastoma. Clin Neurol Neurosurg 115(1):37–43
Dilek O, Ihsan A, Tulay H (2011) Anaphylactic reaction after fluorescein sodium administration during intracranial surgery. J Clin Neurosci 18:430–431
Erdogan N, Tucer B, Mavili E, Menku A, Kurtsoy A (2005) Ultrasound guidance in intracranial tumor resection: correlation with postoperative magnetic resonance findings. Acta Radiol 46:743–749
Ichioka T, Miyatake S, Asai N, Kajimoto Y, Nakagawa T, Hayashi H, Kuroiwa T (2004) Enhanced detection of malignant glioma xenograft by fluorescein-human serum albumin conjugate. J Neurooncol 67:47–52
Koc K, Anik I, Cabuk B, Ceylan S (2008) Fluorescein sodium-guided surgery in glioblastoma multiforme: a prospective evaluation. Br J Neurosurg 22:99–103
Kremer P, Fardanesh M, Ding R, Pritsch M, Zoubaa S, Frei E (2009) Intraoperative fluorescence staining of malignant brain tumors using 5-aminofluorescein-labeled albumin. Neurosurgery 64, ons53-60; discussion ons60-51
Kubben PL, ter Meulen KJ, Schijns OE, ter Laak-Poort MP, van Overbeeke JJ, van Santbrink H (2011) Intraoperative MRI-guided resection of glioblastoma multiforme: a systematic review. Lancet Oncol 12:1062–1070
Kuhnt D, Becker A, Ganslandt O, Bauer M, Buchfelder M, Nimsky C (2011) Correlation of the extent of tumor volume resection and patient survival in surgery of glioblastoma multiforme with high-field intraoperative MRI guidance. Neuro Oncol 13:1339–1348
Kuroiwa T, Kajimoto Y, Ohta T (1998) Development of a fluorescein operative microscope for use during malignant glioma surgery: a technical note and preliminary report. Surg Neurol 50:41–48, discussion 48–49
Kuroiwa T, Kajimoto Y, Ohta T (1999) Comparison between operative findings on malignant glioma by a fluorescein surgical microscopy and histological findings. Neurol Res 21:130–134
Kuroiwa T, Kajimoto Y, Ohta T (1999) Surgical management for supratentorial astrocytic tumors. Minim Invasive Neurosurg 42:182–186
Lee J, Baird A, Eliceiri BP (2011) In vivo measurement of glioma-induced vascular permeability. Methods Mol Biol 763:417–422
Okuda T, Kataoka K, Yabuuchi T, Yugami H, Kato A (2010) Fluorescence-guided surgery of metastatic brain tumors using fluorescein sodium. J Clin Neurosci 17:118–121
Orringer D, Lau D, Khatri S, Zamora-Berridi GJ, Zhang K, Wu C, Chaudhary N, Sagher O (2012) Extent of resection in patients with glioblastoma: limiting factors, perception of resectability, and effect on survival. J Neurosurg 117(5):851–859
Patchell RA, Tibbs PA, Regine WF, Dempsey RJ, Mohiuddin M, Kryscio RJ, Markesbery WR, Foon KA, Young B (1998) Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA 280:1485–1489
Rabb MF, Burton TC, Schatz H, Yannuzzi LA (1978) Fluorescein angiography of the fundus: a schematic approach to interpretation. Surv Ophthalmol 22:387–403
Salvati M, Pichierri A, Piccirilli M, Floriana Brunetto GM, D’Elia A, Artizzu S, Santoro F, Arcella A, Giangaspero F, Frati A, Simione L, Santoro A (2012) Extent of tumor removal and molecular markers in cerebral glioblastoma: a combined prognostic factors study in a surgical series of 105 patients. J Neurosurg 117(2):204–211
Sanai N, Berger MS (2008) Glioma extent of resection and its impact on patient outcome. Neurosurgery 62:753–764, discussion 264–756
Schackert G, Steinmetz A, Meier U, Sobottka SB (2001) Surgical management of single and multiple brain metastases: results of a retrospective study. Onkologie 24:246–255
Senft C, Bink A, Franz K, Vatter H, Gasser T, Seifert V (2011) Intraoperative MRI guidance and extent of resection in glioma surgery: a randomised, controlled trial. Lancet Oncol 12:997–1003
Shinoda J, Yano H, Yoshimura S, Okumura A, Kaku Y, Iwama T, Sakai N (2003) Fluorescence-guided resection of glioblastoma multiforme by using high-dose fluorescein sodium. Technical note. J Neurosurg 99:597–603
Smith JS, Chang EF, Lamborn KR, Chang SM, Prados MD, Cha S, Tihan T, Vandenberg S, McDermott MW, Berger MS (2008) Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol 26:1338–1345
Stummer W, Meinel T, Ewelt C, Martus P, Jakobs O, Felsberg J, Reifenberger G (2012) Prospective cohort study of radiotherapy with concomitant and adjuvant temozolomide chemotherapy for glioblastoma patients with no or minimal residual enhancing tumor load after surgery. J Neurooncol 108:89–97
Stummer W, Novotny A, Stepp H, Goetz C, Bise K, Reulen HJ (2000) Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid-induced porphyrins: a prospective study in 52 consecutive patients. J Neurosurg 93:1003–1013
Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ (2006) Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 7:392–401
Tanahashi S, Lida H, Dohi S (2006) An anaphylactoid reaction after administration of fluorescein sodium during neurosurgery. Anesth Analg 103:503
Wirtz CR, Albert FK, Schwaderer M, Heuer C, Staubert A, Tronnier VM, Knauth M, Kunze S (2000) The benefit of neuronavigation for neurosurgery analyzed by its impact on glioblastoma surgery. Neurol Res 22:354–360
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schebesch, KM., Proescholdt, M., Höhne, J. et al. Sodium fluorescein–guided resection under the YELLOW 560 nm surgical microscope filter in malignant brain tumor surgery—a feasibility study. Acta Neurochir 155, 693–699 (2013). https://doi.org/10.1007/s00701-013-1643-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-013-1643-y