Abstract
Background
The prognosis of severe traumatic brain injury (sTBI) is important. The International Mission on Prognosis in Traumatic Brain Injury (IMPACT) study group has developed a prediction calculator for the outcome of patients with sTBI, and this has been made available on the World Wide Web. We have studied the use of the IMPACT calculator on sTBI patients treated with an ICP-targeted therapy based on the Lund concept.
Method
The individual clinical data of patients in a prospective sTBI protocol-driven trial of the treatment of sTBI using the Lund concept were entered into the prognosis calculator, and the individual prognosis for each patient was calculated and compared with the actual outcome at 6 months.
Findings
The use of the IMPACT calculator led to an overestimation of mortality and of an unfavourable outcome. Compared with the IMPACT database, the absolute risk reduction (ARR) for mortality was 13.6 %. There is a statistically significant probability for the prediction of mortality and unfavourable outcome. A ROC curve analysis shows an area under the curve (AUC) in the Core model for mortality of 0.744 and of unfavourable outcome of 0.731, in the Extended model of 0.751 and 0.721 respectively, and in the Lab model of 0.779 and 0.810 respectively.
Conclusions
The IMPACT prognosis calculator should be used with caution for the prediction of outcome for an individual patient with sTBI treated with an ICP-targeted therapy based on the Lund concept. We conclude that we have to initiate treatment in all patients with blunt sTBI and an initial ICP > 10 mmHg. It seems that the outcome in sTBI patients treated in this fashion is better than would have been expected from the IMPACT prognosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Severe traumatic brain injury (sTBI) is the most common cause of death and morbidity in young adults. This is a fact known not only within the profession, but also to the general public. It is commonly known that people who have suffered from sTBI have a high risk of dying or being severely chronically injured as a result. The treating physician often has to answer the question from relatives about the patient's prognosis. The treating physician wonders about the prognosis to help with making the decision of whether or not to treat the patient, or how long to continue an ongoing treatment.
The question of the prognosis of a certain disease has been a concern since the dawn of the medical profession. One of the oldest documents discussing the prognosis of head injury is the Edwin Smith Papyrus (1600 B.C.) in which the treatment of several different types of head injury together with their prognoses is discussed. In the writings of Hippocrates, the prognosis of head injuries is also discussed [4].
In modern times, studies have been done finding, for example, that bilateral fixed and dilated pupils, older age, lower initial Glasgow Coma Scale (GCS) score, hypotension and hypoxia are all related to poorer outcome [3, 5, 16]. All these correlations have been established on a group level.
The immense work of the IMPACT study group has developed a prognostic model for TBI [9]. The IMPACT group collected data from 11 brain injury studies. They then statistically analysed the material and constructed models for prognostication [5, 16]. Using these data they also constructed a prognosis calculator as an attempt at prognostication on the individual level. The calculator is available on the home page of the IMPACT group (http://tbi-impact.org/).
The prognosis model of the IMPACT study group has three levels. The first level is the basic level or the Core level, which is based on basic clinical data, i.e. age, GCS motor score and pupillary reaction. The second level, the Core+CT or the Extended model, is based on of the Core level with the addition of physiological data and data from the CT investigation, i.e. the presence of hypoxia and/or hypotension, the CT scan scored according to Marshall [12], the presence of subarachnoid haemorrhage on the CT scan and the presence of epidural haematoma. The third and last level is the Core+CT+Lab, or Lab model, which consists of the two previous levels to which some laboratory data are added, namely glucose and haemoglobin levels. Table 1 illustrates the different levels of the prognosis calculator and their variables. The IMPACT group developed a scoring system based on these factors, and from this a prediction of outcome at the individual level should be possible. The correctness of the prognostication is supposed to be better the more factors that are added in the scoring [5, 13, 16]. The prognosis model has been validated against a head injury trial, Corticosteroid Randomisation after Significant Head Injury (CRASH) [16].
The aim of this study was to investigate the accuracy and usability of the IMPACT prognosis calculator for the prognosis of individual patients with sTBI treated with ICP-targeted therapy based on the Lund concept.
Materials and methods
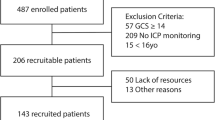
Patients included in a randomised placebo-controlled double-blind study on the effect of prostacyclin in the treatment of sTBI using an ICP-targeted therapy based on the Lund concept were included in the study. Details of the study have been published previously [14]. Inclusion criteria in the study were: verified blunt head trauma, GCS at intubation and sedation ≤8 and age 15–70 years. Exclusion criteria were: penetrating head injury, pregnant or breast-feeding women or a first measured CPP < 10 mmHg. Patients with dilated, fixed pupils and/or GCS 3 were included provided that their first measured ICP was 10 mmHg or higher.
Treatment
We treated all patients in a protocol-guided fashion (Fig. 1) based on the principles of the Lund concept [2]. Details of the treatment guidelines have been published elsewhere [14, 15]. The patients were sedated, using midazolam and fentanyl, and mechanically ventilated (PaO2 > 12 kPa; PaCO2 4.5–5.5 kPa). They were kept normovolemic using infusions of packed red blood cells, albumin, glucose and Ringer’s acetate. The ICP was continuously monitored using an intraparenchymal pressure-measuring device (Codman MicroSensor™, Johnson & Johnson Professional Inc., Raynham, MA, USA). Blood pressure was continuously measured using an arterial line. The reference level for the blood pressure was set at the heart level. All patients were treated in a supine position and without head elevation. After the establishment of normo-volemia, metroprolol and clonidine were administered to normalise the arterial blood pressure and lower the general level of stress generated by the sympathetic nervous system. Further steps in the treatment included the use of thiopental sodium, ventricular drainage and hemicraniectomy.
Data collection
At the time of patients' admittance to our department, we collected the data from the primary receiving hospital. The items collected included: GCS, pupillary reaction, blood pressure, oxygen saturation, CT scan, haemoglobin and blood glucose. We collected the data prospectively, and the data were noted in the patient’s study file.
Independent staff evaluated the clinical outcome of the patients treated at 6 months after injury, using a structured interview based on the Extended Glasgow Outcome Scale score [7, 17]. We report the outcome as Glasgow Outcome Scale (GOS) score [6].
Data analysis
During the month of June 2011, we entered the data in the “Prognostic Calculator” of the IMPACT group available on the homepage (http://tbi-impact.org/). We made an analysis at each level, i.e. (1) the Core level, (2) the Extended level and (3) the Lab level.
The calculator gives the probability as a percentage of mortality (GOS 1) and of an unfavourable outcome (GOS 1–3). We decided to use the information of the primary receiving hospital for this study, the first CT examination being scored for this study.
Statistical analysis
Continuous variables are reported as mean ± SEM and discrete variables as median and range. Logistic fit and receiver-operater curve statistics are used as indicated. The statistical software used is JMP 9.0.0 (SAS Institute Inc.).
Ethics
The local ethics committee at Umeå University approved the study (Dnr 00–175). The study was also approved by the Swedish Medical Products Agency (151:633/01), and the study is registered as a clinical trial (Clinical Trial gov indentifier NCT0133583).
Results
A total of 48 patients were included in the study (17 females and 31 males). The mean age was 35.5 ± 2.2 years. The median GCS at intubation and sedation was 6 (3–8). There was no statistically significant difference in the clinical outcome at 6 months between the prostacyclin group and the placebo group. Further, there was no statistically significant difference in GCS, age, injury severity score, Marshall CT grading, pupillary reaction, the presence of hypoxia or hypotension, the presence of traumatic subarachnoid haemorrhage or epidural haematoma, glucose level or haemoglobin level between the two treatment groups [14]. Based on these findings, we decided to treat the two groups as a single group for this analysis.
Table 1 lists the characteristics of patients relevant for the IMPACT model. The primary receiving hospital for 13 of the patients was our hospital; the other patients were transferred to the Department of Neurosurgery from other hospitals in our catchment area. From the primary receiving hospital, all necessary data were available, except for the laboratory data for ten patients.
The mean prediction using the IMPACT calculator for different levels of prediction is shown in Table 2. The actual mortality in the study at 6 months was 14.6 % (n = 7) and the actual unfavourable outcome (GOS 1 – 3) at the same time was 45.8 %, and there was thus a favourable outcome of 54.2 %.
The analysis of the absolute risk reduction (ARR) of mortality between the IMPACT database using the figures of the IMPACT study database as given by Steyerberg et al. (table page 1254) [16] and our study gave an ARR for mortality of 13.6 %. The same analysis for unfavourable outcome gave an ARR of 4.2 %.
Figure 2 illustrates the IMPACT probability of mortality for each patient according to the different prediction models plotted against the actual GOS at 6 months. The figure also illustrates the linear correlation between the predicted risk of death and the clinical outcome. The figure illustrates further that a large number of patients have a relatively high risk of mortality but have a better outcome. Figure 3 compares the IMPACT risk of mortality according to the different prediction models to the actual outcome dead or alive. The median mortality risk for the deceased patients was 57 % (10–70 %) for the Core model, 62 % (18–84 %) for the Extended mode and 62 % (18–85 %) for the Lab model. The 75 % percentiles for the different models were 75 %, 82 % and 82 % respectively.
Figure 4 illustrates the IMPACT probability of an unfavourable outcome. The median risk for unfavourable outcome for the patients with an actual unfavourable outcome was 73.5 % (11–95 %) for the Core model, 79 % (17–97 %) for the Extended model and 78 % (27–96 %) for the Lab model. The 75 % percentiles for the different models were 84.5 %, 91 % and 93 % respectively.
A logistic fit of the probability of mortality was statically significant for the prediction of mortality using all three levels of the model (p = 0.032 for the Core and Extended models, p = 0.02 for the Lab model). A ROC curve analysis for prediction of outcome shows an AUC for the probability of survival of 0.74422 for the Core model, 0.7509 for the Extended model and 0.7788 for the Lab model. Corresponding logistic fits of the probability of an unfavourable outcome show a statistical significance at all three levels (p = 0.003 for the Core model, p = 0.007 for the Extended model and p = 0.0005 for the Lab model). The ROC curve analyses for prediction of an unfavourable outcome show an AUC of 0.7308 for the Core model, 0.7212 for the Extended model and 0.8097 for the Lab model.
Discussion
Using the IMPACT calculator to analyse all the data and to calculate the mean prognostication for the group as is done in Table 2, we found that our patients had different outcomes than expected.
Another way of demonstrating that the survival in our data differs from that in the IMPACT database is to analyse the ARR. We showed a relatively large ARR for mortality even though our patients had more severe traumatic brain injury than those in the IMPACT database with regard to GCS. The patients in our study had to have a GCS of ≤8, whereas the IMPACT study database has GCS ≤ 12 as an inclusion criterion. We also included patients with bilateral dilated and fixed pupils and / or GCS 3, whereas some of the studies in the IMPACT database did not allow for this or did not allow for severe hypotension [1, 10, 11, 18].
The issue of prognosis is complicated and a daily concern in clinical practice. The prognosis of a disease is applicable at the group level, but seldom for the individual patient. For example, the prognosis at group level will state whether the illness is dangerous and whether there is a high risk of mortality. For the actual patient, this prognosis says little or nothing except whether the disease is dangerous or not dangerous. For the treating physician the information is the same. It does not help the treating physician in making decisions about, for example, whether or not to treat the patient.
The IMPACT calculator makes it possible for the clinician to try to prognosticate mortality and/or an unfavourable outcome for sTBI at the individual level. We retrospectively used this possibility on our own sTBI patients.
The median risk of mortality or of an unfavourable outcome according to the data was prognosticated to be higher than 50 %, whereas the actual outcome was 14.6 % and 45.8 % respectively. This means for example that in the Core model the predicted value for mortality was too high.
We show that there was a statistically significant correlation between the calculated/prognosticated outcome by the IMPACT calculator and the actual outcome in our study. Carrying this analysis one step further using ROC statistics, we showed that the AUC was relatively large, but not large enough to permit for prognostication at the individual level.
One of the important uses of a good prognostication model is to help the clinician to decide whether to treat or not. This puts very high demands on the prognostic tool used, similar to the demands put on a diagnostic laboratory test. A tool fulfilling these criteria would be a great help for the treating physician(s) and for the relatives of the injured person, and would save the medical system large sums of money. In our study, the IMPACT calculator did not allow for individual prognostication and even less for individual treatment decisions.
An immense amount of work lies behind the IMPACT calculator. It is an interesting approach to develop a prognostic instrument by pooling data from several studies and finding parameters that taken together can predict outcome. This is probably the best way to try to develop a prognostic tool. The problem of individualising the prediction has still not been solved. The work of the IMPACT study group has definitely demonstrated that certain simple clinical signs, the CT examination and simple laboratory findings are strong prognostic factors.
One of the problems in the methodology of the IMPACT study group could be that the data were pooled from studies using different treatment protocols. One might ask whether this methodology indirectly states that the different treatment protocols used have no influence on the actual outcome. Most of the protocols used were more or less CPP-guided protocols based on the Brain Trauma Foundation’s guidelines before the 2007 revision. None of the studies used the Lund concept as a treatment paradigm. The authors of this article belive that the choice of treatment protocol/guidelines does influence the outcome at both the group and the individual level. It must therefore be questionable to develop a prognostic tool that is the same regardless of the treatment used.
In our opinion, the IMPACT database prognosis calculator is a step forward in the difficult process of prognostication. However, we find that the treating physician must use it with great caution if it is to guide the treating physician’s way of informing the relatives about the actual prognosis for the patient. On an individual level, it does not provide much help for the actual prognosis of outcome in a specific severely brain-injured patient.
On the home page of the IMPACT study group, it states that the prognosis calculator should be used with caution, when counselling in the individual case. In our opinion, this caution cannot be too strongly emphasised.
The IMPACT study group has, based on its important work, published recommendations and suggestions about how to design studies and trials for the treatment of sTBI in the future [8]. These recommendations have to be considered whenever we are thinking of undertaking or planning to undertake the task of performing a study or trial in sTBI.
Conclusion
The IMPACT prognosis calculator is an interesting tool. Nevertheless, it cannot guide the individual prognostication of outcome in a patient with sTBI treated with an ICP-targeted therapy based on the Lund concept, and thus it cannot tell us which patients to treat or not to treat.
Our conclusion must be that all patients with a severe blunt traumatic brain injury admitted within 24 h of the trauma and with an initial measured CPP of ≥10 mmHg must be treated regardless of other clinical findings.
Compared with the IMPACT database it seems that the outcome in sTBI patients treated in this fashion is better than would have been expected from the IMPACT prognostication.
References
(1994) A multicenter trial of the efficacy of nimodipine on outcome after severe head injury. The European Study Group on Nimodipine in Severe Head Injury. J Neurosurg 80:797–804
Asgeirsson B, Grände PO, Nordström CH (1994) A new therapy of post-trauma brain oedema based on haemodynamic principles for brain volume regulation. Intensive Care Med 20:260–267
Chesnut RM, Marshall LF, Klauber MR, Blunt BA, Baldwin N, Eisenberg HM, Jane JA, Marmarou A, Foulkes MA (1993) The role of secondary brain injury in determining outcome from severe head injury. J Trauma 34:216–222
Hippocrates (1928) Hippocrates Volume III: on wounds in the head, In the Surgery, Fractures, Joints, Moclion translated by E.T. Withington Harvard University Press, Cambridge, MA, London England, pp 7–51
Hukkelhoven CW, Steyerberg EW, Habbema JD, Farace E, Marmarou A, Murray GD, Marshall LF, Maas AI (2005) Predicting outcome after traumatic brain injury: development and validation of a prognostic score based on admission characteristics. J Neurotrauma 22:1025–1039
Jennett B, Bond M (1975) Assessment of outcome after severe brain damage. Lancet 1:480–484
Jennett B, Snoek J, Bond MR, Brooks N (1981) Disability after severe head injury: observations on the use of the Glasgow Outcome Scale. J Neurol Neurosurg Psychiatry 44:285–293
Maas AI, Steyerberg EW, Marmarou A, McHugh GS, Lingsma HF, Butcher I, Lu J, Weir J, Roozenbeek B, Murray GD (2010) IMPACT recommendations for improving the design and analysis of clinical trials in moderate to severe traumatic brain injury. Neurotherapeutics 7:127–134
Marmarou A, Lu J, Butcher I, McHugh GS, Mushkudiani NA, Murray GD, Steyerberg EW, Maas AI (2007) IMPACT database of traumatic brain injury: design and description. J Neurotrauma 24:239–250
Marmarou A, Nichols J, Burgess J, Newell D, Troha J, Burnham D, Pitts L (1999) Effects of the bradykinin antagonist Bradycor (deltibant, CP-1027) in severe traumatic brain injury: results of a multi-center, randomized, placebo-controlled trial. American Brain Injury Consortium Study Group. J Neurotrauma 16:431–444
Marshall LF, Maas AI, Marshall SB, Bricolo A, Fearnside M, Iannotti F, Klauber MR, Lagarrigue J, Lobato R, Persson L, Pickard JD, Piek J, Servadei F, Wellis GN, Morris GF, Means ED, Musch B (1998) A multicenter trial on the efficacy of using tirilazad mesylate in cases of head injury. J Neurosurg 89:519–525
Marshall LF, Marshall SH, Klauber MR, van Berkum CM, Eisenberg HM, Jane JA, Lueressen TG, Marmarou A, Foulkes MA (1991) A new classification of head injury based on computerized tomography. J Neurosurg 75:s14–s20
Murray GD, Butcher I, McHugh GS, Lu J, Mushkudiani NA, Maas AI, Marmarou A, Steyerberg EW (2007) Multivariable prognostic analysis in traumatic brain injury: results from the IMPACT study. J Neurotrauma 24:329–337
Olivecrona M, Rodling-Wahlström M, Naredi S, Koskinen L-OD (2009) Prostacylin treatment in severe traumatic brain injury—a microdialysis and outcome study. J Neurotrauma 26:1251–1262
Olivecrona M, Rodling-Wahlström M, Naredi S, Koskinen LO (2007) Effective ICP reduction by decompressive craniectomy in patients with severe traumatic brain injury treated by an ICP-targeted therapy. J Neurotrauma 24:927–935
Steyerberg EW, Mushkudiani N, Perel P, Butcher I, Lu J, McHugh GS, Murray GD, Marmarou A, Roberts I, Habbema JD, Maas AI (2008) Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. PLoS Med 5:e165, discussion e165
Wilson JT, Pettigrew LE, Teasdale GM (1998) Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. J Neurotrauma 15:573–585
Young B, Runge JW, Waxman KS, Harrington T, Wilberger J, Muizelaar JP, Boddy A, Kupiec JW (1996) Effects of pegorgotein on neurologic outcome of patients with severe head injury. A multicenter, randomized controlled trial. JAMA 276:538–543
Acknowledgement
We thank our research nurses Kristin Nyman and Anna-Lena Östlund, without whose help this study would not have been made.
This study was financially supported by the Foundation for Clinical Neurosciences at Umeå University, Umeå University Hospital, Tore Nilsson Foundation, the Kempe Foundation, and Capio Research Foundation.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Comment
M. Olivecrona and L-O Koskinen provide a paper describing the results of using the IMPACT (International Mission on Prognosis in Traumatic Brain Injury) prognosis calculator in 48 patients with severe traumatic brain injury treated with an ICP-targeted therapy based on the Lund concept. They found that the IMPACT calculator led to an overestimation of mortality and of an unfavourable outcome.
In my opinion, this is a concisely written, interesting, and important paper:
Scoring systems and so-called prognosis calculators are becoming increasingly important in the management of critically ill patients. However, one has to consider that such systems have to be used with care—they are based on statistical data that are difficult or even impossible to transfer to an individual patient. They provide us with probabilities, not with definite predictions. This is especially true in the prediction of death or the time of death.
Another important point of the paper is that the patients treated based on the described concept seem to have an outcome better than the patients included in the IMPACT database. However, only 48 patients were included in the present study, and the statistical power should therefore not be overestimated. Prospective, controlled studies should answer this question.
Marcus Reinges
Gießen, Germany
Rights and permissions
About this article
Cite this article
Olivecrona, M., Koskinen, LO.D. The IMPACT prognosis calculator used in patients with severe traumatic brain injury treated with an ICP-targeted therapy. Acta Neurochir 154, 1567–1573 (2012). https://doi.org/10.1007/s00701-012-1351-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-012-1351-z