Abstract
Background
Cerebrospinal fluid (CSF) shunt-associated infection is one of the most frequent complications of CSF shunt surgery. We evaluated our institutional guideline for the treatment of shunt-associated infections.
Methods
We retrospectively analysed all 92 episodes of shunt-associated infections in 78 patients treated in our institution from 2002 to 2008. All patients underwent urgent surgery, i.e. removal of the complete shunt hardware or externalisation of the distal tubing in cases with an infection restricted to the distal shunt (10 %), placement of an external ventricular drainage as necessary and antibiotic therapy. Standard empirical first-line antibiotic treatment consisted of a combination of flucloxacillin and cefuroxime.
Results
We observed 38 % early (<1 month after shunt surgery) and 20 % late infections (> 1 year after shunt placement). Coagulase-negative staphylococci (CoNS) were isolated in 38 %. In 38 % no pathogens could be isolated. Of cases with a first shunt infection, 58 % were initially treated with flucloxacillin/cefuroxime. Only 53 % of all infections were treated successfully with the first course of antibiotics. Only 51 % of bacterial isolates were sensitive to empirical first-line antibiotics. Twenty percent of infections caused by sensitive bacterial isolates nevertheless required second-line antibiotic therapy.
Conclusions
Urgent surgery for shunt removal and antibiotic therapy will usually cure a shunt-associated infection. The choice of antibiotics should reflect the spectrum of pathogens seen at one’s institution, paying particular attention to the role of CoNS isolates, and in vitro sensitivity testing results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Implantation of a cerebrospinal fluid (CSF) shunt system is the most frequent operation performed for the treatment of hydrocephalus. Shunt malfunction and shunt-associated infection are the most common complications encountered in CSF shunt surgery. CSF shunt-associated infection is a major complication causing substantial morbidity and mortality. More recent studies have reported infection rates between 1 and 18 % [1, 15, 26, 27]. Clinical symptoms may vary and may be unspecific. Patients often present with fever and shunt malfunction [32]. Several important risk factors for shunt-associated infections have been identified. The most important ones are probably postoperative CSF leakage after CSF shunt implantation, preterm birth, previous shunt infection, duration of the shunt placement operation, and the neurosurgeon’s clinical experience [3, 17, 21].
Most CSF shunt-associated infections are caused by opportunistic pathogens, which form part of the human skin flora. They most often develop because of intraoperative bacterial contamination of the shunt hardware, i.e. pathogens are inoculated into the wound at the time of the shunt placement operation [2]. Wound breakdown and postoperative CSF fistulas might also cause shunt-associated infections [17]. Staphylococci are the most frequently detected pathogens. Staphylococci were responsible for 50–89 % of CSF shunt infections in several large published series [13, 17, 19]. These organisms form biofilms, enabling them to colonise implanted devices [8]. The microbiological diagnosis may be challenging. Improper acquisition and handling of the microbiological specimen can easily result in a contamination due to frequent colonisation of the patients’ skin flora with staphylococci [7, 16, 23, 34].
The literature contains various studies evaluating clinical and microbiological features, risk factors and treatment of CSF shunt-associated infections [4, 6, 26, 33]. Nevertheless, there are no universally accepted guidelines for the treatment of CSF shunt-associated infections. At the authors’ institution, treatment of CSF shunt-associated infection follows a standardised protocol, which includes surgery in all but exceptional cases. For the present paper we retrospectively analysed all surgically treated episodes of CSF shunt-associated infections encountered from 2002 to 2008 in order to evaluate this institutional guideline.
Patients and methods
Institutional guideline
All patients with a suspected shunt-associated infection undergo an aseptic puncture of the CSF shunt system, or a lumbar puncture. The diagnosis of a CSF shunt-associated infection is based on the modified CDC criteria for hospital acquired infections [11]:
-
1.
Growth of pathogens in the CSF, shunt tips or in wounds overlying the shunt material
and/or
-
2.
Clinical symptoms like fever (>38°), headache, nausea, neck stiffness, focal neurological deficits, physician initiation of an appropriate antimicrobial therapy for shunt-associated infection and a laboratory finding of a CSF leucocyte count of > 5 cells/μl CSF
Patients with shunt infections routinely undergo surgical treatment usually on the day of diagnosis (i.e. on an emergency basis). We try to distinguish between proximal and distal infections of the CSF shunt system. A proximal infection is defined by infectious CSF and/or bacterial colonisation of the proximal shunt up to the valve (i.e. in close proximity to or involving the CSF compartment). In patients with a distal infection, there are no infectious CSF findings and the infection is clinically confined to the distal shunt catheter. Conceptually, patients with a distal infection do not have meningitis and their proximal shunt is still sterile. Hence, an attempt is made in these cases to retain the proximal shunt. The distal catheter is externalised, and the remainder of the shunt is only explanted if the infection persists or spreads proximally. Patients with a proximal shunt-associated infection routinely have their complete shunt removed. If continuous drainage of CSF is needed, we simultaneously implant an external ventricular drain.
Intravenous antibiotics are administered directly after CSF samples have been obtained and infected shuntware has been removed as described above. Per protocol, as the primary antibiotic treatment a combination of intravenous flucloxacillin (flu; 4 × 1 g/day; 4 × 50 mg/kg/day in children) and cefuroxime (cef; 3 × 1.5 g/day; 3 × 50 mg/kg/day in children) is prescribed for 7 days. This choice of antibiotics was based on an earlier internal review of neurosurgical infections, revealing a high rate of infections caused by Staphylococcus aureus. Individualised primary antibiotic treatment is administered in the presence of a second infectious focus (e.g. pneumonia) which requires treatment or if clinical circumstances suggest a non-staphylococcal aetiology. The antibiotic treatment is routinely modified according to the results of in vitro sensitivity testing of the causative pathogens.
The CSF shunt is replaced 2–5 days following discontinuation of antibiotic treatment if repeat CSF analysis does not reveal any infectious findings and the patient remains asymptomatic. Since patients with an external CSF drain are at risk of developing superinfections, and culturing some low-virulence organisms may take longer than 2–3 days, we do not routinely require a sterile culture obtained in the absence of antibiotic treatment.
Patient characteristics
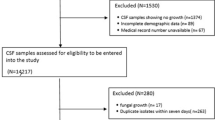
For the present study we analysed all consecutive 92 episodes of shunt-associated infections in 78 patients (75 first infections, 17 recurrent infections) treated surgically from 1 January 2002 until 31 December 2008 at the authors’ institution. Seven patients had one recurrent infection, three patients had two and one patient had four recurrent shunt-associated infections. During the same period, 666 CSF shunts were implanted (range, 81–126 shunt placements annually). The Department of Neurosurgery at the University of Bonn is the principal provider of neurosurgical care in the greater Bonn area serving a population of approximately 1,000,000.
Median patient age at the time of diagnosis was 48 years (range, 3 months to 86 years). There were 13 patients <18 years, and eight patients < 1 year. The series was 58 % female. There were five (5 %) infected ventriculo-atrial shunt systems, and 87 (95 %) infections of ventriculo-peritoneal shunts. Post-haemorrhagic hydrocephalus was the most common indication for shunt placement (27/78 patients, 35 %; Table 1).
Clinical and microbiological data collection, follow-up and statistical analysis
Pertinent clinical and microbiological data were retrieved from the respective patients’ charts. In addition, all patients and/or their families were contacted by phone and asked to provide information on further shunt-related complications, subsequent neurosurgical hospital admissions or any other subsequent neurosurgical procedure. Median follow-up duration was 45 months (range, 5–86 months). Statistical analysis was supported by commercially available software (SPSS version 17.0).
Results
Clinical and radiological presentation
There were 35 early (38 %, < 1 month following shunt placement), 38 delayed (41 %, > 1 month and < 1 year) and 19 (20 %, > 1 year) late infections. The infection was restricted to the distal part of the shunt in nine cases (10 %). One patient presented with a shunt infection 216 months after surgery. Fever was present at the time of hospital admission in 78 (85 %) cases. Forty-three patients (47 %) presented with a depressed level of consciousness or focal neurological signs. Twenty patients (22 %) presented with seizures. A computed axial tomography (CAT) scan of the head showed a hydrocephalic configuration of the ventricular system in 28 cases (30 %). In 28 (30 %) episodes, no cranial CAT scans were obtained. Abdominal sonography (26 cases; 28 %) or a CAT scan of the abdomen (nine cases; 10 %) revealed pathological findings in 16 (17 %) and five (5 %) cases, respectively (Table 1).
Laboratory findings
A CSF sample was obtained in all patients (72 % shunt punctures). Excluding distal infections, a positive CSF leucocyte count (> 5 cells/μl CSF) was observed in 89 % of samples (median, 414 ± 464; median, 231; range 9–2,000 cells/μl CSF). Total CSF protein was available in 69 of these patients (mean, 1,108 ± 1,355 mg/l; median, 736; range, 10–6,685 mg/l). The mean CSF leucocyte count was significantly higher in spinal taps than in CSF samples obtained through shunt punctures (628 ± 475 vs 351 ± 442; P < 0.05, Student’s t-test), whereas there was no significant difference in CSF total protein. Elevated C-reactive protein (CRP) levels (> 3 mg/l) were seen in 98 % of cases (mean, 81 ± 67; median, 61; range, 2.2–340 mg/l). However, a substantially increased CRP > 50 mg/l paralleling findings in, for example, postoperative meningitis was seen in only 59 % [29]. Elevated blood leucocyte counts were recorded in 49 % of all cases (mean, 11 ± 5; median, 10; range, 0.42–32 G/l).
Microbiology findings
Overall, microbiological organisms were detected in 57 episodes of shunt-associated infections (62 %). The most frequently detected pathogens were coagulase-negative staphylococci (CoNS; 35 cases; 38 %, see Table 2). Multiple organisms were identified in 11 infections (12 %). In ten of these cases, the microbiological analysis also detected CoNS. The rate of infections caused by CoNS decreased with time after shunt placement, while the rate of culture-negative infections increased. However, microbiological findings did not differ statistically significantly between first and recurrent infections (chi-squared test/Fisher’s exact test), or between early, delayed and late infections (Armitage trend test; Table 2). Twenty-seven of the 35 patients (77 %) in which no bacteria could be detected were recently treated with antibiotics.
CSF specimens were microbiologically positive in 33 % (87/262 samples). In contrast, pathogens could be isolated from 48/90 (53 %) of ventricular, 12/21 (57 %) of peritoneal and 4/5 (80 %) of atrial catheters.
Surgical treatment
In 83 (90 %) cases the shunt system was completely removed. An external ventricular drain was inserted simultaneously in 75 of these patients (82 %). The peritoneal shunt catheter was externalised in nine cases (10 %, ‘distal infections’). Secondary removal of the remainder of the shunt was performed in two (22 %) of these cases. Shunts were replaced after a mean of 27 ± 21 days (median, 18; range, 7–131 days). Five patients (5 %) did not undergo CSF shunting after their shunt-associated infection was treated successfully (Table 1).
Antibiotic treatment
Overall, only 49 (53 %) cases were cured by a single course of antibiotics. In part, this may reflect the fact that the pathogens identified through the microbiological work-up were sensitive to the empirical first line antibiotic therapy in only 29/57 (51 %) cases. However, the correlation between the results of the in vitro sensitivity testing and the clinical response was not absolute. Only 28/35 (80 %) culture-positive cases treated with first line antibiotics targeting the causative microorganisms according to the in vitro sensitivity testing results were cured with just one course of antibiotics. Infections caused by CoNS responded in only 34 % to empirical first-line antibiotics. Interestingly, 84 % of cases without positive microbiological findings were cured by the first course of antibiotics.
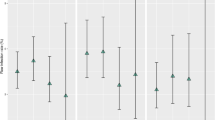
Flu/cef was administered per protocol as first line antibiotic treatment in overall 53 episodes (70 %) of first shunt-associated infections (Fig. 1). In 31 cases (58 %) this treatment was successful, i.e. only one course of antibiotics cured the infection. Notably, this number includes 21 cases (68 %) for which the causative organism could not be identified. The majority of the 22 infections not responding to flu/cef were caused by resistant pathogens (17/18 = 94 % of the microbiologically positive cases). Flu/cef targeted only 10/28 (36 %) of all pathogens tested for sensitivity to flu/cef, including only 8/18 (44 %) of all CoNS isolates. Of all CoNS isolates, 68 % were methicillin-resistant Staphylococcus epidermidis (MRSE). For comparison, at the authors’ institution approximately two-thirds of all CoNS isolates are MRSE.
Efficacy of Flu/cef treatment for first CSF shunt-associated infections. Fifty-three (70 %) of all 75 first shunt-associated infections were initially treated with a combination of flucloxacillin and cefuroxime (flu/cef). Treatment was successful in 31/53 (58 %) of cases. Notably, the microbiological work-up identified pathogens in the majority (18/22 = 82 %) of treatment failures, all but one of which proved resistant to flu/cef upon in vitro testing. Also note the high number of culture-negative flu/cef responders (21/31 = 68 %). Even though the microbiological organisms responsible for shunt-associated infections quite often escaped detection by routine microbiological techniques in this patient subset (25/53 = 47 %), empirical treatment with flu/cef was nevertheless successful (21/25 = 84 % of microbiology negative cases)
The responder rate for first line vancomycin alone (seven cases) or in combination (eight cases) (overall 15 cases = 16 %) was 80 %. Second line treatment with vancomycin alone (12 cases) or in combination (six cases) resulted in a response rate of 83 %. Of all pathogens tested, 49/56 (86 %) were sensitive to vancomycin. Specifically, all CoNS isolates were sensitive to vancomycin.
Treatment outcomes
The 2-month recurrence rate after a first shunt infection was 13 %, and we did not observe any recurrent infections more than 2 months after successful treatment of a first shunt infection. Hence, we operationally defined a cure as no signs and symptoms of a recurrent shunt-associated infection > 3 months after shunt replacement surgery. During her hospital stay, one patient died of multi–organ failure due to nosocomial pneumonia and sepsis. At the time of death, the hydrocephalus was treated with an external ventricular drain; no signs of ongoing meningitis were recorded. All other patients were eventually cured of their shunt-associated infection. Median duration of antibiotic treatment was 14 days (mean, 17 ± 12; range, 4–80 days). Median length of hospital stay was 29 days (mean, 42 ± 35; range, 11–194 days). There were no statistically significant differences between first and recurrent infections (Student’s t-test).
Discussion
Shunt-associated infections complicate the clinical course of 1-18 % of patients with CSF shunts and may cause significant morbidity and even mortality [1, 15, 26, 27]. The present paper describes a large (92 episodes in 78 patients) mono-institutional experience with shunt-associated infections in a predominantly adult (83 % >18 years) patient cohort. Detailed data on adult patients are scarcely found in the literature [6, 14, 21]. Treatment was standardised and included routine removal of the colonised shunt hardware and empirical first-line antibiotic treatment (flu/cef) targeting primarily staphylococci (unless clinical circumstances suggested a non-staphylococcal aetiology or a second infectious focus).
Diagnosing a CSF shunt infection may not be trivial. In our experience, patients with a CSF shunt-associated infection often present with non-specific clinical signs. Fever was seen in only 85 % of the study population. Neurological signs were present in only 43 (47 %) episodes. Shunt-associated infections were not rarely complicated by shunt malfunction. A CAT scan of the head was obtained in 60 % of all episodes, and a hydrocephalic configuration of the ventricular system was diagnosed in 30 % of all patients.
The laboratory diagnosis of a shunt infection can be difficult [6, 16, 29]. Distal shunt infections will not present with infectious CSF findings (10 % of shunt infections in this series). CSF leucocyte counts differ significantly between sampling sites, which has to be taken into account during the diagnostic work-up. We found higher cell counts in spinal taps when compared with samples obtained through shunt punctures. The retrospective character of this study prevented a more comprehensive analysis of additional CSF parameters such as CSF lactate. CSF lactate was within normal limits in 20 % of the cases in two other studies [6, 18].
Notably, we observed elevated serum CRP values in virtually all (98 %) patients. Hence, a normal CRP would strongly argue against the diagnosis of a shunt infection. However, this finding is of very limited diagnostic value, since in everyday practice clinically irrelevant low-level CRP elevations paralleling findings in many shunt-associated infections (e.g. CRP < 30 mg/dl in 23 % of cases in this series) are commonly observed. A substantially increased CRP > 50 mg/l as seen in, for example, postoperative meningitis was seen in only 59 % [29]
The spectrum of pathogens identified is in accordance with the literature with the notable absence of anaerobic microorganisms such as Propionibacterium spp. and the low prevalence of S. aureus [6, 14, 17, 30–32]. Pathogens could be cultured in only 62 % of cases. This may reflect recent antibiotic treatment in 77 % of our culture-negative patients. Interestingly, the rate of infections caused by CoNS decreased with time after shunt placement, while the rate of culture-negative infections increased. However, the respective figures failed to reach statistical significance (Table 2).
Some of the most frequent pathogens which cause shunt-associated infections such as staphylococci (and Propionibacterium spp.) form biofilms after colonisation of implanted devices, making them relatively inaccessible to antibiotics [8]. This may explain why non-surgical treatment for shunt infections has met with limited success at best [5, 12, 22]. In contrast, all but one of our patients were eventually cured from their shunt infection after urgent surgical removal of the colonised shuntware, suggesting that early surgery may be a crucial step in the successful therapy of shunt-associated infections. We do not have experience with systematic non-surgical treatment for shunt-associated infections. Patients with infections involving only the peritoneal catheter may be successfully managed by externalisation of the distal shunt (7/9 cases in this series).
In contrast to most studies, we extensively analysed antibiotic therapy for shunt infections. Empirical antibiotic treatment with flu/cef directed primarily against Staphylococcus spp. cured only 58 % of all episodes of first shunt-associated infections. This was probably mainly due to the relatively high number of resistant CoNS isolates (68 % MRSE) and the low prevalence of infections caused by S. aureus. Of note, many of the culture-negative infections responded to first line treatment with flu/cef. Many of these patients may have in fact suffered from infections caused by Propionibacterium spp. which are usually sensitive to penicillins and cephalosporines (e.g. Propionibacterium acnes). Primary empirical treatment with vancomycin may have resulted in somewhat better infectiological outcomes. The response rate in cases treated with first-line vancomycin was 80 %. Second-line treatment with vancomycin resulted in a response rate of 83 %. Propionibacterium spp. are usually sensitive to vancomycin [24]. Overall, 86 % of all bacterial isolates were sensitive to vancomycin in vitro. Some consider vancomycin the standard antibiotic agent for device-related infections [10, 20, 28].
A combination of vancomycin with a third-generation cephalosporin would cover almost all pathogens causing shunt infections. These theoretical benefits of broad antibiotic coverage have to be balanced against its side effects and costs. Side effects of vancomycin treatment include substantial ototoxicity and nephrotoxicity, which usually requires monitoring of serum levels [9, 25]. Interestingly, the overall treatment results in this series, relying mainly on empirical treatment with the relatively non-toxic combination of flu/cef, were reasonably good. Antibiotic treatment for shunt-related infections can be optimised once in vitro sensitivity results become available, and whenever there is no appropriate clinical response. Nevertheless, the results of this study and in particular the high rate of β-lactam-antibiotic-resistant CoNS isolates have led us to change our institutional protocol and replace primary empirical antibiotic treatment with flu/cef by primary vancomycin therapy. We would encourage others to re-evaluate their first-line antibiotic treatment of shunt-associated infections based on a systematic and regular review of culture results.
In summary, our data support early routine removal of infected shunt hardware in combination with antibiotics as the treatment of choice for all but the exceptional patient with a shunt-associated infection. The choice of (first-line) antibiotics should reflect the spectrum of pathogens seen at one’s institution, paying particular attention to the role of CoNS isolates.
References
Albright AL, Haines SJ, Taylor FH (1988) Function of parietal and frontal shunts in childhood hydrocephalus. J Neurosurg 69:883–886
Baird C, O’Connor D, Pittman T (1999) Late shunt infections. Pediatr Neurosurg 31:269–273
Borgbjerg BM, Gjerris F, Albeck MJ, Borgesen SE (1995) Risk of infection after cerebrospinal fluid shunt: an analysis of 884 first-time shunts. Acta Neurochir (Wien) 136:1–7
Brook I (2002) Meningitis and shunt infection caused by anaerobic bacteria in children. Pediatr Neurol 26:99–105
Brown EM, Edwards RJ, Pople IK (2006) Conservative management of patients with cerebrospinal fluid shunt infections. Neurosurgery 58:657–665, discussion 657–665
Conen A, Walti LN, Merlo A, Fluckiger U, Battegay M, Trampuz A (2008) Characteristics and treatment outcome of cerebrospinal fluid shunt-associated infections in adults: a retrospective analysis over an 11-year period. Clin Infect Dis 47:73–82
Dougherty SH (1988) Pathobiology of infection in prosthetic devices. Rev Infect Dis 10:1102–1117
Fey PD (2010) Modality of bacterial growth presents unique targets: how do we treat biofilm-mediated infections? Curr Opin Microbiol 13:610–615
Forouzesh A, Moise PA, Sakoulas G (2009) Vancomycin ototoxicity: a reevaluation in an era of increasing doses. Antimicrob Agents Chemother 53:483–486
Gandelman G, Frishman WH, Wiese C, Green-Gastwirth V, Hong S, Aronow WS, Horowitz HW (2007) Intravascular device infections: epidemiology, diagnosis, and management. Cardiol Rev 15:13–23
Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM (1988) CDC definitions for nosocomial infections, 1988. Am J Infect Control 16:128–140
James HE, Walsh JW, Wilson HD, Connor JD, Bean JR, Tibbs PA (1980) Prospective randomized study of therapy in cerebrospinal fluid shunt infection. Neurosurgery 7:459–463
James HE, Bejar R, Gluck L, Coen R, Merritt A, Mannino F, Bromberger P, Saunders B, Schneider H (1984) Ventriculoperitoneal shunts in high risk newborns weighing under 2000 grams: a clinical report. Neurosurgery 15:198–202
Kanev PM, Sheehan JM (2003) Reflections on shunt infection. Pediatr Neurosurg 39:285–290
Key CB, Rothrock SG, Falk JL (1995) Cerebrospinal fluid shunt complications: an emergency medicine perspective. Pediatr Emerg Care 11:265–273
Kontny U, Hofling B, Gutjahr P, Voth D, Schwarz M, Schmitt HJ (1993) CSF shunt infections in children. Infection 21:89–92
Kulkarni AV, Drake JM, Lamberti-Pasculli M (2001) Cerebrospinal fluid shunt infection: a prospective study of risk factors. J Neurosurg 94:195–201
Leib SL, Boscacci R, Gratzl O, Zimmerli W (1999) Predictive value of cerebrospinal fluid (CSF) lactate level versus CSF/blood glucose ratio for the diagnosis of bacterial meningitis following neurosurgery. Clin Infect Dis 29:69–74
Mancao M, Miller C, Cochrane B, Hoff C, Sauter K, Weber E (1998) Cerebrospinal fluid shunt infections in infants and children in Mobile, Alabama. Acta Paediatr 87:667–670
McCann MT, Gilmore BF, Gorman SP (2008) Staphylococcus epidermidis device-related infections: pathogenesis and clinical management. J Pharm Pharmacol 60:1551–1571
McGirt MJ, Zaas A, Fuchs HE, George TM, Kaye K, Sexton DJ (2003) Risk factors for pediatric ventriculoperitoneal shunt infection and predictors of infectious pathogens. Clin Infect Dis 36:858–862
McLaurin RL, Frame PT (1987) Treatment of infections of cerebrospinal fluid shunts. Rev Infect Dis 9:595–603
Odio C, McCracken GH Jr, Nelson JD (1984) CSF shunt infections in pediatrics. A seven-year experience. Am J Dis Child 138:1103–1108
Oprica C, Nord CE (2005) European surveillance study on the antibiotic susceptibility of Propionibacterium acnes. Clin Microbiol Infect 11:204–213
Pritchard L, Baker C, Leggett J, Sehdev P, Brown A, Bayley KB (2010) Increasing vancomycin serum trough concentrations and incidence of nephrotoxicity. Am J Med 123:1143–1149
Prusseit J, Simon M, von der Brelie C, Heep A, Molitor E, Volz S, Simon A (2009) Epidemiology, prevention and management of ventriculoperitoneal shunt infections in children. Pediatr Neurosurg 45:325–336
Rowin ME, Patel VV, Christenson JC (2003) Pediatric intensive care unit nosocomial infections: epidemiology, sources and solutions. Crit Care Clin 19:473–487
Sabatier C, Ferrer R, Valles J (2009) Treatment strategies for central venous catheter infections. Expert Opin Pharmacother 10:2231–2243
Schuhmann MU, Ostrowski KR, Draper EJ, Chu JW, Ham SD, Sood S, McAllister JP (2005) The value of C-reactive protein in the management of shunt infections. J Neurosurg 103:223–230
Shapiro S, Boaz J, Kleiman M, Kalsbeck J, Mealey J (1988) Origin of organisms infecting ventricular shunts. Neurosurgery 22:868–872
Spanu G, Karussos G, Adinolfi D, Bonfanti N (1986) An analysis of cerebrospinal fluid shunt infections in adults. A clinical experience of twelve years. Acta Neurochir (Wien) 80:79–82
Thompson TP, Albright AL (1998) Propionibacterium [correction of Proprionibacterium] acnes infections of cerebrospinal fluid shunts. Childs Nerv Syst 14:378–380
Younger JJ, Christensen GD, Bartley DL, Simmons JC, Barrett FF (1987) Coagulase-negative staphylococci isolated from cerebrospinal fluid shunts: importance of slime production, species identification, and shunt removal to clinical outcome. J Infect Dis 156:548–554
Zimmerli W, Lew PD, Waldvogel FA (1984) Pathogenesis of foreign body infection. Evidence for a local granulocyte defect. J Clin Invest 73:1191–1200
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
von der Brelie, C., Simon, A., Gröner, A. et al. Evaluation of an institutional guideline for the treatment of cerebrospinal fluid shunt-associated infections. Acta Neurochir 154, 1691–1697 (2012). https://doi.org/10.1007/s00701-012-1329-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-012-1329-x