Abstract
Background
Darbepoetin-alpha (DA) is a novel erythropoiesis-stimulating agent developed for treating anemia. In animal models, recombinant human erythropoietin has been reported to be beneficial for neuroprotection. In this study, we determined whether DA would protect the spinal cord against ischemia-reperfusion injury in a rabbit model.
Methods
Forty rabbits were randomized into five groups of eight animals each: group 1 (sham), group 2 (ischemia), group 3 (vehicle), group 4 (30 mg/kg methylprednisolone), group 5 (30 μg/kg DA). Only laparotomy was performed in the sham group. In all the other groups, the spinal cord ischemia model was created by a 20-min occlusion of the aorta just caudal to renal artery with an aneurysm clip. The drugs were administered immediately after the clamp was removed. The animals were killed 24 h later. Spinal cord segments between L2 and L5 were harvested for analysis. Neurological evaluation was performed with the Tarlov scoring system just before the animals were killed. Level of tissue malondialdehyde was analyzed as a marker of lipid peroxidation and tissue caspase-3 activity as a marker of apoptosis. Also, histopathological evaluation of the tissues was performed.
Results
Both malondialdehyde and caspase-3 levels were significantly decreased by DA administration. Histopathological evaluation of the tissues also demonstrated decrease in neuronal degeneration and infiltration parameters after DA administration. In the DA group, neurological outcome scores were statistically significantly better compared with the ischemia and the vehicle groups.
Conclusions
Although further studies considering different dose regimens and time intervals are required, DA was shown to be at least as effective as methylprednisolone in spinal cord ischemia/reperfusion model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spinal cord injury (SCI) may have either traumatic or non-traumatic origin; both of them often lead to devastating dysfunction and disability. The neurological damage at the time of insult is called “primary injury”. After primary injury, endogenous substances cause additive damage, which is called “secondary damage”. Inflammation and free radical formation play an important role in pathological mechanisms involved in secondary damage [11, 16, 21]. Ischemic injury, which is aggravated by reperfusion, results in lipid peroxidation and further degeneration [7, 30]. Glutamate-mediated excitotoxicity, formation of reactive oxygen species and lipid peroxidation are important events contributing to neuronal dysfunction and cell loss following traumatic and ischemic injury to the central nervous system [2, 17].
After thoracic or abdominal aorta surgery, there is a well-known risk of paraplegia due to SCI caused by ischemia/reperfusion (I/R) [18]. It is thought to be caused by the lowering or blockage of blood flow from the intercostal arteries to the spinal cord [45]. There are various agents and methods used for spinal cord protection during the ischemic period.
Erythropoietin (EPO) is a glycoprotein hormone that is the primary regulator of erythropoiesis [26]. Recombinant human erythropoietin (rHuEPO) has been used to treat anemia associated with chronic renal failure, chemotherapy for cancer patients, and HIV infections [31]. EPO is neuroprotective in a variety of rodent models of hypoxic/ischemic central nervous system disorders [35, 37, 42]. Darbepoetin-alpha (DA) is a novel erythropoiesis-stimulating agent with additional sialic acid-containing oligosaccharide compared with EPO, and an extended circulatory half-life, and increased in vivo biological activity [12]. DA is now being used extensively to treat anemia associated with chronic renal failure and chemotherapy [34, 40]. Because DA activates the EPO receptors (EPO-R), here we have hypothesized that it should also confer neuroprotection in I/R model of the spinal cord.
Materials and methods
Experimental groups
All experimental procedures used in this investigation were reviewed and approved by the ethical committee of the Ministry of Health Refik Saydam Hıfzıssıha Institution. Forty adult male New Zealand white rabbits, weighing 2,500-3,850 g, were divided randomly into five groups of eight rabbits each:
-
Group 1
Sham group (n = 8); laparotomy only. Rabbits underwent laminectomy and non-ischemic spinal cord samples were obtained immediately after surgery.
-
Group 2
Ischemia group (n = 8). Rabbits underwent transient global spinal cord ischemia. After laminectomy, spinal cord samples were removed 24 h post-ischemia.
-
Group 3
Vehicle group (n = 8). As for group 2, but rabbits received 2 ml vehicle solution (saline) intravenously immediately after the clamp was removed.
-
Group 4
Methylpredinisolone (MP) group (n = 8). As for group 2, but rabbits received a single intravenous dose of 30 mg/kg MP (Prednol, Mustafa Nevzat, Turkey) immediately after the clamp was removed.
-
Group 5
DA group (n = 8). As for group 2, but rabbits received a single intravenous dose of 30 μg/kg DA (Aranesp, Amgen Europe, Netherlands) immediately after the clamp was removed.
Anesthesia and surgical procedure
The animals were kept at optimal (18-21°C) room temperature and fed with the standard diet, where a 12-h light–dark cycle was implemented. Free access to food and water was allowed. The animals were anesthetized by intramuscular administration of 70 mg/kg ketamine (Ketalar, Parke Davis Eczacıbaşı, Turkey) and 5 mg/kg xylazine (Rompun, Bayer, Turkey) and allowed to breath spontaneously. Body temperatures were measured and maintained at 37°C with a heating pad. Animals were placed in the supine position. After sterile preparation, a 10-cm midline incision was made and the abdominal aorta was exposed through a transperitoneal approach. Heparin (150 U/kg) was administered through the intravenous route 5 min before clamping for anticoagulation. Approximately 1 cm below the renal artery, the aorta was clamped using an aneurysm clip of 70 g closing force (Yasargil, FE721, Aesculap, Germany) under a surgical microscope. Cross clamp time was 20 min. At the end of the occlusion period, the clips were removed and restoration of blood flow was visually verified. The drugs were administered immediately after the clamp was removed. Free access to food and water was allowed 2 h after the operation. Crede’s maneuver was performed on animals with neurogenic bladder at least two times a day. The animals were killed 24 h after operation by injection of pentobarbital (200 mg/kg); then, spinal cord segments between L2 and L5 were removed by carefully performed laminectomy for biochemical and histopathological analysis.
Caspase-3 activity
Tissues were homogenized in physiological saline (1 g in 5 ml) and centrifuged at 4,000 g for 20 min. The upper layer of clear supernatant was removed and used in the analyses. Before analysis, the supernatant samples were adjusted so that they contained equal protein concentrations. The protein concentrations of the supernatant samples were measured using the Lowry method. The Lowry method depends on the reactivity of the nitrogen in peptides with copper ions under alkaline conditions and the subsequent reduction of the Folin-Ciocalteau phosphomolybdic-phosphotungstic acid to heteropolymolybdenum blue by the copper catalyzed oxidation of aromatic amino acids. Absorbance measurements were made at 700 nm using a spectrophotometer. The protein concentration of the sample was determined using a protein calibrator. The caspase-3 activity of the tissue samples was measured using the Caspase-3 Colorimetric Detection Kit (907–013; Assay Designs, Ann Arbor, MI, USA). The kit involves the conversion of a specific chromogenic substrate for caspase-3 (acetyl-Asp-Glu-Val-Asp-p-nitroanilide), followed by colorimetric detection of the product (p-nitroaniline) at 405 nm. The absolute value for caspase-3 activity can be determined by comparison with a signal given by the p-nitroaniline calibrator. Activity measurements were quantified by comparing the optical densities obtained with standards with the p-nitroaniline calibrator. One unit of caspase-3 activity was defined as the amount of enzyme needed to convert 1 pmol of substrate per min at 30°C. The results were expressed as U/mg protein.
Tissue malondialdehyde (MDA) analysis
MDA is formed from the breakdown of polyunsaturated fatty acids, and serves as an important and reliable index for determining the extent of peroxidation reactions [30]. Tissue MDA levels were determined by a method based on the reaction with thiobarbituric acid (TBA). Briefly, the samples were mixed with two volumes of cold saline solution containing 0.001% butylated hydroxytoluene (BHT) (200 μl 0.01% BHT solution in methanol) and 0.07% sodium dodecyl sulfate (SDS) (20 μl 7% SDS). Then 1-ml samples were added to 500 μl 0.01 M NH2SO4 and 500 μl thiobarbituric acid reagent (0.67% thiobarbituric acid in 50% aceticacid) to precipitate protein. The samples were heated in boiling water for 60 min. After cooling, an equal volume (2 ml) of n-butanol was added to each test tube and mixed. The mixture was centrifuged at 4,000 rpm for 10 min at room temperature. The absorbance of the organic layer in 1 ml cell was read at 535 nm (Molecular Devices Corporation, Sunnyvale, CA, USA). MDA concentrations were expressed as nmoles per gram tissue wet weight.
Histopathology
The cord specimens obtained at 24 h post-injury were prepared for histological study. Each cord segment was immersed in 4% paraformaldehyde in 0.1 mol/l phosphate buffer and stored at 4°C. The species were then embedded in paraffin, cut into sections of 5 μm thickness, and stained with hematoxylin-eosin (H&E). The specimens were examined under a light microscope by a neuropathologist, who was blinded to the study design.
Neurologic evaluation
Neurologic status of the animals was scored by assessment of hind-limb neurologic function 24 h after the procedure, using the modified Tarlov Scoring System [23]. A score of 0 to 5 was assigned to each animal as follows: 0, no voluntary hind-limb movement; 1, movement of joints perceptible; 2, active movement but unable to sit without assistance; 3, able to sit but unable to hop; 4, weak hop; 5, complete recovery of hind-limb function.
Neurologic evaluations were performed by a medical doctor who was blinded to the experimental groups.
Statistical analysis
All data collected were coded, recorded and analyzed using SPSS 10.0.1 for Windows (SPSS, Chicago, IL, USA). All data are presented as mean ± standard error (SE). One-way analysis of variance (ANOVA) for parametric data was used for comparing differences between two or more groups. Tukey’s test was used to determine differences between groups.
The differences among the groups in terms of Tarlov scores were determined by nonparametric statistical analysis using the Kruskal-Wallis test. A p value less than 0.05 was considered statistically significant.
Results
Caspase-3 activity
There were statistically significant differences between the control and both the ischemia and the vehicle groups with regard to mean caspase-3 activity (p < 0.01); however this data showed that I/R injury clearly elevated caspase-3 activity in the damaged tissue. When the DA group was compared both with the ischemia and the vehicle groups, there were a statistically significant decrease in caspase-3 levels were determined (p < 0.01). As in the DA group, the MP group also showed a statistically significant decrease in caspase-3 levels (p < 0.01). This data concluded that both MP and DA prevented an increase in caspase-3 activity and effectively inhibited apoptotic cell death. There was no statistically significant difference between the MP and the DA groups (p = 0.563). The difference between the vehicle group and the ischemia group was not statically significant (p = 0.115). Also, the difference between the DA group and the sham group was statically significant (p < 0.01) (Fig. 1).
MDA analysis
When mean tissue MDA levels were compared between the control and both the ischemia and the vehicle groups, there were statistically significant differences observed (p < 0.01); so we concluded that after I/R injury, due to elevated lipid peroxidation, tissue MDA levels were increasing. When we compared the ischemia and the vehicle groups with the DA group, there were statistically significant differences were observed (p < 0.01). As in the DA group the comparison between the MP and both the ischemia and the vehicle groups, there were statistically significant differences were also observed (p < 0.01). These data showed that both MP and DA prevented spinal cord tissues from an increase in MDA levels. The DA group had lower mean tissue MDA levels (0.4 ± 0.17) compared with the MP group (0.6 ± 0.08); but this difference was not statistically significant (p = 0.093). The difference between the vehicle group and the ischemia group was not statically significant (p = 0.211). Also, the difference between the DA group and the sham group was statically significant (p < 0.01) (Fig. 2).
Histopathology
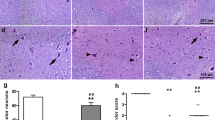
Light microscopic examinations of the spinal cord samples from the sham group were normal (Fig. 3a). Both in the ischemia (Fig. 3b) and the vehicle groups, diffuse hemorrhage and congestion in the gray matter were observed at 24 h after I/R injury. There were marked necrosis and widespread edema in both white and gray matter. In the damaged portion there were infiltrating polymorphonuclear leukocytes, lymphocytes, and plasma cells. Neuronal pyknosis, a loss of cytoplasmic features and cytoplasmic eosinophilia were also observed both in the ischemia and the vehicle groups. In the DA group, the cord tissues were protected from I/R injury, as in the MP group (Fig. 3c, d).
Photomicrographs of 5–µm-thick spinal chord tissue sections from the different treatment groups (H&E, ×10 obj.). a Sham group, showing regular spinal cord parenchyma. b Ischemia group, showing degenerated neurons (hollow arrows) in the edematous surface. c MP group, showing less edema and degenerated neurons (hollow arrows); note the normal appearing neurons (filled arrows). d Photomicrograph of the DA group, showing less degenerated neurons (hollow arrows), and more normal appearing neurons (filled arrows). The cord tissues were well protected from injury
Tarlov score
The mean Tarlov score of the DA group (2.8 ± 0.4) was significantly higher than both the ischemia (0.4 ± 0.5) and the vehicle groups (0.2 ± 0.4) (p < 0.01). The mean Tarlov score of the MP group was 2.4 ± 0.5, and this value was significantly higher than both the ischemia and the vehicle groups (p < 0.01). There was no statistically significant difference between the DA and the MP groups.
Discussion
Recombinant human EPO has been shown to be an exceedingly safe drug, which has been used more than 15 years for treatment of anemia. About a decade ago, it was generally believed that EPO acts only on erythroid precursor cells, but because erythropoietin receptors (EPO-R) have been found in many other tissues, including brain, spinal cord, heart and testis, there is an emerging consensus that EPO may help nonerythroid cells to survive and proliferate [27, 46]. EPO has been showed to have antiapoptotic, antioxidant, antiinflammatory and angiogenic effects, which are providing tissue protector effects [1]. The neuroprotective effect of EPO has been demonstrated in numerous experimental studies [22, 32, 36, 37, 42]. Also, EPO administration to the patients with ischemic stroke showed significant improvement in clinical outcome and reduced the infarct size [13]. There are several studies showed that EPO administration reduces injury caused by I/R of the spinal cord [6], eye [21], gut [43], lung [47], and liver [41] in animals. As a result, it is increasingly agreed that EPO has potent tissue protective effects. The exact mechanism of the EPO’s protective effect against I/R injury is not fully understood. The receptor associated tyrosine kinase (jaus-kinase 2) is the main intracellular pathway for the effect of EPO on hematopoiesis and neuroprotection [9, 25]. Evidence from recent animal studies has shown that EPO reduces apoptosis via Akt-mediated pathway involving a decrease in active caspase-3 [5, 33, 44].
The EPO analogue, DA, is an erythropoiesis-stimulating agent that exerts similar physiological responses by affecting EPO-R [12]. Convincing evidence is available that DA as well as EPO acts as a neurothrophic and neuroprotector in the brain. Banks et al. [3] reported that DA crosses the blood–brain barrier by way of the extracellular pathways in amounts that could account for the neuroprotective effect. In animal studies, both agents have been reported to be beneficial in treating global and focal ischemia, reducing nervous system inflammation and improving neurological outcome [4, 6, 37, 42]. As an EPO-derivate agent, we hypothesized that DA may have neuroprotective effects on I/R model of the spinal cord. The rabbit aortic cross clamping method, which we used, is a useful method for this research, and the ischemic period of 20 min was chosen in order to achieve enough injury [48]. DA had demonstrated its protective effects against myocardial ischemia when infused both during the clamping, post clamping and also 24 h after reperfusion. The dosage of the DA used in this study was obtained from the past studies [15].
Necrosis and apoptosis are the other two major pathways of neuronal death resulting from I/R injury [38]. Acute ischemia usually leads to necrosis because of a reduction in spinal blood flow accompanied by depletion of adenosine triphosphate reserves and the development of edema [19, 38]. Although it has been showed that mild or severe I/R injury triggers apoptosis, which leads to later cell death [20, 28]. Apoptosis is activated by cysteine protease family known as capases [14]. Caspase-3 is an interleukin-converting enzyme, and has been suggested to be the principal effectors in the mammalian apoptotic and inflammatory pathways [24]. Sakurai et al. [38] demonstrated an increase in caspase-3 immunoreactivity in the motor neurons of the spinal cord after 15 min of ischemia. As a result of ischemic events, the peak increase in caspase-3 induction triggers DNA fragmentation [28]. DNA fragmentation and apoptotic cells have also been identified in the ischemic hemisphere after focal and forebrain ischemia [29]. All these past studies identified the caspase-3 activity as a reliable method in reflecting the apoptotic activity of I/R injury. It has also been shown that apoptosis has occurred at 24 h after I/R injury [10]. In the present study, we demonstrated that I/R injury increased tissue caspase-3 activity in the spinal cord of the both ischemia and vehicle groups when compared with the sham group, which is consistent with previous observations [10, 18, 38]. In this study, we demonstrated that both DA and MP have statistically significant effects on lowering caspase-3 activity when compared with the ischemia and the vehicle groups, so we showed that DA and MP have antiapoptotic effects.
The central nervous system consists largely of lipids, which are easily damaged by free-radical-induced lipid peroxidation [39]. Following spinal cord ischemia and during reperfusion, lipid peroxidation occurs within the cell membrane. However, lipid peroxidation is recognized as one of the main pathophysiological mechanism involved in secondary damage [8]. MDA is formed from the breakdown of polyunsaturated fatty acids, and serves as an important and reliable index for determining the extent of peroxidation reactions. MDA rises after spinal cord ischemia, demonstrating lipid peroxidation, which is thus considered to be evidence of reperfusion injury [35]. Our study showed that, after I/R injury, levels of MDA had dramatically increased in the both ischemia and vehicle groups when compared with the sham group. When compared with the ischemia and the vehicle groups, a statistically significant effect of DA and MP on lowering MDA levels after I/R injury has been shown in this study.
Histopathological evaluation includes neuronal and axonal damage and microglia infiltration. The sham group had normal spinal cords. Both in the ischemia and vehicle groups, diffuse hemorrhage, congestion, neuronal and axonal damage in the gray matter were observed at 24 h after I/R injury. Both the DA and the MP groups showed better morphological results compared with the ischemia and vehicle groups. These results suggest that DA and MP have beneficial effects on preserving normal spinal cord morphology, both by reducing lipid peroxidation and inhibiting apoptotic events.
We found that aortic clamping caused paraplegia in almost all animals in the ischemia and the vehicle groups. DA and MP infusions previous to I/R injury protect the spinal cord, which was shown by improved neurological function, determined by Tarlov scores.
Conclusions
In conclusion, neurological outcome, histopathological and biochemical analysis revealed that DA exhibits meaningful neuroprotective activity over I/R injury of the spinal cord. DA is shown to be at least as effective as MP; so we propose that the DA treatment could be useful in I/R injury.
However, this study has some limitations. The number of rabbits in each group and the time period for neurological assessment may be augmented; and the dose-dependent results may be investigated; with delayed neurological and histopathological assessment (more than 24 h) of the I/R injury of the spinal cord will increase DA’s value in treatment. Also, preconditioning in a similar model may have additive effects. Further studies based on our findings may be more helpful for investigating this promising medication for I/R injury of the spinal cord.
References
Ates E, Yalcin AU, Yilmaz S, Koken T, Tokyol C (2005) Protective effect of erythropoietin on renal ischemia and reperfusion injury. ANZ J Surg 75:1100–1105
Azbill RD, Mu X, Bruce-Keller AJ, Mattson MP, Springer JE (1997) Impaired mitochondrial function, oxidative stress and altered antioxidant enzyme activities following traumatic spinal cord injury. Brain Res 765:283–290
Banks WA, Jumbe NL, Farrell CL, Niehoff ML, Heatherington AC (2004) Passage of erythropoietic agents across the blood–brain barrier: a comparison of human and murine erythropoietin and the analog darbepoetin alfa. Eur J Pharmacol 505:93–101
Belayev L, Khoutorova L, Zhao W, Vigdorchik A, Belayev A, Busto R, Magal E, Ginsberg MD (2005) Neuroprotective effect of darbepoetin alfa, a novel recombinant erythropoietic protein, in focal cerebral ischemia in rats. Stroke 36:1071–1076
Calvillo L, Latini R, Kajstura J, Leri A, Anversa P, Ghezzi P, Salio M, Cerami A, Brines M (2005) Recombinant human erythropoietin protects the myocardium from ischemia-reperfusion injury and promotes beneficial remodeling. Proc Natl Acad Sci USA 100:4802–4806
Celik M, Gokmen N, Erbayraktar S, Akhisaroglu M, Konakc S, Ulukus C, Genc S, Genc K, Sagiroglu E, Cerami A, Brines M (2002) Erythropoietin prevents motor neuron apoptosis and neurologic disability in experimental spinal cord ischemic injury. Proc Natl Acad Sci USA 99:2258–2263
Chronidou F, Apostolakis E, Papapostolou I, Grintzalis K, Georgiou CD, Koletsis EN, Karanikolas M, Papathanasopoulos P, Dougenis D (2009) Beneficial effect of the oxygen free radical scavenger amifostine (WR-2721) on spinal cord ischemia/reperfusion injury in rabbits. J Cardiothorac Surg 4:50
Diaz-Ruiz A, Rios C, Duarte I, Correa D, Guizar-Sahagun G, Grijalva I, Madrazo I, Ibarra A (2000) Lipid peroxidation inhibition in spinal cord injury: cyclosporin-A vs methylprednisolone. Neuroreport 11:1765–1767
Digicaylioglu M, Lipton SA (2001) Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-kappaB signalling cascades. Nature 412:641–647
Dolgun H, Sekerci Z, Turkoglu E, Kertmen H, Yilmaz ER, Anlar M, Erguder IB, Tuna H (2010) Neuroprotective effect of mesna (2-mercaptoethane sulfonate) against spinal cord ischemia/reperfusion injury in rabbits. J Clin Neurosci 17:486–489
Dumont RJ, Okonkwo DO, Verma S, Hurlbert RJ, Boulos PT, Ellegala DB, Dumont AS (2001) Acute spinal cord injury, part I: pathophysiologic mechanisms. Clin Neuropharmacol 24:254–264
Egrie JC, Browne JK (2001) Development and characterization of novel erythropoiesis stimulating protein (NESP). Br J Cancer 84:3–10
Ehrenreich H, Hasselblatt M, Dembowski C, Cepek L, Lewczuk P, Stiefel M, Rustenbeck HH, Breiter N, Jacob S, Knerlich F, Bohn M, Poser W, Rüther E, Kochen M, Gefeller O, Gleiter C, Wessel TC, De Ryck M, Itri L, Prange H, Cerami A, Brines M, Sirén AL (2002) Erythropoietin therapy for acute stroke is both safe and beneficial. Mol Med 8:495–505
Emery E, Aldana P, Bunge MB, Puckett W, Srinivasan A, Keane RW, Bethea J, Levi AD (1998) Apoptosis after traumatic human spinal cord injury. J Neurosurg 89:911–920
Gao E, Boucher M, Chuprun JK, Zhou RH, Eckhart AD, Koch WJ (2007) Darbepoetin alfa, a long-acting erythropoietin analog, offers novel and delayed cardioprotection for the ischemic heart. Am J Physiol Heart Circ Physiol 293:H60–H68
Genovese T, Cuzzocrea S (2008) Role of free radicals and poly (ADP-ribose)polymerase-1 in the development of spinal cord injury: new potential therapeutic targets. Curr Med Chem 15:477–487
Gunasekar PG, Kanthasamy AG, Borowitz JL, Isom GE (1995) NMDA receptor activation produces concurrent generation of nitric oxide and reactive oxygen species: implication for cell death. J Neurochem 65:2016–2021
Hagl C, Ergin MA, Galla JD, Lansman SL, McCullough JN, Spielvogel D, Sfeir P, Bodian CA, Griepp RB (2001) Neurologic outcome after ascending aorta-aortic arch operations: effect of brain protection technique in high-risk patients. J Thorac Cardiovasc Surg 121:1107–1121
Hayashi T, Sakuria M, Abe K, Sadahiro M, Tabayashi K, Itoyama Y (1998) Apoptosis of motor neurons with induction of caspases in the spinal cord after ischemia. Stroke 29:1007–1013
Hearse DJ, Boli R (1992) Reperfusion induced injury: manifestations, mechanisms, and clinical relevance. Cardiovasc Res 26:101–108
Junk AK, Mammis A, Savitz SI, Singh M, Roth S, Malhotra S, Rosenbaum PS, Cerami A, Brines M, Rosenbaum DM (2002) Erythropoietin administration protects retinal neurons from acute ischemia-reperfusion injury. Proc Natl Acad Sci USA 99:10659–10664
Kaptanoglu E, Solaroglu I, Okutan O, Surucu HS, Akbiyik F, Beskonakli E (2004) Erythropoietin exerts neuroprotection after acute spinal cord injury in rats: effect on lipid peroxidation and early ultrastructural findings. Neurosurg Rev 27:113–120
Katircioglu SF, Ulus AT, Gökçe P, Sürücü S (2000) Iloprost protects the spinal cord during aortic cross-clamping in a canine model. J Cardiovasc Surg 41:89–93
Keane RW, Kraydieh S, Lotocki G, Bethea JR, Krajewski S, Reed JC, Dietrich WD (2001) Apoptotic and anti-apoptotic mechanisms following spinal cord injury. J Neuropathol Exp Neurol 60:422–429
Klingmuller U (1997) The role of tyrosine phosphorylation in proliferation and maturation of erythroid progenitor cells signals emanating from the erythropoietin receptor. Eur J Biochem 249:637–647
Krantz SB (1991) Erythropoietin. Blood 77:419–434
Lappin T (2003) The cellular biology of erythropoietin receptors. Oncologist 8:15–18
Li M, Ona VO, Chen M, Tenneti L, Zhang X, Stieg PE, Lipton SA, Friedlander RM (2000) Functional role and therapeutic implications of neuronal caspase-1 and −3 in a mouse model of traumatic spinal cord injury. Neuroscience 99:333–342
Li Y, Chopp M, Jiang N, Yao F, Zaloga C (1995) Temporal profile of in situ DNA fragmentation after transient middle cerebral artery occlusion in the rat. J Cereb Blood Flow Metab 15:389–397
Lukacova N, Halat G, Chavko M, Marsala J (1996) Ischemia–reperfusion injury in the spinal cord of rabbits strongly enhances lipid peroxidation and modifies phospholipid profiles. Neurochem Res 21:869–873
Markham A, Bryson HM (1995) Epoetin alfa. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic use in nonrenal applications. Drugs 49:232–254
Marti HH, Bernaudin M, Petit E, Bauer C (2000) Neuroprotection and angiogenesis: dual role of erythropoietin in brain ischemia. News Physiol Sci 15:225–229
Parsa CJ, Matsumoto A, Kim J, Riel RU, Pascal LS, Walton GB, Thompson RB, Petrofski JA, Annex BH, Stamler JS, Koch WJ (2003) A novel protective effect of erythropoietin in the infarcted heart. J Clin Invest 112:999–1007
Patton J, Reeves T, Wallace J (2004) Effectiveness of darbepoetin alfa versus epoetin alfa in patients with chemotherapy-induced anemia treated in clinical practice. Oncologist 9:451–458
Qian H, Liu D (1997) The time course of malondialdehyde production following impact injury to rat spinal cord as measured by microdialysis and high pressure liquid chromatography. Neurochem Res 22:1231–1236
Sadamoto Y, Igase K, Sakanaka M, Sato K, Otsuka H, Sakaki S, Masuda S, Sasaki R (1998) Erythropoietin prevents place navigation disability and cortical infarction in rats with permanent occlusion of the middle cerebral artery. Biochem Biophys Res Commun 253:26–32
Sakanaka M, Wen TC, Matsuda S, Masuda S, Morishita E, Nagao M, Sasaki R (1995) In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc Natl Acad Sci USA 95:4635–4640
Sakurai M, Nagata T, Abe K, Horinouchi T, Itoyama Y, Tabayashi K (2003) Survival and death-promoting events after transient spinal cord ischemia in rabbits: induction of Akt and caspase3 in motor neurons. J Thorac Cardiovasc Surg 125:370–377
Schmidley JW (1990) Free radicals in central nervous system ischemia. Stroke 21:1086–1090
Schwartzberg L, Shiffman R, Tomita D, Stolshek B, Rossi G, Adamson R (2003) A multicenter retrospective cohort study of practice patterns and clinical outcomes of the use of darbepoetin alfa and epoetin alfa for chemotherapy-induced anemia. Clin Ther 25:2781–2796
Sepodes B, Maio R, Pinto R, Sharples E, Oliveira P, McDonald M, Yaqoob M, Thiemermann C, Mota-Filipe H (2006) Recombinant human erythropoietin protects the liver from hepatic ischemia-reperfusion injury in the rat. Transpl Int 19:919–926
Siren AL, Fratelli M, Brines M, Goemans C, Casagrande S, Lewczuk P, Keenan S, Gleiter C, Pasquali C, Capobianco A, Mennini T, Heumann R, Cerami A, Ehrenreich H, Ghezzi P (2001) Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci USA 98:4044–4049
Squadrito F, Altavilla D, Squadrito G, Campo GM, Arlotta M, Quartarone C, Saitta A, Caputi AP (1999) Recombinant human erythropoietin inhibits iNOS activity and reverts vascular dysfunction in splanchnic artery occlusion shock. Br J Pharmacol 127:482–428
Tramontano AF, Muniyappa R, Black AD, Blendea MC, Cohen I, Deng L, Sowers JR, Cutaia MV, El-Sherif N (2003) Erythropoietin protects cardiac myocytes from hypoxia-induced apoptosis through an Akt-dependent pathway. Biochem Biophys Res Commun 308:990–994
Umeda Y, Mori Y, Takagi H, Iwata H, Matsuno Y, Hirose H (2003) Surgical outcome of abdominal aortic aneurysm repair in patients undergoing chronic hemodialysis. Heart Vessels 18:7–11
Wright GL, Hanlon P, Amin K, Steenberger C, Murphy E, Arcasoy MO (2004) Erythropoietin receptor expression in adult rat cardiomyocytes is associated with an acute cardioprotective effect for recombinant erythropoietin during ischemia-reperfusion injury. FASEB J 18:1031–1033
Wu H, Ren B, Zhu J, Dong G, Xu B, Wang C, Zheng X, Jing H (2006) Pretreatment with recombined human erythropoietin attenuates ischemia-reperfusion-induced lung injury in rats. Eur J Cardiothorac Surg 29:902–907
Zivin JA, DeGirolami U (1980) Spinal cord infarction: a highly reproducible stroke model. Stroke 11:200–202
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Comment
Although historically used for the treatment of anemia, EPO has emerged as a neuroprotective agent in different conditions of neuronal damage (traumatic brain injury, ischemia, spinal cord compression, peripheral neuropathy, retinal damage, epilepsy, and Parkinson’s disease, among others). The neuroprotective effect has been reported in a large number of studies, with some controversies.
The authors used darbepoetin, an erythropoietic derivative of EPO, of which much fewer studies are available. As stated by the authors, this derivative of EPO has an extended circulatory half-life and increased in vivo biological activity compared with EPO. This enhanced erythorpoietic activity is, however, considered detrimental by most researchers. A major concern is the risk of thrombosis after the administration of multiple doses of this glycoprotein. The risk in a single administration model, like that proposed in this study, should be less relevant. Nonetheless, the tissue-protective functions of EPO have been separated from its hematopoietic actions, leading to the development of EPO derivatives and mimetics. Many studies are focusing on non-erythropoietic erythropoietin derivatives, investigating their anti-apoptotic potential and anti-inflammatory function as well as their role in restoring vascular integrity. Carbamylated erythropoietin (CEPO) and asialo erythropoietin (asialoEPO) are structural derivatives of EPO that have no effect on erythrocyte mass, whereas they maintain a neuroprotective capability.
Alfredo Conti
Messina, ITALY
Comment
The authors describe a well-designed experiment which demonstrates the neuroprotective activity of an EPO analogue. The efficacy was similar to MP in this rabbit model. The results may be of interest to vascular surgeons and may lead to further research using the compound in traumatic spinal cord injury.
Daniel Resnick
Wisconsin, USA
Rights and permissions
About this article
Cite this article
Yilmaz, E.R., Kertmen, H., Dolgun, H. et al. Effects of darbepoetin-alpha in spinal cord ischemia-reperfusion injury in the rabbit. Acta Neurochir 154, 1037–1044 (2012). https://doi.org/10.1007/s00701-012-1298-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-012-1298-0