Abstract
Background
We present the long-term results of a consecutive series of patients with meningiomas treated by LINAC-radiosurgery using the micro-multi-leaf collimator technique (μMLC).
Methods
Between May 2001 and July 2009, 78 patients (m:f = 24:54; median age, 56.8 years; range, 20.1–81 years) with 87 intracranial meningiomas (78 WHO I, seven WHO II, two WHO III) were treated with μMLC-LINAC radiosurgery at our institution, either as a primary or salvage treatment following one or more microsurgical procedures. Fifty-eight of 87 tumors (66.7%) were located in the skull base. The remaining 29 meningiomas (33.3%) were located in the convexity of the brain. The median tumor volume was 4.8 ml (range, 0.2–18.3 ml). The median tumor surface dose, maximal dose, and therapeutic isodose were 12 Gy, 16 Gy, and 75%, respectively.
Results
For retrospective evaluation, we included 70 patients (78 tumors) with a minimum radiological follow-up of 24 months. After a median follow-up of 79.7 months (range, 24.2–109.1 months), 24 patients (34.3%) improved in their clinical status (paresis of N. abducens 18/48, facial paresis 4/8, and hemiparesis 2/9), 41 patients remained stable (58.6%), three patients had treatment-related temporary complaints (4.3%); two patients developed vertigo, and one had a left-sided hemihypesthesia. All complaints recovered completely after steroid medication within 2 weeks. Two patients (2.8%) developed permanent trigeminal neuralgia. Follow-up MR images showed a partial remission in 21 tumors (26.9%) and a stable tumor size in 55 cases (70.5%). Two patients with high-grade meningiomas showed a tumor progression (one WHO II and one WHO III meningioma). At the end of follow-up (July 2010), the actuarial 5- and 9-year progression-free survival after radiosurgery were 98 and 96%, respectively. There was no treatment-related mortality.
Conclusions
LINAC radiosurgery using a micro multi-leaf collimator for complex shaped intracranial meningiomas is effective yielding a high local tumor control, whereas the treatment-related morbidity remains low.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Meningiomas are usually benign neoplasms, representing the second most common brain tumor in adults, mainly affecting women over 40 years [2].
At present, microsurgery is the treatment of choice in the management of large meningiomas, even when total resection cannot be achieved [1, 2, 30]. However, other options also exist; radiotherapy and radiosurgery. Surgery and radiation therapy differ considerably in the definition of outcomes: local control after resection implies complete removal of the tumor without evidence of recurrence on follow-up evaluations. In contrast, local control after radiosurgery or radiotherapy implies stabilization of the tumor with no evidence of progression on follow-up evaluations.
For small- to medium-sized meningiomas, radiosurgery offers a non-invasive and promising primary treatment modality when resection would be associated with high patient morbidity. In addition, radiosurgery is now routinely used as an adjunct therapy for residual or recurrent meningiomas after surgical removal [33].
In this report, we present the long-term results of 78 patients with 87 meningiomas treated by LINAC radiosurgery (LINAC-RS) using a computer-controlled micro-multi-leaf collimator (μMLC). The advantages of the μMLC in the radiosurgery of, in the majority of cases complexly configured meningiomas, are also assessed.
Methods
Between May 2001 and July 2009, 78 patients with 87 intracranial meningiomas were treated with LINAC-RS at our institution either as a primary treatment or following one or more microsurgical procedures. All patients (m/f ratio: 24/54, median age, 56.8 years; range, 20.1–81 years) had a median follow-up of 76.7 months (range, 11.8–109.1 months). Seventy patients (m/f ratio: 22/48, median age, 54.5 years; range, 20.1–81 years) with a follow-up of more than 2 years (median follow-up 79.7 months; range, 24.2–109.1 months) were considered for retrospective evaluation (Table 1).
Prior to μMLC-LINAC-RS, the leading symptoms were headache in 31 of the 78 patients, diplopia in 17 patients, and trigeminal neuralgia in ten patients. Forty-six of 78 patients underwent one or more open surgeries before radiosurgery. Of this group (n = 46 patients), 20 patients had a relapse documented on follow-up MR images and radiosurgery was indicated. The remaining 32 of 78 patients underwent LINAC-RS as the primary treatment. The tumors were located as follows: falx (n = 20; 23%), cavernous sinus (n = 19; 21.8%), sphenoid wing (n = 14; 16.1%), convexity (n = 9; 10.3%), tentorium (n = 9; 10.3%), CPA (n = 8; 9.2%), petroclival (n = 3; 3.5%) and other locations (n = 5; 5.8%). All patients were treated with LINAC-RS using computed tomography and magnetic resonance imaging as a basis for stereotactic planning. Radiosurgery was indicated in patients with well-circumscribed tumors with a diameter ≤4 cm on CT- and /or MR-images and without a considerable brainstem compression. The median tumor volume was 4.8 ml (range, 0.2–18.3 ml). The statistical analysis was performed using PASW Statistics 18 (SPSS Inc., Chicago, IL, USA).
Technical data
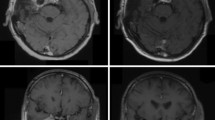
Irradiations were performed with an Elekta SL25 linear accelerator adapted for stereotactic radiosurgery. The patients were fixed in a modified Riechert-Mundinger stereotactic frame [32]. On stereotactic CT scans and fused MR images (contrast-enhanced axial T1- and T2-weighted sequences) the tumor border was delineated. A dural tail was counted as part of the clinical target volume in case of a visible or presumable border to the adjacent healthy tissue, or of approximately 2 mm in case of dural thickness. A computer-controlled μMLC with 1.5-mm lamella width (maximal field size 72 × 68 mm, Siemens, Heidelberg, Germany) was used to shape the 6MV photon beams [22, 24]. For each target volume, 10–20 non-coplanar beams were applied and each beam was shaped to match tumor shape in its beam’s eye view to achieve highly conformal dose distributions. Treatment planning based on CT (contrast medium enhanced, slice thickness 1.25 mm) and MRI (axial T1-weighted, gadolinium-enhanced and axial T2-weighted, both slice thickness 2 mm) was performed using the planning software VIRTUOSO 3.0 (Leibinger, Freiburg, Germany). Figure 1 shows a representative μMLC treatment plan. A cumulative tumor surface dose ranging between 10 and 15 Gy (median, 12 Gy; median isodose, 75%; range, 50–90%; median maximum dose, 16 Gy; range, 16.9-23.3 Gy) was applied. Our treatment and dosimetric parameters are shown in Table 1; the conformity index are defined according to Paddick [23].
The upper line shows a treatment plan showing a contrast-enhanced tumor in the right sphenoid bone infiltrating the cavernous sinus (red line) in a T1-weighted axial, coronal, and sagittal MRI [Isodose: 80% ( yellow dotted line), 50% (green line), and 30% (blue line)]. The lower line shows a 3-D configuration of the tumor (red) in relation to risk structures (right trigeminal nerve in green and right optical nerve in violet)
Follow-up
The clinical follow-up data were obtained from the patients and the referring physicians for a median follow-up period of 79.7 months (range; 24.2–109.1 months). Follow-up MR images for patients with WHO I meningioma were requested at a 6-month interval and at 1-year intervals thereafter. In patients with WHO II or III, meningioma MR images were scheduled for 3-month intervals during the first year and for 6-month intervals thereafter. Tumor response was evaluated by measuring the maximum diameters of the tumor in three dimensions. The response was classified according to the MacDonald criteria [18].
Results
Clinical follow-up
Clinically, 24 (34.3%) patients improved (paresis of N. abducens in 18 of 48 cases; facial paresis in four of eight cases (according to House & Brackmann: two patients from grade IV to II and two patients from grade V to IV) and hemiparesis 2/9), 41 patients were stable (58.6%), three patients had treatment-related temporary complaints (4.3%); two patients developed vertigo and one suffered transiently from a left-sided hemihypesthesia. All complaints recovered completely after steroid medication within 2 weeks. Two patients (2.9%) developed permanent trigeminal neuralgia 6 and 9 months after treatment. The trigeminal neuralgia of both patients was well controlled by pain medication.
At the end of follow-up time (July 2010), five patients had died; four due to old age and one due to pulmonary embolism. There was no treatment-related mortality.
Radiological follow-up
Follow-up MR images showed a partial remission in 21 tumors (26.9%, Fig. 2) and a stable tumor size in 55 cases (70.5%). Two patients showed a tumor progression (one WHO II and one WHO III meningioma).
The overall, actuarial 5- and 9-year progression-free survival following radiosurgery was 98 and 96%, respectively (Fig. 3).
Discussion
Meningiomas account for 15–25% of all primary brain tumors [1, 2]. They have generally an unspectacular and indolent natural history and present themselves with minimal, mild symptoms, even when the tumor is large. This indolent course renders the tumor undiagnosed until late stages when it has already invaded the dura mater and bone, making complete resection impossible in many cases [30]. In addition, the vicinity of these tumors to the vital neurovascular structures of the brain complicates microsurgical resection [2, 3].
It is widely agreed that the best chance of cure is total surgical removal of the tumor and its nidus of origin [2, 30].
Al-Mefty et al. reported a 62% incidence of new or worsened neurologic deficits following surgical resection of petroclival meningiomas in 13 patients [1].
Couldwell et al., reporting on the largest series of petroclival meningiomas to date, noted a complication rate of 35% and mortality rate of 4% [5].
Postoperative external beam radiation therapy improves long-term local control of subtotally resected or recurrent meningiomas [1, 7, 17, 20, 34].
Goldsmith et al. reported on a significant decline of meningioma regrowth of 29% following subtotal resection and adjuvant radiotherapy compared to 74% with subtotal resection alone [9].
However, fractionated radiotherapy of meningiomas carries the possibility of long-term side-effects, such as loss of vision, pituitary dysfunction, delayed radiation-induced severe adverse effects (SAEs), and the development of secondary neoplasms after irradiation for benign CNS tumors [17, 20].
Favorable results have also been shown with stereotactic radiosurgery for intracranial meningiomas using linear accelerator- or gamma knife techniques [3–6, 28, 29, 33, 34]. Meningiomas are ideal radiobiological targets because single-fraction radiation has a high biologically effective dose.
Furthermore, radiosurgery is particularly suitable for the treatment of meningiomas because they are well defined by computed tomography or MR imaging and usually do not invade the brain [25–27, 33].
The importance of the dural origin of meningiomas is well established in surgical practice, as reflected by Simpson’s grades, but may be equally significant in radiosurgical practice [8, 10–12, 30]. Three different factors were considered in selecting the cases for radiosurgical treatment in our patients: 1st tumor size, 2nd possibility of the surgical resectibility of the tumor and 3rd adjacency to critical structures and the chance of preservation of the normal brain tissues. Using these criteria, we had a tumor control rate of more than 97%, which was predictable due to the comparability of our treatment procedure and selection criteria to those of the international studies [10, 11, 22, 25, 26, 28, 29].
For patients with hemispheric meningiomas, the indication for SRS should be limited, as they have a higher risk of treatment related edema compared to patients with basal meningiomas. Kondziolka et al., reported peritumoral imaging changes in 5% either due to edema or adverse radiation effects and an overall complication rate of 9.6% in their series of convexity meningiomas [14].
Furthermore, a highly conformal irradiation can provide effective treatment to the tumor while sparing the surrounding brain. Since meningiomas are often complexly configured, beam shaping with a μMLCs offers an effective way to generate homogeneous and conformal dose distributions.
In our study, three patients had treatment-related temporary complaints (4.3%); two patients developed vertigo, and one had a left-sided hemihypesthesia. All untoward effects recovered completely after steroid medication within 2 weeks. Two patients (2.9%) developed permanent trigeminal neuralgia, which was treated with pain medication. To date, the trigeminal neuralgia is well controlled in both patients.
Although ideally an excellent method, radiosurgery certainly does have its limitations. One is the size (tumor diameter should be less than 4 cm in diameter); the precise definition of the target may be another restrictive factor [15, 16]. Brainstem mass effect and encasement of basilar perforators are also important considerations, with possible risk of brain stem injury as a result of micro vascular ischemia [13, 19–21, 31].
Conclusions
LINAC radiosurgery using a micro multi-leaf collimator for meningiomas is effective and safe, yielding a high local tumor control and a low treatment-related morbidity. This treatment modality is proper for surgically inaccessible small- to moderate-sized meningiomas as a primary and adjuvant treatment or after recurrent tumors.
References
Al-Mefty O, Kersh JE, Routh A, Smith RR (1990) The long-term side effects of radiation therapy for benign brain tumors in adults. J Neurosurg 73:502–512
Barnholtz-Sloan JS, Kruchko C (2007) Meningiomas: causes and risk factors. Neurosurg Focus 23:E2
Chang SD, Adler JR Jr (1997) Treatment of cranial base meningiomas with linear accelerator radiosurgery. Neurosurgery 41:1019–1025, discussion 1025–1017
Chin LS, Szerlip NJ, Regine WF (2003) Stereotactic radiosurgery for meningiomas. Neurosurg Focus 14:e6
Couldwell WT, Fukushima T, Giannotta SL, Weiss MH (1996) Petroclival meningiomas: surgical experience in 109 cases. J Neurosurg 84:20–28
Duma CM, Lunsford LD, Kondziolka D, Harsh GRT, Flickinger JC (1993) Stereotactic radiosurgery of cavernous sinus meningiomas as an addition or alternative to microsurgery. Neurosurgery 32:699–704, discussion 704–695
Elia AE, Shih HA, Loeffler JS (2007) Stereotactic radiation treatment for benign meningiomas. Neurosurg Focus 23:E5
Friedman WA, Murad GJ, Bradshaw P, Amdur RJ, Mendenhall WM, Foote KD, Bova FJ (2005) Linear accelerator surgery for meningiomas. J Neurosurg 103:206–209
Goldsmith BJ, Wara WM, Wilson CB, Larson DA (1994) Postoperative irradiation for subtotally resected meningiomas. A retrospective analysis of 140 patients treated from 1967 to 1990. J Neurosurg 80:195–201
Hakim R, Alexander E 3rd, Loeffler JS, Shrieve DC, Wen P, Fallon MP, Stieg PE, Black PM (1998) Results of linear accelerator-based radiosurgery for intracranial meningiomas. Neurosurgery 42:446–453, discussion 453–444
Kondziolka D, Flickinger JC, Perez B (1998) Judicious resection and/or radiosurgery for parasagittal meningiomas: outcomes from a multicenter review. Gamma Knife Meningioma Study Group. Neurosurgery 43:405–413, discussion 413–404
Kondziolka D, Kano H, Kanaan H, Madhok R, Mathieu D, Flickinger JC, Lunsford LD (2009) Stereotactic radiosurgery for radiation-induced meningiomas. Neurosurgery 64:463–469, discussion 469–470
Kondziolka D, Levy EI, Niranjan A, Flickinger JC, Lunsford LD (1999) Long-term outcomes after meningioma radiosurgery: physician and patient perspectives. J Neurosurg 91:44–50
Kondziolka D, Madhok R, Lunsford LD, Mathieu D, Martin JJ, Niranjan A, Flickinger JC (2009) Stereotactic radiosurgery for convexity meningiomas. J Neurosurg 111:458–463
Kotapka MJ, Kalia KK, Martinez AJ, Sekhar LN (1994) Infiltration of the carotid artery by cavernous sinus meningioma. J Neurosurg 81:252–255
Liscak R, Simonova G, Vymazal J, Janouskova L, Vladyka V (1999) Gamma knife radiosurgery of meningiomas in the cavernous sinus region. Acta Neurochir (Wien) 141:473–480
Lunsford LD (1994) Contemporary management of meningiomas: radiation therapy as an adjuvant and radiosurgery as an alternative to surgical removal? J Neurosurg 80:187–190
Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277–1280
Morita A, Coffey RJ, Foote RL, Schiff D, Gorman D (1999) Risk of injury to cranial nerves after gamma knife radiosurgery for skull base meningiomas: experience in 88 patients. J Neurosurg 90:42–49
Newman SA (1994) Meningiomas: a quest for the optimum therapy. J Neurosurg 80:191–194
Ojemann SG, Sneed PK, Larson DA, Gutin PH, Berger MS, Verhey L, Smith V, Petti P, Wara W, Park E, McDermott MW (2000) Radiosurgery for malignant meningioma: results in 22 patients. J Neurosurg 93(Suppl 3):62–67
Otto-Oelschlager S, Schlegel W, Lorenz W (1994) Different collimators in convergent beam irradiation of irregularly shaped intracranial target volumes. Radiother Oncol 30:175–179
Paddick I (2000) A simple scoring ratio to index the conformity of radiosurgical treatment plans. Technical note. J Neurosurg 93(Suppl 3):219–222
Pastyr O, Hartmann GH, Schlegel W, Schabbert S, Treuer H, Lorenz WJ, Sturm V (1989) Stereotactically guided convergent beam irradiation with a linear accelerator: localization-technique. Acta Neurochir (Wien) 99:61–64
Pollock BE (2003) Stereotactic radiosurgery for intracranial meningiomas: indications and results. Neurosurg Focus 14:e4
Pollock BE (2009) Stereotactic radiosurgery of benign intracranial tumors. J Neurooncol 92:337–343
Pollock BE, Stafford SL, Utter A, Giannini C, Schreiner SA (2003) Stereotactic radiosurgery provides equivalent tumor control to Simpson Grade 1 resection for patients with small- to medium-size meningiomas. Int J Radiat Oncol Biol Phys 55:1000–1005
Roche PH, Regis J, Dufour H, Fournier HD, Delsanti C, Pellet W, Grisoli F, Peragut JC (2000) Gamma knife radiosurgery in the management of cavernous sinus meningiomas. J Neurosurg 93(Suppl 3):68–73
Shafron DH, Friedman WA, Buatti JM, Bova FJ, Mendenhall WM (1999) Linac radiosurgery for benign meningiomas. Int J Radiat Oncol Biol Phys 43:321–327
Simpson D (1957) The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry 20:22–39
Stafford SL, Pollock BE, Foote RL, Link MJ, Gorman DA, Schomberg PJ, Leavitt JA (2001) Meningioma radiosurgery: tumor control, outcomes, and complications among 190 consecutive patients. Neurosurgery 49:1029–1037, discussion 1037–1028
Sturm V, Pastyr O, Schlegel W, Scharfenberg H, Zabel HJ, Netzeband G, Schabbert S, Berberich W (1983) Stereotactic computer tomography with a modified Riechert-Mundinger device as the basis for integrated stereotactic neuroradiological investigations. Acta Neurochir (Wien) 68:11–17
Subach BR, Lunsford LD, Kondziolka D, Maitz AH, Flickinger JC (1998) Management of petroclival meningiomas by stereotactic radiosurgery. Neurosurgery 42:437–443, discussion 443–435
Torres RC, Frighetto L, De Salles AA, Goss B, Medin P, Solberg T, Ford JM, Selch M (2003) Radiosurgery and stereotactic radiotherapy for intracranial meningiomas. Neurosurg Focus 14:e5
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
El Majdoub, F., Elawady, M., Bührle, C. et al. μMLC-LINAC radiosurgery for intracranial meningiomas of complex shape. Acta Neurochir 154, 599–604 (2012). https://doi.org/10.1007/s00701-012-1278-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-012-1278-4