Abstract
Object
The object of surgical treatment for hemifacial spasm (HFS) is the exclusion of pulsatile neurovascular compression of the root exit zone (REZ). However, spasm persists transiently or permanently in some cases even after complete decompression. In particular, we mainly experience these results when the vertebral artery (VA) is the offender. Hence, we verified color changes of the nerve and indentations from within the operative field in HFS patients with the VA as the offender. So, we reviewed retrospectively the records of those patients who were treated with microvascular decompression (MVD) in order to assess the relationship between operative findings and clinical results.
Methods
A total of 232 patients with HFS associated with the VA were treated with MVD between January 1994 and January 2009 at our institution. The patients were classified into one of the following three categories based on compression severity: Group I, mild; Group II, moderate; Group III, severe. The patients were also classified into one of the following four categories based on the existence of indentation and discoloration of nerve VII: Group A (−/−), B (+/−), C (−/+), or D (+/+).
Results
A total of 94.2% and 96.6% of the patients in Groups I and II, respectively, had improved to grades I-II at the last follow-up. The surgical outcomes of Group III were slightly poorer than those of Groups I and II. Group A showed the poorest outcomes with 60% of the patients classified as grades III-IV. In Group B, 98.4% of the patients showed a marked improvement and Groups C and D showed relatively poor outcomes compared with those of Group B.
Conclusions
Severe deviations and color changes of the facial nerves may be the risk factors for poor surgical outcomes. Future studies with larger sample sizes and investigations of the pathophysiology underlying these findings are needed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gardner et al. [5] reported that the pathophysiological mechanism of hemifacial spasm (HFS) is due to chronic pulsatile compression of the facial nerve root exit zone (REZ). As a result, denervation of the nerve sheath occurs and produces nerve action potentials below the normal level. Reports of lateral excitation of facial axons due to ephaptic transmission [7, 25] and hyperexcitability of facial motor neurons also exist [15, 19]. Microvascular decompression (MVD) has been suggested to be the best treatment for HFS; it has an excellent potential for achieving a disease-free state by removing vascular compression [1, 14]. However, several controversial issues are associated with MVD for HFS, including the mechanism of recurrence and persistent symptom despite surgery [11, 23, 31], and many questions remain unanswered. One of our questions was inspired by an uncommon observation regarding a color change coinciding with events such as venous congestion or ischemia of the facial nerve. Such color change is seen intraoperatively when the offending vessels are transposed. The object of surgical treatment for HFS has been accepted as the exclusion of pulsatile neurovascular compression of the REZ. However, some cases show persistent spasms either transiently or permanently even after complete decompression of the REZ. In particular, we experience these results when the vertebral artery (VA) with strong pulsatile force and intense compression severity is the offender of HFS. Therefore, this study was designed to investigate factors contributing to clinical outcomes in HFS patients with the VA as the offender according to the degree of compression force and color change in the nerve, with a focus on the intraoperative view (Fig. 1). We reviewed retrospectively the records of HFS patients with the VA the offender who were treated with MVD to assess the relationships between operative findings and clinical results.
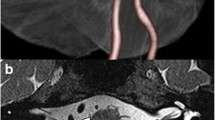
Preoperative magnetic resonance imaging (MRI) series of a 49-year-old woman with a 5-year history of left hemifacial spasm. Two-dimensional fast spin echo image and 3D TOF MRA (b) showing the left vertebral artery and the posterior inferior cerebellar artery compressing the facial nerve at the root exit zone (a and b) and tortuosity of the vertebral artery (c). Operation view shows severe compression by the vertebral artery and the posterior inferior cerebellar artery (d). When decompressed, we could see the indentation and color change of the facial nerve (e)
Materials and methods
Patient population
Between January 1994 and January 2009, 1,729 consecutive patients underwent MVD for HFS at Yonsei University Severance Hospital. Among these, 1,663 patients were followed-up for 2 years, and 109 of 1,663 patients had HFS caused by the VA alone and 123 of 1,663 patients had HFS caused by the VA plus multiple offenders. The mean patient age was 52.32 years (age range, 15–79). The male-to-female ratio was 1:3.5 with a predominantly female occurrence and left-sided preponderance. The mean preoperative symptom duration was 6.0 years (range, 0.7-40 years), and the mean follow-up duration was 23.83 months (range, 3.20-199.95 months).
MRI evaluation and monitoring technique
Preoperative and postoperative three-dimensional magnetic resonance angiography (3D-TOF MRA) evaluations were performed with a 1.5-Tesla imaging system (Signa; GE Medical Systems, Milwaukee, WI, USA), and vascular compression and the status of the REZ were evaluated using the same criteria that we reported previously [4]. The REZ was identified as vascular compression at the pontomedullary junction. Pure tone audiometry (PTA) and facial electromyelography (EMG) were performed preoperatively. Brainstem auditory evoked potential and facial EMG were monitored intraoperatively.
Surgical procedures
Under general anesthesia, all procedures were performed via a lateral retrosigmoid suboccipital approach. The lateral pontomedullary membrane was carefully dissected while assessing the low cranial nerve complexes. Approximately 1-3 mm superior to the nineth cranial nerve, the REZ of the facial nerve was located. The compression of the VA and other blood vessels was identified near the REZ. When small perforating arteries were branching towards the brain stem or the cerebellar peduncle directly, careful manipulation was essential because traction injuries might cause severe consequences. We used Teflon felt and ball to reposition the offending vessels and surgical glue to fix the repositioned vessels in place. Then the surface of the facial nerve sheath was enveloped using surgical glue.
Clinical assessment and follow-up review
All surgical procedures were performed by two surgeons, and a database was assembled which included the surgeons’ intraoperative records, photographs, and videos. Compression severity was categorized as follows: Group I (mild) = only contact without nerve indentation, Group II (moderate) = indentation of the facial nerve without deviation from its course, and Group III (severe) = deviation from the natural course of the facial nerve (Fig. 2). The existence of indentation and discoloration of nerve VII was determined by the senior surgeon. In order to compare the surgical outcomes with the presence of indentation and/or color change, we categorized the participants into four groups. Group A included patients without indentation and color change. Patients with indentation alone were placed in Group B. Group C contained patients with only color change, and Group D included patients with both indentation and color change upon intraoperative view (Fig. 3). Our ratings for the improvement of facial spasm postoperatively were as follows: excellent (I), complete resolution of spasm; good (II), more than 90% improvement; fair (III), 50–90% improvement; poor (IV), less than 50% improvement; bad (V), no improvement. Clinical outcomes were evaluated immediately after the surgery and at outpatient visits during the subsequent follow-up period. When patients could not visit, we used a telephone questionnaire. All patients included in this study were followed-up for at least 24 months after MVD surgery. When classifying postoperative complications, an “immediate” cranial nerve deficit was defined as a cranial nerve deficit occurring within 24 h. An occurrence of a cranial nerve deficit 24 h postoperatively was defined as “delayed.” If a patient presented with complications, such as a hearing impairment, facial weakness, low cranial symptoms, and surgical complications, the patient was evaluated to determine the complication severity and recovery state.
We categorized the patients into four groups based on color and indentation differences. Group A included patients without indentation or color change. Group B included patients with only indentation, and Group C was made up of patients with only color change. Patients in Group D had both indentation and color change
Statistical analysis
Pearson’s chi-square test was used to compare transient or permanent surgical complications between the VA group and the overall group. Pearson’s chi-square and Fisher’s exact test were used to compare neurological outcomes and improvement depending upon the severity of compression, indentation, and/or color change between each group. The comparison of each group was adjusted as Bonferroni correction. All statistical analyses were performed using SAS (SAS ver. 9.2; SAS Institute, Cary, NC, USA). The data were presented as the mean values ± standard deviations (SD); p values <0.05 were considered statistically significant.
Results
Clinical outcomes after MVD for HFS
Among those with HFS caused by the VA, grades I-II were observed in 221 cases (95.2%), grades III-IV were seen in 22 cases (3.5%), and grade V were identified in three cases (1.3%) after 2 years of follow-up. Overall, grades I-II were observed in 1,363 cases (95.2%), grades III-IV were found in 58 cases (4.1%) and grade V was identified in ten cases (0.7%). The surgical success rate of MVD for HFS caused by the VA was statistically similar to the overall surgical success rates (Table 1).
Surgical complications
Permanent facial palsy was seen in three patients (1.3%) and hearing impairment was noted in five patients (2.2%). Low cranial nerve palsy occurred in 15 patients (6.5%) transiently, and two of these 15 patients (0.9%) had permanent palsies at the follow-up 2 years later. One patient experienced infection, another patient had hemorrhaging, and cerebral spinal fluid (CSF) leakage occurred in seven patients in the VA group. Overall, 16 patients (1.1%) experienced permanent facial palsy, and 31 patients (2.2%) had hearing impairment. Low cranial nerve palsy occurred in 24 patients (1.7%) transiently and was permanent in two of these 24 patients (0.1%). Infection was noted in 35 cases (2.5%), hemorrhage in 18 cases (1.3%), and CSF leakage in 13 cases (0.9%). The frequencies of transient lower cranial palsy and CSF leakage in patients with HFS associated with the VA were significantly different from the overall frequencies (p < 0.001 and 0.145, respectively). Overall, transient facial palsy was commonly observed (p = 0.0033). However, the occurrence of permanent complications in those with VA-associated HFS did not differ from the overall occurrence of permanent complications (Table 2).
Neurological outcomes and improvement with respect to compression severity
In Groups I and II, 94.2% and 96.6% of patients, respectively, had improved to grades I-II at the last follow-up. Grades III-V were observed at a rate of 5.8% in Group I and 3.4% in Group II. Intergroup comparisons of surgical outcomes revealed no statistically significant differences between Group I and II (p > 0.9999), Group I and III (p > 0.9999), and Group II and III (p = 0.7608). However, patients in Group III experienced a lower degree of post-surgical improvement compared with those in Groups I and II; 90.6% were grades I-II, 6.3% were grades III-IV, and 3.1% were grade V (Table 3, Fig. 4).
Neurological outcomes and improvement with respect to indentation and/or color change
Patients in group A experienced poor outcomes; 40% of the participants were categorized as grades I-II and 60% were grades III-V. A marked improvement was observed in 98.4% of Group B patients, and only three patients were classified as grades III-IV. Patients in Groups C and D had poorer outcomes than those in Group B (grade III-V: 15.8% vs 11.8%, respectively). Significant differences in clinical outcomes were noted between Groups A and B (p < 0.001). Although Groups B and C (p = 0.0636) and Groups B and D (p = 0.1458) revealed no statistically significant differences, Groups C and D patients in whom a color change was observed also experienced relatively poor outcomes (Table 4, Fig. 5).
Discussion
Pathophysiology
Despite many studies, the pathophysiology of HFS has still not been completely established. Previous studies of HFS have suggested that ectopic excitation and ephaptic transmission may be important factors underlying hyperexcitability [20]. The cranial nerves are most sensitive to pulsatile vascular contact and can undergo degenerative changes near REZ. The facial nerve is particularly vulnerable due to the lack of the epineurium and interfascular connective tissue [21]. The REZ is thought to be the region where there is a transition from central to peripheral axonal myelination within the most proximal few millimeters. For these reasons, MVD is known to be an effective method of therapy for HFS because it prevents the transmission of pulsatile signals regardless of their compressed pattern or strength. Many functional neurosurgeons have reported high success rates following MVD surgery, ranging from 79% to 97% [1, 8, 9, 17, 23, 24]. However, efforts have not been made to produce a more complete surgical outcome when the VA is the offender. Recently, studies have analyzed the patterns of neurovascular compression, and the areas of compression by offending vessels in HFS have also been reported. According to Park et al. [22], neurovascular compression patterns can be categorized into six different types and no significant differences among these compression patterns in terms of clinical outcomes have been reported. Campos-Benitez and Kaufmann [3] suggested that the common neurovascular compression theory should be expanded to include not only REZ compression but also severe pulsatile neurovascular compression on the peripherally myelinated portion of the facial nerve; these were not related to compression location, severity, or vessel type. However, we think that a treatment for HFS caused by large vessels (such as vertebral or basilar arteries) should be carefully considered. Nerve compression by the VA is frequently a result of atherosclerosis, making transposition of the VA difficult. In cases of VA-associated HFS, the REZ of the facial nerve is subjected to high pressure and it is likely that pulsatile signals may still remain after surgery. In addition, a tortuous perforating artery makes it difficult to completely transpose the parent artery. Therefore, many authors have suggested modified methods over conventional MVD [2, 12, 13, 16, 27, 28]. Nevertheless, the outcomes of surgical treatment for facial spasm caused by the VA remain unsatisfactory. Therefore, we sought to determine whether a higher compression pressure and the degree of facial nerve distortion influence clinical outcomes in cases of VA-associated HFS. The results of our study indicate that the compressive force measured as the degree of indentation did not influence the surgical outcomes statistically. However, a severe deviation of the natural course of the facial nerve might be minimally related with surgical outcomes, although these findings were not statistically significant. We hypothesized that deviations of nerve VII may produce ischemia of the nerve and gradually cause nerve degeneration. Hatem et al. [6] suggested that a delay in improvement after surgery strongly supports the hypothesis that HFS is not only due to the mechanical pulsations of the elongated artery against the REZ of the facial nerve but also due to demyelination of the nerve and/or hyperactivity of facial motor nuclei generated by neurovascular compression over extended periods of time. High pulsatile pressure or other causes induce severe degeneration of the nerve and do not allow complete resolution of spasm. Based on this theory, we explored color change as another indicator of nerve degeneration and analyzed the relationship between clinical outcomes and these changes identified in the operative field. In previous studies of peripheral nerves, researchers have found alterations in nerve excitability due to changes in membrane potential and ion channel function that resulted from nerve ischemia and hyperventilation [10, 18]. Wang [30] studied ten facial nerve root specimens obtained from patients with HFS using electron microscopy. The axons showed a particular pattern of degeneration, and the axonal mitochondria and smooth endoplasm showed irregular vacuolization. The myelin sheath showed irregular vacuolization, replication, convolution, and extensive segmental demyelination, and the Schwann cells were apparently in a state of degeneration. Sapmaz et al. [26] investigated functional and histopathological changes in the facial nerves due to the application of physical and electrical stimuli. Axonal degeneration, macrovacuolization, and vascular congestion in these specimens were assessed by light microscopy. A severe compression induced by large vessels that is sufficient to deviate the facial nerves can sometimes result in degeneration of the nerve itself or ischemia of a perforating artery. Takeda et al. [29] reported that in an animal model of ischemic facial palsy, the regeneration process did not always occur normally in cases of severe nerve damage, and a decrease in the number of fibers and irregularly-shaped fibers were noted in animals with incomplete recovery. Also, in terms of the technical aspect, the VA offenders are often atherosclerotic and perforating arteries make it difficult to transpose the parent artery. It could, therefore, be inferred that complete decompression of the offending vessels may not have been achieved with our procedures. The most likely cause of incomplete decompression of MVD associated with the VA is a perforating artery. MVD associated with the VA should be done very carefully because even the slightest damage to a perforating artery could create serious complications. When a perforating artery was present, we used a Teflon felt to lift the VA and then placed small Teflon pieces carefully and minimally between the facial nerve and the perforating artery. We then prepared a space between the Teflon pieces and the facial nerve. Subsequently, the facial nerve sheath was enveloped using surgical glue. However, it should be noted that an excess amount of surgical glue can cause vasospasm of the vessel and also occlusion of the surrounding small arteries or perforating artery. We performed this procedure under the assumption that surgical glue can protect the nerve from ischemia in addition to achieving complete decompression and fixation of the VA. Such neuroprotective management using surgical glue may be helpful during the intraoperative and perioperative periods. Based on our results, we concluded that the compression force as measured by the degree of indentation did not influence the surgical outcomes. Also, the existence of a color change in the facial nerve might be a risk factor for poor surgical outcomes. Interestingly, patients without indentation or color change in our study experienced poor outcomes. We think that this may be due to a wrong diagnosis or the possibility of another unknown mechanism. A limitation of our study was that we did not assess treatment outcomes for HFS according to all the different offending vessels. However, our study aimed to assess the hypothesis that severe pulsatile force and compression affect the facial nerve. Hence, we chose HFS patients associated the VA. Also, the minimal differences in treatment outcomes observed in this study may be due to the degree of transposition or characteristics of the perforating artery influencing the VA and difficulty in the surgical technique. Moreover, this study has limitations because it is a non-randomized, retrospective study. Furthermore, indentation and color change are subjective parameters and may vary depending on the operating surgeon. However, we think that our results provide a meaningful discussion because all surgeries were performed by only two surgeons.
Conclusions
Although compression severity revealed no statistically significant differences, a severe deviation of the facial nerve may affect the clinical outcomes. Group A without indentations or color changes showed poor outcomes postoperatively, while Group B with only indentations showed excellent outcomes (p < 0.001). Although Groups B and C (p = 0.0636) and Groups B and D (p = 0.1458) revealed no statistically significant differences, Group C and D patients in whom a color change was observed also experienced relatively poor outcomes. In the future, a greater number of cases and investigation of the pathophysiology of these findings will be required to further understand the outcomes of MVD.
References
Barker FG 2nd, Jannetta PJ, Bissonette DJ, Shields PT, Larkins MV, Jho HD (1995) Microvascular decompression for hemifacial spasm. J Neurosurg 82:201–210
Bejjani GK, Sekhar LN (1997) Repositioning of the vertebral artery as treatment for neurovascular compression syndromes. Technical note. J Neurosurg 86:728–732
Campos-Benitez M, Kaufmann AM (2008) Neurovascular compression findings in hemifacial spasm. J Neurosurg 109:416–420
Chang JW, Chang JH, Choi JY, Kim DI, Park YG, Chung SS (2002) Role of postoperative magnetic resonance imaging after microvascular decompression of the facial nerve for the treatment of hemifacial spasm. Neurosurgery 50:720–725, discussion 726
Gardner WJ (1962) Concerning the mechanism of trigeminal neuralgia and hemifacial spasm. J Neurosurg 19:947–958
Hatem J, Sindou M, Vial C (2001) Intraoperative monitoring of facial EMG responses during microvascular decompression for hemifacial spasm. Prognostic value for long-term outcome: a study in a 33-patient series. Br J Neurosurg 15:496–499
Hopf HC, Lowitzsch K (1982) Hemifacial spasm: location of the lesion by electrophysiological means. Muscle Nerve 5:S84–S88
Illingworth RD, Porter DG, Jakubowski J (1996) Hemifacial spasm: a prospective long-term follow up of 83 cases treated by microvascular decompression at two neurosurgical centres in the United Kingdom. J Neurol Neurosurg Psychiatry 60:72–77
Iwakuma T, Matsumoto A, Nakamura N (1982) Hemifacial spasm. Comparison of three different operative procedures in 110 patients. J Neurosurg 57:753–756
Kugelberg E (1948) Activation of human nerves by ischemia; Trousseau's phenomenon in tetany. Arch Neurol Psychiatry 60:140–164
Kureshi SA, Wilkins RH (1998) Posterior fossa reexploration for persistent or recurrent trigeminal neuralgia or hemifacial spasm: surgical findings and therapeutic implications. Neurosurgery 43:1111–1117
Kurokawa Y, Maeda Y, Toyooka T, Inaba K (2004) Microvascular decompression for hemifacial spasm caused by the vertebral artery: a simple and effective transposition method using surgical glue. Surg Neurol 61:398–403
Kyoshima K, Watanabe A, Toba Y, Nitta J, Muraoka S, Kobayashi S (1999) Anchoring method for hemifacial spasm associated with vertebral artery: technical note. Neurosurgery 45:1487–1491
Lovely TJ, Getch CC, Jannetta PJ (1998) Delayed facial weakness after microvascular decompression of cranial nerve VII. Surg Neurol 50:449–452
Martinelli P, Giuliani S, Ippoliti M (1992) Hemifacial spasm due to peripheral injury of facial nerve: a nuclear syndrome? Mov Disord 7:181–184
Miyazono M, Inoue T, Matsushima T (2003) A surgical case of hemifacial spasm caused by a tortuous, enlarged, and calcified vertebral artery. No Shinkei Geka 31:437–441
Moffat DA, Durvasula VS, Stevens King A, De R, Hardy DG (2005) Outcome following retrosigmoid microvascular decompression of the facial nerve for hemifacial spasm. J Laryngol Otol 119:779–783
Mogyoros I, Kiernan MC, Burke D, Bostock H (1997) Excitability changes in human sensory and motor axons during hyperventilation and ischaemia. Brain 120(Pt 2):317–325
Moller AR (1991) Interaction between the blink reflex and the abnormal muscle response in patients with hemifacial spasm: results of intraoperative recordings. J Neurol Sci 101:114–123
Nielsen VK (1984) Pathophysiology of hemifacial spasm: I. Ephaptic transmission and ectopic excitation. Neurology 34:418–426
Nielsen VK (1985) Electrophysiology of the facial nerve in hemifacial spasm: ectopic/ephaptic excitation. Muscle Nerve 8:545–555
Park JS, Kong DS, Lee JA, Park K (2008) Hemifacial spasm: neurovascular compressive patterns and surgical significance. Acta Neurochir (Wien) 150:235–241, discussion 241
Payner TD, Tew JM Jr (1996) Recurrence of hemifacial spasm after microvascular decompression. Neurosurgery 38:686–690, discussion 690–681
Samii M, Gunther T, Iaconetta G, Muehling M, Vorkapic P, Samii A (2002) Microvascular decompression to treat hemifacial spasm: long-term results for a consecutive series of 143 patients. Neurosurgery 50:712–718, discussion 718–719
Sanders DB (1989) Ephaptic transmission in hemifacial spasm: a single-fiber EMG study. Muscle Nerve 12:690–694
Sapmaz E, Kaygusuz I, Alpay HC, Akpolat N, Keles E, Karlidag T, Orhan I, Yalcin S (2010) Histopathologic and functional effects of facial nerve following electrical stimulation. Eur Arch Otorhinolaryngol 267:607–612
Shigeno T, Kumai J, Endo M, Oya S, Hotta S (2002) Snare technique of vascular transposition for microvascular decompression—technical note. Neurol Med Chir (Tokyo) 42:184–189, discussion 190
Suzuki S, Tsuchita H, Kurokawa Y, Kitami K, Sohma T, Takeda T (1990) New method of MVD using a vascular tape for neurovascular compression involving the vertebrobasilar artery—report of two cases. Neurol Med Chir (Tokyo) 30:1020–1023
Takeda S, Takeda T, Okada T, Nakatani H, Hamada M, Kakigi A (2008) An animal model of ischemic facial palsy. Behavioral facial nerve function and ultrastructural changes of the facial nerve. ORL J Otorhinolaryngol Relat Spec 70:215–223
Wang H (1991) Ultrastructural changes in the facial nerve root in patients with hemifacial spasm. Zhonghua Er Bi Yan Hou Ke Za Zhi 26(262–264):316
Yamaki T, Hashi K, Niwa J, Tanabe S, Nakagawa T, Nakamura T, Uede T, Tsuruno T (1992) Results of reoperation for failed microvascular decompression. Acta Neurochir (Wien) 115:1–7
Acknowledgements
We thank Eun Jung Kweon, a research nurse at Severance Hospital, for helping this study. We are also grateful to Dong-Su Jang, a medical illustrator at the Medical Research Support Section, Yonsei University College of Medicine, for his help with the figures.
This study was supported by the Industrial Source Technology Development Program (no.10033812) of the Ministry of Knowledge Economy (MKE) of Korea.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, J.P., Chung, J.C., Chang, W.S. et al. Outcomes of surgical treatment for hemifacial spasm associated with the vertebral artery: severity of compression, indentation, and color change. Acta Neurochir 154, 501–508 (2012). https://doi.org/10.1007/s00701-011-1247-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-011-1247-3