Abstract
Background
Microsurgical, circumferential stripping of intracerebral metastases often proves to be insufficient to prevent local tumor recurrence.
Objective
We were interested in the potential impact of 5-aminolevulinic acid (5-ALA)-induced-fluorescence (5-AIF) as a diagnostic tool for the resection of intracerebral metastases.
Methods
A retrospective analysis was performed for 52 patients who underwent 5-AIF-guided resection for intracerebral mass lesions that histologically corresponded to metastases from tumors outside the central nervous system. The presence of ALA fluorescence in the tumor was determined in each patient. In 42 patients, fluorescence of the resection cavity after tumor removal was additionally recorded. Data were correlated with neuropathological findings in tissue specimens.
Results
A total of 32 of the 52 metastases (62%) exhibited 5-AIF in tumor parts. All 5-AIF-positive metastases exhibited an inhomogeneous fluorescence pattern. 5-AIF was neither associated with the histological type nor with the site of origin of the metastases. Residual fluorescence of the resection cavity was detected after macroscopically complete white light resection in 24 patients with 5-AIF positive metastases. Residual tumor tissue was histologically confirmed in 6 of 18 patients with available tissue specimens from such 5-AIF positive areas (33%).
Conclusions
The majority of metastases (62%) were 5-AIF positive, suggesting a potential impact of 5-AIF for improved visualization of metastatic tumor tissue within the brain. However, residual 5-AIF after macroscopically complete resection of a metastasis needs to be interpreted with caution because of the limited specificity for detection of residual tumor tissue.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intracerebral metastases are the most common intracranial neoplasms in adults, comprising more than half of all brain tumors [5]. Microsurgical resection by circumferential stripping from the surrounding brain tissue, though an established treatment concept, is often insufficient to achieve local tumor control [10]. For malignant gliomas, the introduction of 5-aminolevulinic acid (5-ALA)-derived fluorescence-guided resection has significantly improved local tumor control, as demonstrated by more complete resections and an improved 6-month progression-free survival as compared to conventional white light resection [16–18]. 5-ALA-induced fluorescence (5-AIF) is seen not only in malignant gliomas, but also in various tumors outside the central nervous system, such as lung, prostate, colorectal and bladder carcinomas [1, 7–9, 12, 13, 22]. Various 5-ALA fluorescence-based treatment concepts have been established for these malignancies. As yet, there is no systematic approach to determine the impact of 5-ALA-induced fluorescence for the intraoperative visualisation of intracerebral metastases. Here, we report a retrospective analysis of 5-AIF patterns in intracerebral metastases in order to evaluate the role of this method as a potential adjuvant tool for neurosurgical resection of these tumors.
Material and methods
Between October 2008 and November 2010, a total number of 471 patients with intracerebral lesions preoperatively considered as being suspicuous for high-grade gliomas were treated at our institution by open resection using 5-AIF. Retrospective analysis of this patient cohort revealed 52 patients with intracerebral metastases from primary tumors outside the central nervous system. In 21 of the 52 patients, the respective primary tumors were unknown at the time of neurosurgical intervention and only later discovered upon postoperative clinical staging. The remaining 31 patients had been diagnosed with cancer outside the nervous system before neurosurgery. However, malignant glioma/glioblastoma was raised as a differential diagnosis on preoperative neuroimaging; hence, these patients were subjected to 5-ALA-based resection. None of the patients demonstrated any adverse effects related to the administration of 5-ALA. Retrospective analysis of the patient data was approved by the local ethics committee of the Medical Faculty, Heinrich Heine University (study no. 3307).
Clinical management
In 50 of the 52 patients, 5-ALA was administered 3 h prior to surgery in a dose of 20 mg per kilogram body weight as described elsewhere [18]. Two patients received 5-ALA 24 h before the operation. Intraoperative frozen sections were obtained in all patients. After the histological diagnosis of an intracerebral metastasis had been established based on intraoperative frozen sections, standard circumferential resection was performed. Following complete white light resection, the resection cavity was checked for residual 5-AIF in 42 patients. In 24 patients, areas of residual 5-AIF were noted, and in 18 patients, the neurosurgeon had decided that it was neurosurgically feasible to resect or biopsy these 5-ALA-positive tissue areas.
Histopathological analyses
All tumors were subjected to standard histological classification based on formalin-fixed and paraffin-embedded tissue sections. Routine histological stains comprised hematoxylin-eosin, alcian blue and PAS stains. In addition, immunohistochemical stainings were performed for epithelial markers, including the cytokeratin subtypes 5, 7, 8 and 20, thyroid transcription factor 1 (TTF1), caudal-type homeobox transcription factor 2 (CDX2), estrogen receptors and progesteron receptors, as well as melanocytic markers, including Melan A and HMB-45. Immunohistochemical stainings were performed using the Envision system (Dako, Kopenhagen, Denmark) and 3’3-diaminobenzidine as peroxidase substrate [6]. All available tissue specimens were retrospectively re-assessed with particular attention to each tumor’s delimitation and locally invasive growth pattern. For each tumor, neuropathological findings were correlated with the respective fluorescence behavior as intraoperatively determined by the neurosurgeon.
Statistical analysis
Descriptive statistics were performed using the IBM SPSS version 19. P values of less than 0.05 were considered significant (chi2 test). Adjustment for multiple testing was not performed.
Results
Patients
A retrospective analysis of patients with solitary brain lesions who were operated on at our institution over a period of 26 months between October 2008 and November 2010 revealed 52 patients with singular intracerebral metastases who had been subjected to 5-ALA-guided resection. Clinical data of the 52 patients are summarized in Table 1.
Figure 1 provides an overview of the fractions of 5-AIF-positive tumors according to histological tumor type (a) and site of corresponding primary tumor (b). Poorly differentiated adenocarcinoma metastases were diagnosed in 33 patients, tubulo-papillary or papillary adenocarcinoma metastases in 7 patients, small cell neuroendocrine carcinoma metastases in 4 patients, squamous cell carcinoma metastases in 3 patients, clear cell carcinoma metastases in 2 patients, malignant melanoma metastases in 2 patients, and poorly differentiated transitional cell carcinoma in 1 patient. The respective primary tumor sites were identified by clinical staging. Primary tumors were non-small bronchial cancer in 27 patients, breast carcinomas in 8 patients, renal cell carcinomas in 4 patients, small cell bronchial carcinomas or colorectal carcinomas in 3 patients each, cutaneous malignant melanomas in 2 patients and endometrial, esophageal, ovarian, stomach and urinary bladder cancer in 1 patient each.
The majority of cerebral metastases were positive for 5-ALA-induced fluorescence
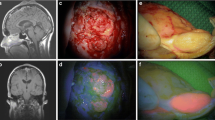
Intraoperative 5-AIF was detected in the majority of patients (Fig. 2). In total, 32 metastases (61.5%) were 5-AIF positive, while 20 metastases (38.5%) were 5-AIF negative. All 5-ALA-positive intracerebral metastases exhibited an inhomogeneous fluorescence pattern with strongly fluorescent parts as well as 5-ALA-negative areas (Fig. 2).
Example of an ALA-positive intracerebral metastasis. ALA-positive metastasis (a white light, b fluorescence light). Normal appearing (c) but strongly fluorescent adjacent brain tissue (d), with expansion of a carcinoma metastasis into the brain tissue upon histological examination. Note the inhomogeneous fluorescence pattern of the cerebral metastasis with small strongly fluorescent parts and 5-ALA-negative areas
Histological entity and primary tumor site were not correlated with 5-ALA fluorescence
Presence of 5-AIF was neither associated with the histopathological type of metastasis nor with the primary tumor site (p = 0.131; p = 0.507 respectively).
Intracerebral metastases may locally extend into the adjacent brain tissue
Areas from the tumor-brain interface were histologically detectable in the resection specimens of 43 patients. A rather sharp border between the metastatic tumor tissue and the adjacent edematous and gliotic brain tissue was seen in 17 patients (39.5%). Ten of these 17 tumors reacted 5-AIF positive (58.8%). Twenty-six of the 43 tumors demonstrated more irregular tumor-brain interfaces with tongue-like extensions of tumor tissue and small perivascular islets of tumor cells in the adjacent brain parenchyma (Fig. 3a). In two instances of intracerebellar metastases from poorly differentiated bronchial adenocarcinomas, histology showed quite pronounced perivascular invasion of tumor cells into the neighboring cerebellar cortex (Fig. 3b). 5-AIF was detected in 18 of the 26 tumors (69.2%) with such irregular tumor-brain interfaces.
Examples of poorly delimitated brain metastases. Examples of irregular tumor-brain interfaces in intracerebral metastases. a Tongue-like tumor extension and small nests of tumor cells in the adjacent brain parenchyma. b Invasive growth of metastatic tumor cells along vessels and disseminated small groups of tumor cells within cerebellar tissue
5-ALA-induced fluorescence and the identification of residual metastatic tissue
The resection cavity after macroscopically complete white light resection was assessed for residual 5-ALA fluorescence in 42 patients. In 24 patients (57.2%) residual fluorescent areas were noted in the resection cavities (Fig. 2c and d). In 18 patients, tissue specimens were taken from such residual fluorescent areas. Histological workup of these samples revealed the presence of residual metastatic tumor tissue in six patients (33.3%, Fig. 2c and d). In two patients who had received 5-ALA already 24 h before surgery, the actual metastases demonstrated only little 5-AIF, while the resection cavity was strongly positive for 5-AIF (Fig. 4). Histological assessment of small tissue biopsies taken from the fluorescent cavity walls did not reveal any residual tumor tissue in both patients.
Metastasis with inhomogeneous fluorescence pattern but strongly fluorescent tumor bed after 24 h. Circumferentially stripped metastasis with inhomogeneous areas of 5-ALA-induced fluorescence (a, b). The tumor bed demonstrates strong 5-ALA-induced fluorescence (c). Biopsy taken from this area (d) was negative. Note that the patient had received 5-ALA 24 h before surgery
Discussion
In this retrospective analysis we report on 5-AIF patterns in 52 patients operated on for intracerebral metastases. Though 5-AIF has been employed for diagnosis and treatment of various tumors outside the central nervous system, such as lung, prostate, colorectal or bladder carcinomas [1, 7–9, 12, 13, 22], there are only a few reports on 5-ALA fluorescence in intracerebral metastases.
Utsuki et al. reported that 9 of 11 (82%) metastatic brain tumors demonstrated 5-AIF [20]. This corresponds to our finding that the majority of intracerebral metastases (61.5%) showed 5-AIF. Our data indicate that 5-AIF is neither associated with the histological type nor with the origin of intracerebral metastases. In addition, the 5-AIF signals in positive metastases were heterogeneously distributed within the tumor tissue.
Both findings indicate that 5-AIF in cerebral metastases is more hetereogenous when compared to 5-AIF in malignant gliomas. It is assumed that 5-AIF is caused by an abnormal accumulation of the fluorescent molecule protoporphyrin IX (PPIX) in the mitochondria. It has been demonstrated that downregulation of the ferrochelatase (FECH) gene, which encodes a key enzyme that catalyzes the conversion of protoporphyrin IX (PpIX) to heme, might be responsible for PpIX accumulation in glioblastoma cells [19]. Kemmner et al. reported a significant downregulation of ferrochelatase mRNA expression in gastric and colorectal carcinomas [11]. Accordingly, in an in vitro model of several carcinoma cell lines, ferrochelatase downregulation and loss of enzymatic activity corresponded to enhanced PpIX-dependent fluorescence. The authors concluded that the FECH gene is transcriptionally downregulated in malignant tumors, which in turn causes endogenous PpIX accumulation [11]. The heterogeneous pattern of 5-AIF might therefore be explained by heterogeneous downregulation of FECH in different patients and even within an individual metastasis. Interestingly, Liu et al. demonstrated that FECH gene expression is upregulated during hypoxia by a mechanism involving hypoxia-inducible factor 1 (HIF-1) [14]. The heterogeneous pattern of oxygenation is a key feature of solid malignant tumors [3, 4], and could thus explain the regionally variable expression of ferrochelatase and hence 5-AIF within the tumor tissue.
Our observation that the majority of intracerebral metastases demonstrate 5-AIF suggests 5-ALA-based resection to be a useful tool to detect residual metastatic tumor tissue. This is of special interest because the assumption that cerebral metastases can be completely removed by circumferential stripping may not apply to each metastasis. However, the majority of intracerebral metastases in our series histologically demonstrated areas with tongue-like expansion of tumor tissue into the adjacent brain parenchyma along vessels and/or the presence of small groups/islets of perivascularly invading tumor cells. Our results are in line with previous studies. For example, based on autopsy findings Baumert et al. reported on an invasive growth of cerebral metastases in more than 50% of the investigated cases [2]. Another study reported on a frequent pseudogliomatous infiltrative growth pattern of intracerebral metastatic anaplastic small cell carcinomas [15].
Albeit the extent of brain infiltration of metastatic lesions is far less pronounced as compared to the majority of primary brain tumors, in particular diffuse gliomas, our findings and those of other investigators [2, 21] suggest that a fraction of intracerebral metastases locally invades into the adjacent brain tissue. Therefore, the surrounding brain tissue needs to be considered as a therapeutic target. Accordingly, Yoo and co-workers achieved significantly better local tumor control by extending the resection of intracerebral metastases (in non-eloquent areas) to a depth of about 5 mm [21].
However, the sensitivity of 5-AIF for the identification of remnants of metastatic tumor tissue in the resection cavity after macroscopically complete resection appears to be limited. Though we detected residual fluorescent tissue at the brain-tumor interface, the majority of biopsies taken from fluorescent areas of the tumor cavity after macroscopically complete resection lacked tumor tissue on histology. In line with our experiences, Utsuki et al. also observed diffuse PPIX fluorescence in samples from tumor-free edematous zones surrounding intracerebral metastases [20]. The authors contributed this observation to a diffuse leakage of PPIX in areas of edema. This observation is supported by findings in two of our patients who had received 5-ALA already 24 h before surgery. Despite strong 5-AIF of the resection cavity in these patients, histological assessment of corresponding tissue biopsies did not demonstrate metastatic tumor tissue.
Conclusion
Our study demonstrates that the majority of cerebral metastases are 5-AIF positive. This observation suggests a potential usefulness of 5-ALA for better visualization of the surgical target, better definition of the margins of resection and new therapeutic options, such as photodynamic therapy. However, in contrast to malignant gliomas, the use of fluorescence-guided resection for cerebral metastases seems to be impeded by the regionally heterogeneous and therefore probably unpredictable patterns of 5-ALA-induced fluorescence within the actual tumor tissue. Moreover, a reliable identification of infiltrating tumor cells appears to be problematic, possibly because of an unspecific leakage of PPIX and hence 5-AIF being detectable in the peritumorous edematous brain tissue in a considerable fraction of patients. Further studies are needed to precisely define the role of fluorescence-guided resection in patients with intracerebral metastases.
References
Baas P, Triesscheijn M, Burgers S, van Pel R, Stewart F, Aalders M (2006) Fluorescence detection of pleural malignancies using 5-aminolaevulinic acid. Chest 129(3):718–724
Baumert BG, Rutten I, Dehing-Oberije C, Twijnstra A, Dirx MJ, Debougnoux-Huppertz RM, Lambin P, Kubat B (2006) A pathology-based substrate for target definition in radiosurgery of brain metastases. Int J Radiat Oncol Biol Phys 66(1):187–194
Brahimi-Horn MC, Chiche J, Pouyssegur J (2007) Hypoxia and cancer. J Mol Med 85(12):1301–1307
Brahimi-Horn MC, Chiche J, Pouyssegur J (2007) Hypoxia signalling controls metabolic demand. Curr Opin Cell Biol 19(2):223–229
DeAngelis LM (2001) Brain tumors. N Engl J Med 344(2):114–123
Felsberg J, Thon N, Eigenbrod S, Hentschel B, Sabel MC, Westphal M, Schackert G, Wilhelm, Kreth F, Pietsch T, Loeffler M, Weller M, Reifenberger G and Tonn JC (2011) Promoter methylation and expression of MGMT and the DNA mismatch repair genes MLH1, MSH2, MSH6, and PMS2 in paired primary and recurrent glioblastomas. Int J Cancer
Gamarra F, Lingk P, Marmarova A, Edelmann M, Hautmann H, Stepp H, Baumgartner R, Huber RM (2004) 5-Aminolevulinic acid-induced fluorescence in bronchial tumours: dependency on the patterns of tumour invasion. J Photochem Photobiol B 73(1–2):35–42
Grimbergen MC, van Swol CF, van Moorselaar RJ, Uff J, Mahadevan-Jansen A, Stone N (2009) Raman spectroscopy of bladder tissue in the presence of 5-aminolevulinic acid. J Photochem Photobiol B 95(3):170–176
Huber RM, Gamarra F, Hautmann H, Häußinger R, Wagner S, Castro M, Baumgartner R (1999) 5-Aminolaevulinic Acid (ALA) for the fluorescence detection of bronchial tumors. Diagn Ther Endosc 5:113–118
Jenkinson MD, Haylock B, Shenoy A, Husband D, Javadpour M (2011) Management of cerebral metastasis: Evidence-based approach for surgery, stereotactic radiosurgery and radiotherapy. Eur J Cancer 47(5):649–655
Kemmner W, Wan K, Ruttinger S, Ebert B, Macdonald R, Klamm U, Moesta KT (2008) Silencing of human ferrochelatase causes abundant protoporphyrin-IX accumulation in colon cancer. FASEB J 22(2):500–509
Krammer B, Plaetzer K (2008) ALA and its clinical impact, from bench to bedside. Photochem Photobiol Sci 7(3):283–289
Leonhard M (1999) New incoherent autofluorescence/fluorescence system for early detection of lung cancer. Diagn Ther Endosc 5:71–75
Liu YL, Ang SO, Weigent DA, Prchal JT, Bloomer JR (2004) Regulation of ferrochelatase gene expression by hypoxia. Life Sci 75(17):2035–2043
Neves S, Mazal PR, Wanschitz J, Rudnay AC, Drlicek M, Czech T, Wustinger C, Budka H (2001) Pseudogliomatous growth pattern of anaplastic small cell carcinomas metastatic to the brain. Clin Neuropathol 20(1):38–42
Pichlmeier U, Bink A, Schackert G, Stummer W (2008) Resection and survival in glioblastoma multiforme: an RTOG recursive partitioning analysis of ALA study patients. Neuro Oncol 10(6):1025–1034
Stummer W, Hassan A, Kempski O, Goetz C (1996) Photodynamic therapy within edematous brain tissue: considerations on sensitizer dose and time point of laser irradiation. J Photochem Photobiol B 36(2):179–181
Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ (2006) Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 7(5):392–401
Teng L, Nakada M, Zhao SG, Endo Y, Furuyama N, Nambu E, Pyko IV, Hayashi Y, Hamada JI (2011) Silencing of ferrochelatase enhances 5-aminolevulinic acid-based fluorescence and photodynamic therapy efficacy. Br J Cancer 104(5):798–807
Utsuki S, Miyoshi N, Oka H, Miyajima Y, Shimizu S, Suzuki S, Fujii K (2007) Fluorescence-guided resection of metastatic brain tumors using a 5-aminolevulinic acid-induced protoporphyrin IX: pathological study. Brain Tumor Pathol 24(2):53–55
Yoo H, Kim YZ, Nam BH, Shin SH, Yang HS, Lee JS, Zo JI, Lee SH (2009) Reduced local recurrence of a single brain metastasis through microscopic total resection. J Neurosurg 110(4):730–736
Zaak D, Sroka R, Khoder W, Adam C, Tritschler S, Karl A, Reich O, Knuechel R, Baumgartner R, Tilki D, Popken G, Hofstetter A, Stief CG (2008) Photodynamic diagnosis of prostate cancer using 5-aminolevulinic acid–first clinical experiences. Urology 72(2):345–348
Conflicts of interest
All authors report no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Comment
5-Aminolevulinic acid (5-ALA)-induced fluorescence in intracerebral metastases: a retrospective study
At the authors’ institute, a total of 471 patients underwent open 5-ALA-guided resection for intracerebral lesions preoperatively considered as high-grade gliomas. It turned out that 52 lesions were actually intracerebral metastases, an unexpected research window. Of the 52 metastases, 32 (62%) exhibited 5-ALA-fluorescence, not associated with the histological type or site of origin of the metastases. Furthermore, in 24 (75%) of the 32 fluorescent metastases, there was recidual fluorescence in the cavity wall after seemingly complete removal under white light, confirmed histologically in 6 of the 18 patients with tissue specimens from fluorescent areas in the cavity wall.
This is an important pilot study, awaiting verification by others. Most importantly, these data support the view that many metastases infiltrate the edematous/gliotic brain around them and therefore require more drastic measures than microsurgical removal in white light only.
Juha E Jääskeläinen
Kuopio, Finland
Rights and permissions
About this article
Cite this article
Kamp, M.A., Grosser, P., Felsberg, J. et al. 5-Aminolevulinic acid (5-ALA)-induced fluorescence in intracerebral metastases: a retrospective study. Acta Neurochir 154, 223–228 (2012). https://doi.org/10.1007/s00701-011-1200-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-011-1200-5