Abstract
Objective
Delayed ischemic brain tissue damage in the time course of cerebral vasospasm in the rat double-subarachnoid hemorrhage (SAH) model has been described before. However, in order to enhance hemodynamic insufficiency during cerebral vasospasm (CVS), we performed—in a modification to the standard double-hemorrhage model—an additional unilateral common carotid artery occlusion (CCAO), expecting aggravation of brain-tissue damage in areas particularly sensitive to hypoxia.
Methods
CVS was induced by injection of 0.25 ml autologous blood twice in the cisterna magna of Sprague-Dawley rats with and without unilateral CCAO. The animals were examined on days 2, 3, 4 and 5, and compared with the sham-operated control group without SAH. The functional deficits were graded between 0 and 3. Perfusion weighted imaging (PWI) at 3 Tesla magnetic resonance (MR) tomography was performed to assess cerebral blood flow (CBF). The brains were fixed, stained and evaluated for histological changes.
Results
On day 5, the neurological state was significantly worse in rats with SAH. The relative CBF/muscle blood ratio was significantly decreased by SAH and lowest in rats with CCAO and SAH (4.5 ± 1.1 vs 2.7 ± 0.6) compared with sham (7.9 ± 1.5; p < 0.001). Basilar artery (BA) diameter was 79 ± 5 μm (SAH) vs 147 ± 4 μm (sham, p < 0.001). Neuronal cell count in the hippocampal areas CA1-CA4 was significantly reduced by SAH on day 5 (p < 0.001) and lowest in rats with SAH and CCAO.
Conclusions
CCAO leads to an aggravation of CVS-related delayed brain tissue damage in the modified rat double-SAH model.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Delayed cerebral vasospasm (CVS) after aneurysmal subarachnoid hemorrhage (SAH) remains to be a substantial reason for poor clinical outcome [8, 14, 33]. Particularly, because the prevention of delayed ischemic deficits after SAH seems to be more capable for potential treatment or prophylaxis than the initial insult, animal models simulating delayed CVS in humans like the rat double-SAH model are necessary for further improvement of the knowledge about pathophysiological mechanisms and for the development of tailored treatment strategies [41].
We described delayed brain tissue damage in the rat double-SAH model before [12]. Brain tissue damage in the phase of CVS was present in the hippocampus and the inner layer of the cerebral cortex without development of territorial infarctions. Pronounced cortical collateralization, bypassing hypoperfusion during pathological conditions, has been described before and held responsible for the avoidance of territorial infarctions [3].
Unilateral common carotid artery occlusion (CCAO) in the rat results in a moderate reduction of cerebral blood flow (CBF) in both cerebral hemispheres without asymmetrical perfusion [6]. However, the reserve capacity of the circulatory system is limited. If the adaptive mechanisms become insufficient, e.g., by CVS, brain tissue may—depending on the severity of hypoperfusion—become hypoxic. In order to enhance hemodynamic insufficiency during CVS, we performed—in a modification to the standard double hemorrhage model—an additional unilateral CCAO at the left side expecting aggravation of brain tissue damage in areas particularly sensitive to hypoxia.
According to recent investigations like the CONSCIOUS-1 trial,the causality between the delayed spasm of proximal cerebral arteries after SAH and the development of delayed ischemic deficits remains to be proven [24]. Accordingly, there is a need for animal models mimicking not only angiographic vasospasm but also neurological deterioration and ischemic brain tissue damage.
The aim of the present investigation was, therefore, to correlate the ischemic brain tissue damage with histological CVS and neurological deterioration in an SAH model with enhanced hemodynamic insufficiency by CCAO. Furthermore, an exact histological characterization of ischemic brain tissue damage after SAH with and without CCAO should be established, for utilization as reference data for the analysis and adjustment of neuroprotective strategies in further studies. Therefore, we analyzed the time course of brain tissue damage in a modified rat double-SAH model using the neuronal cell count method, which was introduced by Soehle et al. [36] and utilized in a former study for the characterization of brain tissue damage in the standard double-SAH model [12]. The neuronal cell count was determined in the cerebral cortex and hippocampus, which are areas differently sensitive to cerebral ischemia.
Materials and methods
Animal preparation
Experimental groups are summarized in Table 1. The present investigations were performed with male Sprague-Dawley rats (n = 70) weighing 280–360 g. Experimental CVS was induced using a modified rat double-hemorrhage model [37] as described in detail previously [12, 41]. Briefly, the animals were anesthetized on day 1 of the experiment by intraperitoneal application of midazolam (1 mg/kg body weight) and ketamine (100 mg/kg body weight). This anesthesia is commonly used in rat SAH models [5, 35, 41], its feasibility is proven for imaging procedures in rats [7] and significant changes of circulation parameters and of CBF due to this medication were not observed [20, 34]. A tube (Portex polythene tube, luminal diameter 0.96 mm) was inserted in the femoral artery for the measurement of blood gas values, for the control of blood pressure, and for blood sample withdrawal. Another tube of the same size was inserted in the femoral vein to administer additional medications or infusions (crystalloids). Body temperature was maintained at 37°C using a heading pad. In rats assigned for CCAO, the left common carotid artery was exposed through a ventral incision in the neck, separated from the vagosympathetic trunks, and ligated. Thereafter, the animals were positioned in a stereotactic frame and the atlanto-occipital membrane was exposed through a small midline approach. After insertion of a catheter (Portex polythene tube, luminal diameter 0.28 mm) in the cisterna magna, 0.1 ml cerebrospinal fluid (CSF) was withdrawn followed by the injection of 0.25 ml autologous blood to induce the first SAH. The wounds were surgically closed. The animals received 5 ml crystalloid solution with 0.0125 mg fentanyl subcutaneously and were kept in a head down position for 15 min. An identical procedure was performed on day 2 to induce the second SAH. In sham operated rats, 0.25 ml physiological saline was inserted instead of blood. After second SAH, the animals were observed clinically and received 5 ml crystalloid solution with 0.0125 mg fentanyl twice a day.
Neurological scoring
All animals underwent neurological examination according to the grading system by Bederson et al. [3] on day 2 after induction of a second SAH as well as on days 3, 4, and 5 thereafter. In brief, rats were gently held by the tail above the floor. Rats without neurological deficit received the grade 0. Rats with flexion of one forelimb without further deficits were grade 1 (mild dysfunction). After placing the rats on a sheet of plastic coated paper, animals were tested for their resistance to lateral push to one side. Rats with consistently reduced resistance were grade 2 (moderate dysfunction). Rats were then allowed to move freely and observed for circling behavior. Rats with consistent circling to one side or with a reduction of vigilance without any movement were deemed grade 3 (severe dysfunction).
Magnetic resonance imaging and cerebral blood flow
The magnetic resonance imaging (MRI) measurements were performed at 3.0 T (Magnetom Trio, Siemens, Germany) with a wrist coil, as described in detail previously [41]. The standardized imaging protocol included axial and sagittal T2-weighted images (WI), and perfusion weighted images (PWI). MRI measurements were performed on day 3 and on day 5—the day of the maximum CVS [41]. In rats with CCAO, the masseter muscles of the contralateral side to the occluded common carotid artery were used for relative regional cerebral blood volume (rrCBV) and rrCBF measurement.
Histology
Histological analysis was performed, as prospectively assigned, on day 3 and day 5 after the surgical procedure to evaluate the time course of histological changes after artificial SAH. The number of animals was estimated according to the mortality rates of the double-SAH model in rats described by Vatter et al. [41] (Table 1). Animals were killed in deep anesthesia and perfused with ice-cold heparinized saline from the left ventricle for 10 min. The brains were removed and postfixed in ice-cold phosphate-buffered 4% paraformaldehyde (PFA) for 24 h, and embedded in paraffin to obtain coronal sections of the parietal region as described previously [12]. Sections, 20 μm thick, were cut and stained with hematoxylin-eosin (H & E). Histological evaluation was performed by light microscopy and photographed using a CCD camera (Sony, Tokyo, Japan). Image software was calibrated with a microscope ruler (Ernst Leitz, Wetzlar, Germany). For quantitative assessment of brain tissue damage, vital cells were counted by two independent observers who were blinded to the previous treatment conditions, in seven nonoverlapping regions of interest (ROIs) in the hippocampus (CA1, CA2, CA3, and CA4) and the adjoining cortex (Cortex1 to Cortex3 without relation to cortex layers) as described elsewhere [12, 36]. The investigated hippocampal areas were 0.02 mm2 and the cortical areas were 0.04 mm2 each (CA1–CA4 and Cortex1–Cortex3). Neurons were classified as non-vital when they exhibited pyknosis, karyorrhexis, karyolysis, cytoplasmic eosinophilia, or loss of affinity for hematoxylin [22]. Unilateral CCAO is known to result in a moderate symmetrical reduction of CBF in both cerebral hemispheres [6]. Accordingly, neuronal cell count between both hemispheres did not differ in the current study. Therefore, neuronal cell count is expressed as average values. For the measurement of the basilar artery diameter (BA), cross-sections of the brainstem were performed on days 3 and 5 and stained for H & E. Computer-assisted morphometry was calculated by using three adjusted straight lines inside the basilar lumen. Of each animal, three sections from different levels of the BA were analyzed (proximal, median, distal).
Data analysis
Data are expressed as mean ± standard deviation (SD). Statistical significance was verified by analysis of variance performed in one-way ANOVA followed by Tukey test for multiple comparisons. Significance of differences between the groups regarding neurologic scores was analyzed by Kruskal-Wallis one-way ANOVA followed by the t-test procedure. A probability value of p < 0.05 was considered statistically significant.
Results
Physiological values of arterial blood pressure, partial pressure of oxygen and carbon dioxide, blood pH, and hemoglobin did not differ among the experimental groups. Experimental groups and mortality are summarized in Table 1.
Mortality
Rats without SAH (groups 1 and 2) survived until being killed on day 3 and day 5. In group 3, three of ten SAH rats (30%) died after second SAH until day 3, and six of 12 rats (50%) until day 5. In group 4, four of ten SAH rats (40%) died after the second SAH until day 3, and seven of 14 SAH rats (50%) until day 5.
Parameters of cerebral vasospasm are summarized in Table 2.
Neurological and neuroradiological evaluation
In the SAH groups 3 and 4, the clinical state after second SAH was worst on day 5 (median: 2.5 and 3), with a significant worsening compared with day 2 (median: 1.5; p < 0.01).
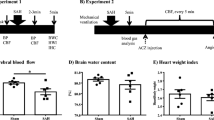
In group 1, rrCBF was 8.1 ± 1.3-fold higher compared with the perfusion of the masseter muscle on day 3. Furthermore, rrCBV was 7.6 ± 1.2-fold higher compared with the perfusion of the masseter muscle. In rats after SAH (group 3), these values were reduced to 56% (CBF) and 63% (CBV) on day 5. In group 4, rrCBF was reduced in both hemispheres symmetrically to 70% on day 3 and to 35% on day 5 (Fig. 1).
MRI axial slices of a sham-operated rat (a, b) and SAH rats without (c) and with CCAO (c) suffering from cerebral vasospasm on day 5. T2-weighted imaging for the demonstration of the anatomic site for rrCBF measurement (a). Blood perfusion (rrCBF) of the central nervous system and the masticatory muscles are plotted on color-coded PWI parameter images indicating reduced rrCBF in the animals suffering from CVS (c, d)
Basilar artery
Basilar artery (BA) diameter on day 3 was 152 ± 10 μm in sham operated rats (group 1) and discrete but significantly reduced to 139 ± 9 μm in group 3 (p = 0.01). On day 5, BA diameter was 147 ± 4 μm in sham operated rats and significantly reduced to 79 ± 5 μm in the SAH group 3 (p < 0.0001).
BA diameter was larger in group 2 compared with group 1 on day 3 (167 ± 11 μm vs 152 ± 10 μm; p = 0.03) and on day 5 (168 ± 8 μm vs 147 ± 4; p = 0.0002) respectively. BA diameter was markedly reduced in group 4 on day 5 compared with day 3 (p < 0.0001).
Morphological changes
Neuronal cell counts obtained from the defined ROIs in the hippocampal areas and the cerebral cortex are shown in Figs. 2 and 3 and in Tables 3 and 4.
Quantitative data on neuronal cell count in the rat hippocampal layers (CA1–CA4) of sham operated and SAH rats with and without CCAO on day 3 (left) and day 5 (right). Neuronal cell count is preserved in all hippocampal layers after SAH with and without CCAO on day 3 and significantly decreased on day 5 (*). Group 1: without SAH, without CCAO; group 2: without SAH, with CCAO; group 3: with SAH, without CCAO; group 4: with SAH, with CCAO
Quantitative data on neuronal cell count in the rat cerebral cortex (Cortex1–Cortex3) of sham-operated and SAH rats with and without CCAO on day 3 (left) and day 5 (right). Neuronal cell count is preserved in all cortical layers after SAH with and without CCAO on day 3 and significantly decreased in the inner cortical layer (Cortex3) on day 5 (*). Group 1: without SAH, without CCAO; group 2: without SAH, with CCAO; group 3: with SAH, without CCAO; group 4: with SAH, with CCAO
On day 3, the neuronal cell count in the hippocampal areas CA1-CA4 and Cortex1-Cortex3 was not significantly different between the groups 1–4.
On day 5, the neuronal cell count in the hippocampal areas CA1-CA4 was significantly reduced by SAH (p < 0.001). CCAO without SAH (group 3) was not associated with reduced neuronal cell count on day 5. CCAO with SAH (group 4) was associated with a marked reduction of neuronal cell count in the hippocampal areas CA1-CA4 on day 5 compared with day 3 (p < 0.001), and in Cortex3 (p = 0.04), whereas no significant difference could be found for Cortex1 (p = 0.2) and Cortex2 (p = 0.5).
CCAO with SAH was associated with a significantly lower neuronal cell count in the CA1 and CA3 region on day 5 compared with the group of SAH rats without CCAO (p = 0.03 and p = 0.02). Neuronal cell count did not differ between both hemispheres in rats with and without CCAO. Histological changes in the hippocampal layers CA1 and CA3 are exemplified in Fig. 4.
Discussion
Delayed cerebral ischemia contributes significantly to morbidity and mortality after aneurysmal SAH [13, 16, 44]. Despite intensive research, the pathogenesis of delayed CVS is still not completely understood. Therefore, animal models are needed for further investigation of pathological changes to develop new therapeutic strategies.
The present data demonstrate the development of neurological impairment in rats with cerebral high risk areas created by unilateral CCAO and induced CVS in an aggravated modified double-hemorrhage model.
In the standard double-hemorrhage model, several authors demonstrated an extended distribution of blood in both the supratentorial and infratentorial basal cisterns after artificial SAH by injection of blood into the cisterna magna. Furthermore, these investigations demonstrated that all major cerebral arteries were surrounded by blood [39–41, 45]. Despite the blood degradation in the basal cisterns until day 3 [39, 41], SAH induced CVS led to a maximum reduction of vessel diameter on days 5–7. Furthermore, CBF was decreased by CVS to 30–70% from the control [12, 19, 39, 41]. Determination of the vessel perimeter revealed for the middle cerebral artery a 36% reduction [17], the posterior communicating artery a 35% reduction, and the BA a 33% reduction [11] compared with the controls. In the measurement of the vessel diameter in histological slices, this reduction amounted to 25-30% for the middle cerebral artery [39, 45], 30% of the posterior cerebral artery [39], and 30-53% for the BA [12, 19, 39, 41]. Therefore, the data from the available literature clearly suggests that a comparable proximal CVS could be induced in all major cerebral arteries in the rat cisterna magna double-hemorrhage model.
In the current study, we analyzed BA diameter on the assumption of the proximal CVS, which is used by the vast majority of such investigations previously [12, 19, 39, 41] and therefore facilitates the comparability of results. Furthermore, reliable measurement of BA diameter at several levels is more reliable to assess by cross-sections of the brainstem compared with the middle cerebral artery, especially when coronal sections of the same animal’s parietal region are needed to evaluate the hippocampus. Therefore, analyzing the BA diameter remains to be the most robust and established measure for estimation of proximal CVS, even if the correlation to neurological impairment and the decreased hippocampal neuronal cell count are not, or only partially, in a brain territory depending on BA supply.
Neurological impairment and cerebral blood flow
The mortality rate of rats with CCAO and SAH (50%) was comparable with data of previous studies using the double-SAH model without CCAO [12, 41]. A significant neurological impairment was induced by SAH, not by CCAO alone. The neurological deterioration within the first days after SAH may be an expression of a transient increase of intracranial pressure and reduced CBF [37, 42].
Mechanisms inducing a reduction of CBF in the acute phase after SAH include acute vasoconstriction, brain edema and decreased cerebral perfusion pressure (CPP) [1, 2, 4]. Early changes after SAH have been described in the prechiasmatic and the perforation SAH model [32] that are used to mimic the acute phase of SAH. In the cisterna magna model used in the present study, early changes do not seem to play a major role. Accordingly, measurements of decreased rrCBF as well as histological confirmation of reduced BA diameter reached their maximum on day 5 in correlation with the severe neurological impairment at that time point in the present investigation, which is consistent with findings in former studies [12, 37, 41]. Therefore the cisterna magna double-hemorrhage model seems to be more suitable to mimick the delayed phase of SAH, including the development of delayed cerebral ischemia.
CCAO was not associated with a significant reduction of neurological impairment compared with SAH rats. Unilateral CCAO is known to result in a moderate reduction of CBF decrease in both cerebral hemispheres without asymmetrical perfusion in animal models of CCAO without SAH [6]. CBF after CCAO is normalized within 24 h after ligation and not different from values found in the control group [6]. In the current study, SAH significantly impaired neurological function of CCAO rats at both day 3 and day 5. Accordingly, rrCBF of CCAO rats was reduced by SAH at day 3 and significantly lower compared with SAH rats without CCAO at day 5. Hence, reflecting the effect of the created high risk area for delayed cerebral ischemia after carotid occlusion and CVS.
MR PWI has been used in the evaluation of rrCBF and rrCBV in the rat double-hemorrhage model before [41]. The correlation of CBF and/or CBV estimation between microsphere technique [43], positron emission tomography (PET) [23, 28, 29], single-photon emission computed tomography (SPECT) [23] and MR PWI has been validated previously. Using radiolabeled microsphere techniques, a stable CBF/masseter muscle blood flow ratio between 6.1 and 7.7 was observed in male Sprague-Dawley rats [10, 31], which is consistent with our data. Therefore, the rrCBF/masseter muscle blood flow ratio may be a good quantitative discriminator between reduced CBF and/or CBV caused by CVS or reduced systemic circulation. In addition, our data demonstrate a significant correlation between the defined ratio (CBF/muscular blood flow and CBV/muscular blood volume) and the histological reduction of BA diameter, suggesting an adequate noninvasive monitoring of CVS by MR PWI.
The time course of BA diameter reduction by CVS has been characterized in the double-hemorrhage model before [41]. The maximal CVS was described to be on day 5 [25, 26, 41], as well as the consecutive reduction of rrCBF and neurological deterioration. The average diameter of the control rat BA was 152 ± 10 μm in the present study, which is in agreement with previously published values between 150 to 250 μm [4, 12]. Furthermore, the reduction of the BA diameter to 79 ± 5 μm after SAH on day 5 is in accordance with formerly reported reduction to 47–55% [12, 41]. The increase of the BA diameter of 9.9% after CCAO in control rats is consistent with previously reported dilatation of the BA of 10 ± 1% after unilateral CCAO by Fuji et al. [9]. Interestingly, at day 3 the BA diameter in SAH rats with CCAO was also 10% larger compared to SAH rats without CCAO, whereas at day 5 the difference of the BA diameter between SAH rats with and without CCAO was reduced to 4% by CVS. While rrCBF was maintained in CCAO rats without SAH at day 3 and day 5 by autoregulatory enlargement of the BA diameter, delayed CVS in CCAO rats led to a significant reduction of BA diameter at day 5 and significantly reduced rrCBF compared with SAH rats without CCAO.
The mortality rates for sham rats (0%) and SAH rats (30% until day 3; 50% until day 5) in the current investigation are consistent with findings of our previous studies (24% until day 3; 47% until day 5; 0% sham rats) [12, 41]. Mortality in CCAO rats was 0% without SAH. Mortality in CCAO rats with SAH was 40% until day 3, and 50% until day 5. Thus, CCAO increased mortality at day 3 but not at day 5.
Morphological changes
We analyzed neuronal integrity after SAH within the hippocampus because of its known particular sensitivity to cerebral ischemia [15, 18, 21, 38] and within the cerebral cortex. We reported previously a CVS-related significant reduction of neuronal cell count after SAH in the hippocampus (CA1-CA4) and the inner layer of the cortex at day 5 [12].
In the current aggravated double-hemorrhage model with CCAO, neuronal cell count in the hippocampus (CA1-CA4) and the inner layer of the cortex was reduced by CVS at day 5, not at day 3. Furthermore, neuronal cell count in SAH rats with CCAO was significantly lower in the CA1 and the CA3 region at day 5 compared with the group of SAH rats without CCAO (p = 0.03 and p = 0.02). The aggravation of brain tissue damage in areas that are particularly vulnerable to ischemia is attributable to the development of cerebral high risk areas by unilateral CCAO and induced CVS and correlates with the development of neurological impairment and reduction of rrCBF at day 5.
Early brain tissue damage after SAH—within 24–72 h after the onset—have already been analyzed qualitatively in the cerebral cortex as well as in the hippocampus of the rat in the endovascular perforation model [4, 30]. Early histological changes included cytoplasmic condensation and nuclear pyknosis as markers of evolving necrosis, as was used for the definition of non-vital neurons in the current study. In contrast to the cisterna magna model used in the current study exhibiting changes of the late SAH phase including CVS [12, 37, 41], the endovascular perforation model is known to exhibit the acute changes after SAH in a distinct fashion with early reduction of CBF [4, 30, 32].
Quantitative data of brain tissue damage in the phase of CVS in the cisterna magna model has been analyzed before [12]. In the current study we provided quantitative data of significantly decreased neuronal cell count in the hippocampus (CA1–CA4) and the inner layer of the cortex (Cortex3) of SAH rats with and without CCAO on day 5 compared with sham operated rats and demonstrated that on day 3—i.e., the early phase—of SAH, no significant decrease of neuronal cell count was present compared with sham rats. Thus, our data indicate a delayed development of the brain tissue damage in both SAH rats with and without CCAO. Furthermore, brain tissue damage was enhanced in SAH rats by CCAO in the CA1 and CA3 region of the hippocampus, which is known to be vulnerable to ischemia [15, 18, 21, 38].
The occurrence of brain tissue damage in areas particularly sensitive to hypoxia, instead of a global or territorial ischemic infarction, may be explained by CVS that is accentuated in the posterior fossa and cortically bypassed by the pronounced cortical collateralization in the rat [3]. Furthermore, CBF in the rat hippocampus is known to be lower compared with the cerebral cortex [32]. The medially located basilar artery and artificial bleeding is not expected to induce lateralized CVS. Unilateral CCAO is known to result in a moderate symmetrical reduction of CBF decrease of both cerebral hemispheres without asymmetrical perfusion in animal models of CCAO without SAH [6]. Accordingly, the surviving rats are expected to exhibit bilateral brain tissue damage especially in areas with low CBF and vulnerability for hypoxia.
Clinical importance
CVS arising after aneurysmal SAH contributes significantly to morbidity and mortality after SAH, leading to brain tissue damage of differing severity [13, 16, 44]. Besides global ischemia, vulnerable brain regions may be affected in humans as well [27]. In contrast to early brain injury after SAH, delayed changes due to ongoing pathophysiological processes are potentially amenable for treatment, e.g., by neuroprotective agents. Therefore the use of animal models simulating not only the acute phase after SAH—i.e., the perforation model—but the time course during CVS are important and have the potential to improve treatment and outcome.
Conclusion
The present investigation provides data that CCAO deteriorates the effects of CVS in the rat double-SAH model. Therefore, our data suggest that the rat double-SAH model in combination with unilateral CCAO is suitable for pharmacological investigations evaluating both the improvement of CBF and neuroprotection during the development of delayed CVS after SAH.
References
Bederson JB, Germano IM, Guarino L (1995) Cortical blood flow and cerebral perfusion pressure in a new noncraniotomy model of subarachnoid hemorrhage in the rat. Stroke 26:1086–1091
Bederson JB, Levy AL, Ding WH, Kahn R, DiPerna CA, Jenkins AL III, Vallabhajosyula P (1998) Acute vasoconstriction after subarachnoid hemorrhage. Neurosurgery 42:352–360
Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H (1986) Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke 17:472–476
Cahill J, Calvert JW, Solaroglu I, Zhang JH (2006) Vasospasm and p53-induced apoptosis in an experimental model of subarachnoid hemorrhage. Stroke 37:1868–1874
Cambj-Sapunar L, Yu M, Harder DR, Roman RJ (2003) Contribution of 5-hydroxytryptamine1B receptors and 20-hydroxyeiscosatetraenoic acid to fall in cerebral blood flow after subarachnoid hemorrhage. Stroke 34:1269–1275
de Ley G, Nshimyumuremyi JB, Leusen I (1985) Hemispheric blood flow in the rat after unilateral common carotid occlusion: evolution with time. Stroke 16:69–73
Dittmar MS, Fehm NP, Vatankhah B, Horn M (2004) Ketamine/xylazine anesthesia for radiologic imaging of neurologically impaired rats: dose response, respiratory depression, and management of complications. Comp Med 54:652–655
Findlay JM, Deagle GM (1998) Causes of morbidity and mortality following intracranial aneurysm rupture. Can J Neurol Sci 25:209–215
Fujii K, Heistad DD, Faraci FM (1991) Flow-mediated dilatation of the basilar artery in vivo. Circ Res 69:697–705
Granstam E, Granstam SO (2003) Involvement of nitric oxide in the regulation of regional hemodynamics in streptozotocin-diabetic rats. Physiol Res 52:159–169
Gules I, Satoh M, Clower BR, Nanda A, Zhang JH (2002) Comparison of three rat models of cerebral vasospasm. Am J Physiol Heart Circ Physiol 283:H2551–H2559
Güresir E, Raabe A, Jaiimsin A, Dias S, Raab P, Seifert V, Vatter H (2010) Histological evidence of delayed ischemic brain tissue damage in the rat double-hemorrhage model. J Neurol Sci 293:18–22
Heros RC, Zervas NT, Varsos V (1983) Cerebral vasospasm after subarachnoid hemorrhage: an update. Ann Neurol 14:599–608
Hop JW, Rinkel GJ, Algra A, van Gijn J (1997) Case-fatality rates and functional outcome after subarachnoid hemorrhage: a systematic review. Stroke 28:660–664
Hossmann KA (1998) Experimental models for the investigation of brain ischemia. Cardiovasc Res 39:106–120
Kassell NF, Torner JC, Haley EC Jr, Jane JA, Adams HP, Kongable GL (1990) The International Cooperative Study on the Timing of Aneurysm Surgery. Part 1: Overall management results. J Neurosurg 73:18–36
Kim DE, Suh YS, Lee MS, Kim KY, Lee JH, Lee HS, Hong KW, Kim CD (2002) Vascular NAD(P)H oxidase triggers delayed cerebral vasospasm after subarachnoid hemorrhage in rats. Stroke 33:2687–2691
Larsson E, Lindvall O, Kokaia Z (2001) Stereological assessment of vulnerability of immunocytochemically identified striatal and hippocampal neurons after global cerebral ischemia in rats. Brain Res 913:117–132
Lee JY, Huang DL, Keep R, Sagher O (2008) Characterization of an improved double hemorrhage rat model for the study of delayed cerebral vasospasm. J Neurosci Methods 168:358–366
Lei H, Grinberg O, Nwaigwe CI, Hou HG, Williams H, Swartz HM, Dunn JF (2001) The effects of ketamine-xylazine anesthesia on cerebral blood flow and oxygenation observed using nuclear magnetic resonance perfusion imaging and electron paramagnetic resonance oximetry. Brain Res 913:174–179
Leifer D, Kowall NW (1993) Immunohistochemical patterns of selective cellular vulnerability in human cerebral ischemia. J Neurol Sci 119:217–228
Li F, Liu KF, Silva MD, Omae T, Sotak CH, Fenstermacher JD, Fisher M, Hsu CY, Lin W (2000) Transient and permanent resolution of ischemic lesions on diffusion-weighted imaging after brief periods of focal ischemia in rats: correlation with histopathology. Stroke 31:946–954
Liu Y, Karonen JO, Vanninen RL, Ostergaard L, Roivainen R, Nuutinen J, Perkio J, Kononen M, Hamalainen A, Vanninen EJ, Soimakallio S, Kuikka JT, Aronen HJ (2000) Cerebral hemodynamics in human acute ischemic stroke: a study with diffusion- and perfusion-weighted magnetic resonance imaging and SPECT. J Cereb Blood Flow Metab 20:910–920
Macdonald RL, Kassell NF, Mayer S, Ruefenacht D, Schmiedek P, Weidauer S, Frey A, Roux S, Pasqualin A (2008) Clazosentan to overcome neurological ischemia and infarction occurring after subarachnoid hemorrhage (CONSCIOUS-1): randomized, double-blind, placebo-controlled phase 2 dose-finding trial. Stroke 39:3015–3021
Meguro T, Clower BR, Carpenter R, Parent AD, Zhang JH (2001) Improved rat model for cerebral vasospasm studies. Neurol Res 23:761–766
Miyagi Y, Carpenter RC, Meguro T, Parent AD, Zhang JH (2000) Upregulation of rho A and rho kinase messenger RNAs in the basilar artery of a rat model of subarachnoid hemorrhage. J Neurosurg 93:471–476
Nau R, Haase S, Bunkowski S, Bruck W (2002) Neuronal apoptosis in the dentate gyrus in humans with subarachnoid hemorrhage and cerebral hypoxia. Brain Pathol 12:329–336
Ostergaard L, Sorensen AG, Kwong KK, Weisskoff RM, Gyldensted C, Rosen BR (1996) High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part II: Experimental comparison and preliminary results. Magn Reson Med 36:726–736
Ostergaard L, Weisskoff RM, Chesler DA, Gyldensted C, Rosen BR (1996) High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part I: Mathematical approach and statistical analysis. Magn Reson Med 36:715–725
Ostrowski RP, Colohan AR, Zhang JH (2005) Mechanisms of hyperbaric oxygen-induced neuroprotection in a rat model of subarachnoid hemorrhage. J Cereb Blood Flow Metab 25:554–571
Palmer GM, Cairns BE, Berkes SL, Dunning PS, Taylor GA, Berde CB (2002) The effects of lidocaine and adrenergic agonists on rat sciatic nerve and skeletal muscle blood flow in vivo. Anesth Analg 95:1080–6, table
Prunell GF, Mathiesen T, Svendgaard NA (2004) Experimental subarachnoid hemorrhage: cerebral blood flow and brain metabolism during the acute phase in three different models in the rat. Neurosurgery 54:426–436
Rabinstein AA, Pichelmann MA, Friedman JA, Piepgras DG, Nichols DA, McIver JI, Toussaint LG III, McClelland RL, Fulgham JR, Meyer FB, Atkinson JL, Wijdicks EF (2003) Symptomatic vasospasm and outcomes following aneurysmal subarachnoid hemorrhage: a comparison between surgical repair and endovascular coil occlusion. J Neurosurg 98:319–325
Rousselle CH, Lefauconnier JM, Allen DD (1998) Evaluation of anesthetic effects on parameters for the in situ rat brain perfusion technique. Neurosci Lett 257:139–142
Satoh M, Parent AD, Zhang JH (2002) Inhibitory effect with antisense mitogen-activated protein kinase oligodeoxynucleotide against cerebral vasospasm in rats. Stroke 33:775–781
Soehle M, Heimann A, Kempski O (1998) Postischemic application of lipid peroxidation inhibitor U-101033E reduces neuronal damage after global cerebral ischemia in rats. Stroke 29:1240–1246
Solomon RA, Antunes JL, Chen RY, Bland L, Chien S (1985) Decrease in cerebral blood flow in rats after experimental subarachnoid hemorrhage: a new animal model. Stroke 16:58–64
Sugawara T, Lewen A, Noshita N, Gasche Y, Chan PH (2002) Effects of global ischemia duration on neuronal, astroglial, oligodendroglial, and microglial reactions in the vulnerable hippocampal CA1 subregion in rats. J Neurotrauma 19:85–98
Takeuchi K, Renic M, Bohman QC, Harder DR, Miyata N, Roman RJ (2005) Reversal of delayed vasospasm by an inhibitor of the synthesis of 20-HETE. Am J Physiol Heart Circ Physiol 289:H2203–H2211
Trandafir CC, Nishihashi T, Wang A, Murakami S, Ji X, Kurahashi K (2004) Participation of vasopressin in the development of cerebral vasospasm in a rat model of subarachnoid haemorrhage. Clin Exp Pharmacol Physiol 31:261–266
Vatter H, Weidauer S, Konczalla J, Dettmann E, Zimmermann M, Raabe A, Preibisch C, Zanella FE, Seifert V (2006) Time course in the development of cerebral vasospasm after experimental subarachnoid hemorrhage: clinical and neuroradiological assessment of the rat double hemorrhage model. Neurosurgery 58:1190–1197
Verlooy J, Van Reempts J, Haseldonckx M, Borgers M, Selosse P (1992) The course of vasospasm following subarachnoid haemorrhage in rats. A vertebrobasilar angiographic study. Acta Neurochir (Wien) 117:48–52
Walsh EG, Minematsu K, Leppo J, Moore SC (1994) Radioactive microsphere validation of a volume localized continuous saturation perfusion measurement. Magn Reson Med 31:147–153
Weir B, Grace M, Hansen J, Rothberg C (1978) Time course of vasospasm in man. J Neurosurg 48:173–178
Widenka DC, Medele RJ, Stummer W, Bise K, Steiger HJ (1999) Inducible nitric oxide synthase: a possible key factor in the pathogenesis of chronic vasospasm after experimental subarachnoid hemorrhage. J Neurosurg 90:1098–1104
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Güresir, E., Vasiliadis, N., Dias, S. et al. The effect of common carotid artery occlusion on delayed brain tissue damage in the rat double subarachnoid hemorrhage model. Acta Neurochir 154, 11–19 (2012). https://doi.org/10.1007/s00701-011-1191-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-011-1191-2