Abstract
Purpose
This study investigates retrospectively the clinical, neuroradiological, pathological and surgical evidence verifying the infundibulo-tuberal topography for craniopharyngiomas (CPs). Infundibulo-tuberal CPs represent a surgical challenge due to their close anatomical relationships with the hypothalamus. An accurate definition of this topographical category is essential in order to prevent any undue injury to vital diencephalic centres.
Methods
A systematic review of all scientific reports involving pathological, neuroradiological or surgical descriptions of either well-described individual cases or large series of CPs published in official journals and text books from 1892 to 2011 was carried out. A total of 1,232 documents providing pathological, surgical and/or neuroradiological evidence for the infundibulo-tuberal or hypothalamic location of CPs were finally analysed in this study.
Findings
For a total of 3,571 CPs included in 67 pathological, surgical or neuroradiological series, 1,494 CPs (42%) were classified as infundibulo-tuberal lesions. This topography was proved in the autopsy of 122 non-operated cases. The crucial morphological finding characterizing the tubero-infundibular topography was the replacement of the third ventricle floor by a lesion with a predominant intraventricular growth. This type of CP usually presents a circumferential band of tight adherence to the third ventricle floor remnants, formed by a functionless layer of rective gliosis of a variable thickness. After complete surgical removal of an infundibulo-tuberal CP, a wide defect or breach at the floor of the third ventricle is regularly observed both in the surgical field and on postoperative magnetic resonance imaging studies.
Conclusions
Infundibulo-tuberal CPs represent a major topographical category of lesions with a primary subpial development at the floor of the third ventricle. These lesions expand within the hypothalamus itself and subsequently occupy the third ventricle; consequently, they can be classified as not strictly intraventricular CPs. A tight attachment to the hypothalamus and remnants of the third ventricle floor is the pathological landmark of infundibulo-tuberal CPs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Craniopharyngiomas (CPs) are histologically benign epithelial lesions which may develop at any point along the pituitary-hypothalamus axis, from the sella turcica to the third ventricle [105, 123, 151, 159]. These lesions remain among the most challenging for neurosurgeons due to the everlasting controversy about the most appropriate therapy to be chosen for each patient. A major reason for this controversy is the high variability regarding the age at onset, topographical relationships, size and consistency, degree of adherence and biological behaviour of these lesions. CPs involving the third ventricle include the lesions that present the utmost difficulty for a complete surgical excision due to their intimate anatomical and functional relationships with the hypothalamus. An accurate definition of the topographical relationships between intraventricular CPs and the functional hypothalamus is essential prior to planning the most appropriate surgical approach and degree of excision, a judicious decision that should take into account the age of the patient and the preoperative presence of symptoms of hypothalamic derangement.
In 2004, we developed a topographical classification system for intraventricular CPs based on a meticulous analysis of the tumour-third ventricle relationships observed on autopsies, surgical procedures and neuroradiological computed tomography (CT) or magnetic resonance imaging (MRI) studies of individual well-described lesions considered as “truly” or “purely” intraventricular [106]. Considering the integrity of the third ventricle floor, in addition to the location of the whole mass within the third ventricle, as the decisive factor proving the strictly intraventricular location of the CP, we were able to reclassify a majority of the previously considered purely intraventricular CPs as not strictly intraventricular lesions. CPs with such topography had originally developed within the neural tissue of the third ventricle floor and had then progressively replaced the floor while growing into the third ventricle cavity [107, 108]. Not strictly intraventricular CPs differ from the classical suprasellar CP as well as from the rare strictly intraventricular type in the wide and circumferential tight adhesion to the hypothalamus they present [46, 78, 99, 136, 143]. Very recently, Qi et al. [115] have reviewed the microsurgical meningeal relationships of 195 CPs operated on in their Department and classified 104 lesions as infundibulo-tuberal CPs, a topography characterized by its subarachnoidal position at the junction of the pituitary stalk with the floor of the third ventricle. A subset of 17 CPs belonging to this topography had a pure or strict third ventricle development, with a variable type of attachment to the floor of the third ventricle [104]. Such a high rate of the infundibulo-tuberal topography is surprising and apparently contradictory with the classical view of CPs as the paradigmatic example of a lesion with a suprasellar location. Given the considerable predominance of infundibulo-tuberal or not strictly intraventricular CPs among the well-described intraventricular CPs included in our previous review, a result parallel to the findings in the work by Qi et al. [115], we decided to investigate all the existing evidence for the infundibulo-tuberal topography of CPs provided since the original categorization of these lesions. In this work, a systematic and comprehensive account of the clinical, pathological, neuroradiological and surgical evidence for the infundibulo-tuberal topography of CPs is presented. The purpose of this survey is to provide a comprehensive synthesis of the accumulated knowledge regarding the specific characteristics and risks associated with this topographical category and help neurosurgeons to properly diagnose and plan the surgical treatment to prevent any undue damage to the hypothalamus.
Methods
A thorough search of embryological, clinical, pathological, neuroradiological and/or surgical studies of CPs providing evidence for the infundibulo-tuberal origin and/or location of the lesion was carried out. The critical morphological finding taken into consideration to categorize a CP within the tubero-infundibular topography was the replacement of the floor of the third ventricle by a lesion with a predominant intraventricular growth. These are the same criteria that define the not strictly intraventricular category of intraventricular CPs as it is differentiated in the classification system of intraventricular CPs by Pascual et al. [106].
This survey comprises CPs presented in both large series of patients and well-detailed individual cases in medical journals obtained from the PubMed, Medline and Scopus databases after introducing the keyword craniopharyngioma. Reference lists of the selected articles as well as CPs displayed in specialized texts and monographs focused on the fields of Neurosurgery, Neurology, Neuroradiology, Neuroophtalmology, Neuroendocrinology, Neurooncology and Neuropathology were also scrutinized. Scientific reports and monographs of interest from the nineteenth and the first half of the twentieth centuries referenced in the articles selected from the previous databases were all retrieved and reviewed from the following medical libraries: The National Library of Medicine, NIH, Bethesda, Maryland, USA; The Francis Countway Library, Harvard School of Medicine, Boston, MA, USA; The Tompkins McCaw Library, Medical College Virginia, Richmond, VA, USA; The New York Academy of Medicine Library, New York, NY, USA; The Harvey Cushing/John Hay Whitney Medical Library, Yale University, New Haven, Connecticut, USA; The Welch Medical Library, Johns Hopkins University, Baltimore, MD, USA; The British Library, London, UK; The Complutense Medical School Library, Madrid, Spain and The Medical libraries of La Paz University Hospital, Ramón y Cajal University Hospital and Gregorio Marañón University Hospital, Madrid, Spain.
A final set of 1,232 scientific documents—articles, monographs and textbooks—including CPs either categorized within the infundibulo-tuberal or not strictly intraventricular topography or providing anatomical, pathological, neuroradiological or surgical evidence for such topography were finally selected and analysed. The database of evidence for the infundibulo-tuberal topography for CPs includes a total of 3,449 CPs reported in 67 surgical, pathological and neuroradiological series and 122 CPs displayed in necropsy studies.
Results
Table 1 presents the number and relative rate of CPs classified within the infundibulo-tuberal or not strictly intraventricular category in the 36 surgical series of CPs providing evidence of such topography published in the scientific literature [3, 24, 30, 31, 35–39, 41, 42, 46, 48, 51, 53, 56, 74, 79, 87, 88, 96, 99, 104, 112, 115, 116, 119, 120, 125, 127, 131, 136, 137, 143, 149, 151, 152, 154, 158–161, 164]. Table 2 presents the list of infundibulo-tuberal CPs found in autopsy studies and included in the corresponding surgical or pathological series from the literature [5, 7, 12, 15, 17, 19, 20, 24, 25, 32, 34–36, 39, 40, 44, 47, 49, 52, 56, 60, 61, 65, 67, 69, 70, 76, 78, 81, 83, 85, 93, 100, 126, 136, 137, 141, 146, 147, 154, 165, 166]. Figures 1, 2, 3, 4, 5, 6, 7 show, respectively, a pictorial collection of anatomical (Fig. 1), embryological (Figs. 2 and 3), histological (Fig. 4), neuroradiological (Fig. 5), surgical (Fig. 6) and radiosurgical (Fig. 7) evidence supporting the concept of the infundibulo-tuberal topography as a separate category of CPs. In addition, remarkable examples of individual cases of CPs providing noteworthy evidence for the infundibulo-tuberal topography from autopsies, MRI studies, surgical procedures or radiosurgical treatments selected from a collection of 2,100 intraventricular CPs published in the literature are included in Figs. 1, 4, 6 and 7.
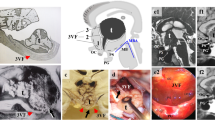
Topographical classification of CPs involving the third ventricle: differentiation of the infundibulo-tuberal or not strictly intraventricular category in necropsy specimens. a, b Strictly or truly third ventricle CP of the squamous-papillary type (Mott and Barrat, 1899 [97]); a midsagittal brain section showing a rounded cystic/basal solid papillary epithelial lesion confined to the third ventricle; b histological detail from the same case showing the morphologically intact floor of the third ventricle (black arrows) and the basal tumour area of attachment to the ependymal lining of the third ventricle floor (white arrows); c artistic illustration showing a solid epithelial mass replacing the floor of the third ventricle (black arrow) and expanding into the third ventricle cavity (Babinski, 1900 [11]). d-i The three major topographycal categories of intraventricular CPs according to the type of involvement of the third ventricle floor: strictly intraventricular (d, g), infundibulo-tuberal or not strictly intraventricular (e, h, j) and pseudointraventricular (f, i). d, g Strictly intraventricular CP located within the third ventricle above a morphologically intact tuber cinereum and infundibulum as observed on coronal (d) and brain undersurface (g) views of the necropsy specimen (Schilder, 1927 [129]). Notice the position of both optic tracts (white arrows) and the cleavage plane for dissection between the CP’s upper pole and the walls of the third ventricle (black arrows). The lesion is not observable from the brain undersurface yet it can be presumed because of the belly-like protrusion of the tuber cinereum (g). e Basal view of a solid CP (T) developed within the neural tissue of the infundibulum and tuber cinereum. Notice the intact pituitary stalk and gland and the lack of tumour expansion into the chiasmatic cistern (Cushing, 1932 [39]). h Artistic reproduction of the sagittal view of a tubero-infundibular, adamantinomatous CP which had replaced the floor of the third ventricle (black arrow) and expanded predominantly within the third ventricle. Notice the intact pituitary stalk below the lesion and the cystic infiltration of the mamillary body (Kaufmann, 1929 [73]). j Undersurface brain view of an infundibulo-tuberal CP diagnosed in a 16-year-old man who died after a 4-year history of severe headache, progressive visual deficit, recurrent seizures and obnubilation followed by bradycardia and respiratory arrest. This solid, adamantinomatous CP was confined into the third ventricle and separated from the suprasellar cistern by a layer of pia mater (Erdheim, 1904 [49]). Note the tumoral papillary protrusions through the tuber cinereum. f Pseudoventricular type of CP. The heterogeneous solid-cystic mass developed from the pituitary stalk is pressing on a morphologically intact third ventricle floor (black arrows) masquerading a truly intraventricular position (Strada, 1911 [138]). i Undersurface brain view showing the concave deformation of the intact third ventricle floor (black arrow) caused by a suprasellar expanding mass mimicking an intraventricular position (Blackburn, 1950 [18])
Embryological basis for the development of infundibulo-tuberal craniopharyngiomas I: embriology of the pars tuberalis of the pituitary gland. a 1 –a 3 Early stages of development of Rathke’s pouch. Rathke’s pouch is developed as a pouch of cube epithelial cells evaginated from the dorsal surface of the primitive mouth. From early stages of embryo development Rathke’s pouch presents two inferior-lateral thickenings of epithelial tissue (black arrows) close to the craniopharyngeal duct which become fused as the flattened disc-shaped pouch bends forward upon itself to transform into a vesicle. a 1 Carnegie stage 17 (6th week of development, embryo about 8 mm long). a 3 Carnegie stage 19 (7th week of development, embryo about 16 mm long) (Henke, 1926 [64]) b Sagittal section of Rathke’s pouch connected by the solid remnant of the craniopharyngeal duct (white arrow) to the roof of the primitive mouth (Carnegie stage 18, 6 and half weeks of development). The black arrow points to the primordia of the pars tuberalis (O’Rahilly, 2001 [102]). c Artistic illustration showing the disc of Rathke’s pouch closely apposed to the floor of the diencephalic evagination at a similar Carnegie stage as shown in b (Rouvière, 1947 [122]). Black arrows point to the inferolateral thickenings adjacent to the craniopharyngeal duct which will transform into the pars tuberalis. d Initial rotation of the pars tuberalis: the lower edge of the Rathke’s pouch is now closed and the pouch transformes into an epithelial vesicle which progressively folds upon itself and becomes apposed against the third ventricle floor. Such rotation could presumably transfer cell remnants of the craniopharyngeal duct or, alternatively, either epithelial cells from the primitive oral mucosa or dental ridge cells, into the third ventricle floor (Dialti, 1910 [45]). e-g Rathke’s pouch vesicle stage. e The pars tuberalis (pt) is observed at the most anterior position after the rotation of Rathke’s pouch (Attwell, 1926 [10]). f Original artistic illustration by von Mihalkovics showing the upward and forward bending of the parts tuberalis (pt) towards the floor of the diencephalon in a dog embryo (von Mihalkovics, 1875 [150]). g Cenital view of Rathke’s pouch as a hollow vesicle (Rouvière, 1947 [122]). h Final embryological stage of Rathke’s pouch development; the pars tuberalis is holding the upper pituitary stalk and extends as a sheet upon the anterolateral infundibulum and the tuber cinereum (Attwell, 1926 [10]). i 1 -i 4 Stages of development of the pars tuberalis in a rabbit embryo; i 1 Rathke’s pouch attached to the primitive mouth (12-day rabbit embryo); i 2 initial stage of migration of Rathke’s pouch toward the floor of the third ventricle. The primordia of the pars tuberalis (pt) is still attached to the remnant of the craniopharyngeal duct (15-day rabbit embryo); i 3 Rathke’s pouch transforms in a vesicle by upward migration of the pars tuberalis to the floor of the third ventricle (17-day rabbit embryo); i 4 The sphenoid bone and sella turcica are formed by organization of the mesenchimal tissue and the craniopharyngeal duct (cdr) is disconnected from the vesicle. The pars tuberalis has completed its rotation toward the diencephalic vesicle and may become embedded within the third ventricle floor. A adenohypophysis, cd craniopharyngeal duct, cdr craniopharyngeal duct remnant, pt pars tuberalis, IIIv floor third ventricle floor, O.m. oral mucosa, Sph sphenoid bone (Attwell, 1918 [9])
Embryological basis for the development of infundibulo-tuberal CPs II: meningeal relationships of the pars tuberalis and epithelial cell nests within this structure. a 1 –a 3 Coronal sections of human embryos at the level of the third ventricle showing the fusion of the upper edges of the pars tuberalis with the neural tissue of the third ventricle floor. a 1 The primordia of the pars tuberalis undergo a movement towards the floor of the diencephalic evagination and become embedded into the primitive meningeal layer covering the floor (black arrows) (18.2 mm embryo); a 2 Rathke’s pouch has become a vesicle whose antero-superior edges are in close contact with the floor of the third ventricle (white arrows) (36 mm fetus); a 3 the pars tuberalis has invaded the neural tissue of the third ventricle floor by trespassing the barrier of the pia mater (white arrows) (36 mm fetus) (Wislocki, 1937 [156]). b Illustrative scheme showing the meningeal relationships of the pars tuberalis. This structure is enclosed between two layers of pia mater, one antero-ventral, covering its outer aspect together with the arachnoidal layer (lower row of black arrows), and one postero-dorsal, corresponding to the piamater formed from the primitive meningeal layer that covers the neural tube (upper row of black arrows) (Attwell, 1926 [10]); c Sagittal section of the pituitary in a human fetus (16 cm sitting height), showing the pars tuberalis ensheated by the two layers of piamater (Wislocki, 1936 [155]); d Medial sagittal section of the pituitary body of a cat embryo showing the pars tuberalis embedded within the neural tissue of the third ventricle floor (black arrow) (Schäfer, 1922 [128]). e 1 Midsagittal section through the infundibulum and pituitary gland of a 49-year-old man showing the presence of squamous-epithelial cell nests within the pars tuberalis (white arrows) (Erdheim, 1904 [49]). Notice the accumulation of squamous cells at the junction between the infundibulum and the optic chiasm, where perforant hypothalamic vessels penetrate the floor of the third ventricle. e 2 Axial section through the infundibulo-chiasmatic junction in the same patient, showing the nests of squamous epithelial cells at this level (E) (Erdheim, 1904 [49]). f Areas of the pars tuberalis where nests of epithelial cells are most frequently observed: at the upper edge (UG) in close contact with the floor of the third ventricle immediately posterior to the optic chiasm and at the junction between the gland and the pituitary stalk (LG) (Simonds, 1922 [133]). g 1 -g 3 Theoretical development of infundibulo-tuberal CPs from tumoural growth of epithelial cell nests at the junctional area between the infundibulum and the optic chiasm (Dott, 1938 [46])
Histopathological relationships between infundibulo-tuberal CPs and the hypothalamus. a One of the earliest depictions of a cystic, infundibulo-tuberal CP of the squamous-papillary type. Notice the basal papillary nodule in the infundibulum (black arrow) and the cyst capsule replacing the floor of the third ventricle (Langer, 1892 [85]). b Sagittal section of a CP similar to that reported by Langer found in an autopsy study. Observe the infundibulo-tuberal origin of the basal papillary nodule (black arrow) and the thin layer of neural tissue surrounding the intraventricular cystic component corresponding to the stretched remnant of the third ventricle floor, as proved by Pan et al. [104] (white arrows) (Zülch, 1975 [165]). c Histological tumor-third ventricle relationships of an infundibulo-tuberal or not strictly intraventricular CP of the adamantinomatous type. The lesion has developed at the infundibulum-chiasm junction and replaced most of the tuber cinereum while expanding into the third ventricle. The arachnoid of the chiasmatic cistern is intact below the basal aspect of the lesion (black arrow); ch optic chiasm, mb mamillary body (Wheatley et al., 1986 [153]). d Artistic picture of an infundibulo-tuberal CP of adamantinomatous type whose lower pole is protruding into the suprasellar area (Ziegler, 1902 [163]). e Histological coronal section of an infundibulo-tuberal CP whose intraventricular component shows no infiltration of the ventricle walls. A clear plane of dissection of the lesion is observed between the tumor capsule and the walls of the third ventricle (white arrows) (Newmark, 1917 [98]). f Midsagital brain section showing the autopsy specimen of a strictly intraventricular papillary CP attached to the third ventricle floor by a short gliovascular pedicle (black arrow). In contrast to the previously shown lesions, an intact third ventricle floor is observed beneath the lesion (Schmidt, 1984 [130]). g 1 -g 2 Macroscopic midsagittal section (g 1 ) and microscopic coronal section of an intraventricular CP in a 30-year-old man who died postoperatively due to hypothalamic haemorrhage. Basal solid portions of the tumour were tightly adhered to the third ventricle floor and walls (black arrows) with areas of haemorrhagic infiltration of the hypothalamus (white arrow) (Kubota et al., 1980 [83]). h 1 -h 3 Coronal macroscopic and microscopic sections of a solid-cystic infundibulo-tuberal CP showing the whole obliteration of the third ventricle. The basal solid portion of the lesion which replaces the infundibulo-tuberal area is surrounded by the arachnoid-pia mater (black arrows); its cystic intraventricular portion is separated from the hypothalamus by a three-layered wall formed by a connective tissue layer (C), the arachnoid (A) and the pia mater (P) (Goldstein, 1928 [58]). i 1 -i 2 Coronal macroscopic and microscopic sections of a cystic third ventricle CP showing the infiltration of the hypothalamus by epithelial finger-like protrusions (black arrows) (Orthner and Rettinger, 1965 [103])
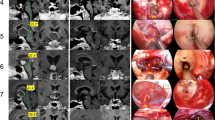
Neuroradiological differentiation of the infundibulo-tuberal or not strictly intraventricular topographical category of CPs. a 1 –a 3 Preoperative midsagital (a 1 ), axial (a 2 ) and coronal (a 3 ) T1-weighted MRI sections after gadolinium administration of an infundibulo-tuberal cystic CP diagnosed in a 49-year-old woman with a history of headache, anorexy, left hemianopsy and loss of libido for the last 2 years. The third ventricle floor is apparently replaced by the lesion, which obliterates both the suprachiasmatic and infundibular recesses of the third ventricle, above a normal pituitary gland. Irregular tumour protrusions are observed expanding within the hypothalamic issue on the axial section (white arrows). a 4 Postoperative midsagittal T1-weighted MRI study after total removal of the lesion. The defective third ventricle floor allows the free CSF flow into the chiasmatic cistern (white arrow. a 5 Preoperative midsagittal T2-weighted MRI section of the same lesion showing the integrity of both the pituitary gland and pituitary stalk (white arrow); a thin layer of neural tissue around the intraventricular portion of the lesion, between the optic chiasm and the compressed mamillary bodies can be observed (black arrows). b 1 -b 3 Preoperative midsagittal (b 1 ), axial (b 2 ) and coronal (b 3 ) T1-weighted MRI sections of an infundibulo-tuberal basal solid/upper cystic craniopharyngioma diagnosed in a 48–year-old man with a history of headache, short memory defect and behaviour disturbance for the last few moths. A normal, free-of-tumour pituitary gland, pituitary stalk and chiasmatic cistern are observed on these sections. The third ventricle floor is apparently replaced by the lesion that fills almost the whole third ventricle. The stretched thin walls of the third ventricle containing the hypothalamic nuclei are observed on the axial section (white arrows). b 4 Postoperative midsagittal T1-weighted MRI study after total removal of the lesion. A defect in the third ventricle floor caused by the mass can be observed (white arrow). b 5 Preoperative midsagittal 3D FIESTA (fast imaging employing steady-state acquisition) MRI image showing the basal, cauliflower-like portion of the lesion replacing the infundibulum and tuber cinereum (white arrow) and the thin layer of neural tissue surrounding its intraventricular cystic portion, between the optic chiasm and the compressed mamillary bodies (black arrows). a 6 -b 6 Proton density-weighted axial MRI sections of both CPs shown above; a marked symmetrical hyperintense signal corresponding to edema along the optic tracts (black arrows) is characteristically caused by infundibulo-tuberal or not strictly intraventricular CPs
Surgical evidence of the infundibulo-tuberal or not strictly intraventricular topographical category of CPs. a 1 –a 9 Giant mixed solid-cystic infundibulo-tuberal CP diagnosed and surgically removed in a 33-year-old man with a history of headache, visual deficit and hyperphagic obesity disorder. a 1 Preoperative midsagittal T1-weighted MRI study after gadolinium administration showing the elliptic lesion filling the whole third ventricle. a 2 Coronal T1-weighted MRI section showing the position of both hypothalami around the equator of the lesion (black arrows). a 3 Postoperative midsagittal T1-weighted MRI study showing a tumor remnant at the level of the infundibulum. a 4 , a 5 Postoperative midsagittal and coronal T1-weighted MRI sections showing the defect in the floor of the third ventricle after total removal of the infundibular CP remnant. a 6 Intraoperative photograph of the initial surgical procedure. The chiasm and optic nerves are exposed through a pterional right approach. Notice the bulged lamina terminalis beneath the A-1 segment of the anterior cerebral artery due to the mass effect of the intraventricular component of the lesion (black arrow). a 7 Intraoperative view of the third ventricle obtained in the second surgical procedure after removing the infundibular CP remnant through the same pterional approach. The inner aspect of the third ventricle left wall is observed, in addition to the bloody rim of attachment of the lesion (black arrows). a 8 -a 9 Histological specimens of the tumor-brain interface showing a functionless band of reactive gliosis (G) around the adamantinomatous CP at the level of the third ventricle floor in the initial (a 8 ) and recurrent (a 9 ) lesions. The thickness of this band ranged from 50 to 600 μm and was similar in both tumour specimens, although it showed a significantly more prominent vascular proliferation in the recurrent CP (black arrows). b 1 -b 4 Intraventricular solid CP originated in the infundibulum and removed through an endoscopically assisted extended transphenoidal approach (de Divitiis, 2007 [41]. Reproduced with permission from Lippincott Williams & Wilkins Publishing group.). b 2 , b 3 Intraoperative endoscopic views of the basal aspect of the lesion which expands at the chiasmatic-infundibular junction. The border of the breach in the third ventricle floor left by the lesion can be observed in the surgical field after removal of the tumor (T) (white arrows) and on the postoperative midsagital MRI study (b 4 , white arrow). c 1 -c 4 Infundibulo-tuberal CP removed through an endoscopically assited extended transphenoidal approach (Kassam, 2008 [72]. Reproduced with permission from JNS Publishing group). The basal aspect of the solid lesion replaces the tuber cinereum with the remnants of the third ventricle floor being visible adjacent to the anteroinferior tumour border (c 2 , white arrow). A wide defect in the tuber cinereum is left after total removal of the CP allowing a view of the inside of the ventricle which presents a rim of blood infiltration (c 4 , black arrows). The breach in the third ventricle floor is observed on postoperative coronal MRI (c 4 )
Radiosurgical evidence of the infundibulo-tuberal or not strictly intraventricular topographical category of CPs. a 1 a1 –a 3 Infundibulo-tuberal solid CP treated with gamma knife (Kobayashi et al., 1994 [77]. Reproduced with the kind permission by S. Karger AG, Basel.). The sequential follow-up MRI studies obtained 6 months and 1 year after the treatment show the progressive shrinkage of the lesion which last remnant remains at the infundibulo-tuberal area, the probable site of origin of this CP. b 1 , b 2 Similar long-term evolution of a basal-solid/upper cystic intraventricular CP following gamma knife radiosurgery showing the progressive shrinkage of the residual solid mass in the floor of the third ventricle, above an intact pituitary stalk (Chung et al., 2000 [26]. Reproduced with permission from the JNS Publishing group.). c 1 , c 2 Eliptic solid CP treated by stereotactic radiosurgery which last remnant is observed embedded within the floor of the third ventricle 4 months following application of the therapy (This figure was published by Steiner L et al: Gamma surgery in cerebral vascular lesions, malformations, tumors and functional disorders of the nervous system, in Schmidek HH & Sweet WH (eds): Operative Neurosurgical Techniques, indications, methods and results, fourth edition, WB Saunders Company, Vol 1 pp 670–706, Copyright Elsevier, 2000. [135])
The infundibulo-tuberal or not strictly intraventricular topographical category of CPs was substantiated by the following six kinds of evidence:
-
1.
Pathological: the tubero-infundibular topography has been confirmed in whole non-operated specimens of CPs obtained from necropsies. Table 2 presents a list of 122 non-operated or biopsed CPs disclosed during autopsy studies. Some additional examples of necropsy specimens showing intraventricular CPs developed at the floor of the third ventricle are presented in Figs. 1 and 4. In these necropsy brain specimens tubero-infundibular CPs are characterized as lesions that replace the floor of the third ventricle, above an identifiable, almost intact pituitary stalk, and whose mass bulk is occupying the lower half or the whole third ventricle chamber. Although the lesion expands predominantly within the third ventricle, its lower pole protrudes into the chiasmatic cistern; therefore, this type of CP corresponds to the not strictly intraventricular category. The walls of the third ventricle containing the hypothalamic nuclei are separated from each other by the mass and identifiable remnants of the hypothalamus are found around the equator or lower pole of the lesion. Nearly all lesions were smoothly rounded or elliptic, lacking the multilobulated pattern of typical suprasellar lesions. This shape pattern is modelled by the expansion of the mass within the boundaries of third ventricle. Three major tumour consistency patterns were observed: pure solid, pure cystic or basal solid/upper cystic. In mixed lesions, the solid component was placed at the floor of the third ventricle, whereas the cystic component occupied the cavity of the ventricle. In both pure solid and pure cystic lesions, the lower pole of the mass had a retrochiasmatic position, within the chiasmatic cistern.
-
2.
Histological: the basal, solid component of tubero-infundibular CPs is usually tightly adhered to the remnants of the infundibulum and tuber cinereum, the neural structures forming the floor of the third ventricle (Table 2). In most histological studies this area of the tumor was surrounded by a circumferential, non-functional layer of rective gliosis of variable thickness, ranging from 0.1 to 1.5 mm. The reactive gliosis can be identified by glial fibrillary acidic protein (GFAP) immunostaining at the boundaries of the lesion in histological samples taken from wholly removed lesions. However, in some cases a direct contact between peripheral finger-like tumour protrusions and the viable hypothalamic nuclei was observed in autopsy specimens. (Fig. 4g-i) A thin layer of neural tissue corresponding to the stretched remnants of the third ventricle floor was identified on the outer border of the lesion in some macroscopic specimens (Fig. 4b). Although the gliotic plane seems to represent an appropriate cleavage plane for tumour dissection, no studies have analysed the relationship between the presence of a gliotic layer around infundibulo-tuberal CPs and the easiness of dissection. The distribution of histologic types in the 85 infundibulo-tuberal CPs diagnosed at autopsy providing this information was 59 adamantinomatous (69.4%) and 26 squamous-papillary (30.6%) lesions, a result that confirms the predominance of the adamantinomatous variety for this topography.
-
3.
Surgical: a tubero-infundibular topography for CPs has been explicitly addressed or described according to intraoperative findings in 1,372 cases from 36 surgical or neuroradiological series in the literature including a total of 2,907 CPs (Table 1). This figure represents 47.2% of CPs. Considering the age distribution of cases, the infundibulo-tuberal topography corresponds to 56% and 54.5% of paediatric and adult CPs, respectively. These lesions are usually hidden at a retrochiasmatic position within the anteroinferior portion of the third ventricle, causing the ballooning of the lamina terminalis. With an apparently tumour-free suprasellar space, the lower pole of the CP was the only part observable under the optic chiasm or through the optic-carotid triangle using a basal pterional or subfrontal approach. Tubero-infundibular CPs have been defined in some of the series included in Table 1 with diverse terms such as retrochiasmatic-intraventricular [112, 120, 149, 151, 160, 164], suprasellar-intraventricular [41, 48, 53, 125, 147, 158], or extra-intraventricular [136, 137], following alternative anatomical criteria for classification. Nevertheless, the anatomical relationships of such lesions confirmed during surgery or at necropsy, with replacement of the third ventricle floor by a mass that expands predominantly into the third ventricle, allowed their unequivocal ascription to the infundibulo-tuberal topography. Checking a breach or defect in the third floor after removing the lesion through a translamina-terminalis or transcallosal transventricular approach was the surgical finding allowing the neurosurgeon to confirm the removal of a tubero-infundibular CP (Fig. 6). With the introduction of the extended transphenoidal approach, a direct view of the CP’s lower pole, replacing the infundibulo-tuberal area, is possible during the initial stages of surgery (Fig. 6b,c). Following tumour removal through this approach, the same defect in the third ventricle floor is observed from the chiasmatic cistern, allowing illumination of the third ventricle chamber and identification of its inner structures with the aid of the endoscope.
-
4.
Neuroradiological: tubero-infundibular CPs can hardly be differentiated from strictly intraventricular lesions preoperatively because of the the impossibility of a proper identification of the limits of the third ventricle in most MRI studies. Gadolinium administration usually obscures the delimitation of the neural structures from the tumour capsule. The use of T2-weighted, heavily T2-weighted and/or 3D-FIESTA MRI sequences has proved useful for the identification and differentiation of the remnants of the third ventricle floor, pituitary stalk and tumour capsule [124]. Postoperative MRI studies after total removal of the lesion show a characteristic breached or defective floor of the third ventricle without evidence of injury extending into the hypothalamus (Fig. 5). This defect in the floor, easily observed in midsagittal and coronal MRI sequences, corresponds to the area of neural tissue that becomes replaced by the expansion of the CP and not to an iatrogenic injury.
-
5.
Radiosurgical: the use of stereotactic radiosurgery in the treatment of intraventricular CPs shows a progressive shrinkage of the tumour mass accompanied by normalization of the third ventricle’s shape. A small solid tumour remnant occupying the infundibulum and/or the tuber cinereum is displayed on long-term follow-up MRI studies following the application of this therapeutic modality, which presumably represents the epithelial nests’ original growth site within the third ventricle floor (Fig. 7). A sequence of regressive changes in the lesion induced by radiation might reverse the CP growth stages, allowing identification of the tumour’s original area of development.
-
6.
Embryological: tubero-infundibular CPs are presumably originated from nests of epithelial cells incorporated or derived from metaplasic transformation of glandular cells of the pars tuberalis of the pituitary gland [10, 49, 123, 159]. The body of evidence for this hypothesis is presented in Figs. 2 and 3. The inferior-lateral thickenings of Rathke’s pouch, precursors of the pars tuberalis, are adjacent to the area of the primitive mouth from which the craniopharyngeal duct is evaginated [10]. These paired primordia of the pars tuberalis rotate and move upwards into the primitive meningeal layer covering the floor of the diencephalic vesicle. Such an embryological course might move epithelial cells from the primitive dental ridge, the oral mucosa or the craniopharyngeal duct into a subpial position, within the floor of the third ventricle [9, 27, 49, 150]. Nests of epithelial cells, presumably originated from a metaplastic transformation of glandular cells of the adenohypophysis, have been found predominantly within the uppermost area of the pars tuberalis, which covers the infundibulum and tuber cinereum [23, 49, 68, 92].
Discussion
The concept of the infundibulo-tuberal topography for CPs: historical background
The original description of hypophyseal duct tumours or CPs was made by the Austrian pathologist Jakob Erdheim in 1904 on the basis of the analysis of a collection of suprapituitary lesions in autopsy brain specimens, proving unequivocally their intimate relationship to the substance of the third ventricle floor and the hypothalamus (Fig. 1j) [49]. Such overlooked evidence is contrary to the classical prevailing notion of a general, extra-axial suprasellar location for CPs. Many of the earliest reports on CPs displayed accurate artistic depictions of the anatomical situation of these lesions as they were observed on a view of the undersurface of the brain and on midsagittal and coronal sections made through the third ventricle. Most of these illustrations show brain specimens whose neural tissue constituting the basal components of the hypothalamus—the infundibulum and the tuber cinereum—had been replaced or had became encroached by the CP to a degree incompatible with life [11, 40, 49, 58, 73, 85, 98, 163]. This category of CPs respected the anatomical integrity of the pituitary gland and the pituitary stalk, structures easily identifiable under the lesion (Fig. 1e,h and Table 2). Many of these lesions were diagnosed in adults showing unequivocal symptoms of hypothalamic derangement, such as drowsiness, apathy, behaviour alterations, severe memory defects and gait disturbances [11, 46, 49, 85, 154]. Aware of this kind of tumour expansion within the diencephalus, some neurosurgeons considered in the following decades that these CPs should be topographically classified as hypothalamic lesions rather than as suprasellar ones (Figs. 1, 4) [13, 14, 46, 70, 99, 143]. J.H. Biggart and Norman Dott were the first authors to consider typical epidermoid suprasellar tumours—actual CPs—as lesions developing at the junction of the optic chiasm and the infundibular recess (Fig. 3g) [16]. Although hypothalamus-centered CPs were considered inoperable or not amenable to surgical dissection with the methods and techniques employed at that time, Norman Dott reported the successful removal of four such lesions placed in the substance of the third ventricle floor for which he devised specific surgical strategies to minimize the risk of hypothalamic injury [46]. The tight attachment of CPs to the hypothalamus was considered the major cause for incomplete resection and poor patient outcomes before the use of corticoid therapy and microsurgical techniques [70, 120].
Douglas Northfield in the UK and V. Grekhov in the USSR were the first authors to explicitly propose the topographical category of tuberal CPs as the subgroup of tumors with an intrapial origin which had replaced the floor and/or anterior walls of the third ventricle [59, 99]. Northfield considered CPs as epitheliomas originated from small nests of squamous cells commonly found in the pars tuberalis of the pituitary. Biggart’s concepts about CPs agreed with Northfield’s by employing the term “epidermoid tumors of the pars tuberalis” to define the lesions presumably originated from the pars tuberalis that developed a cauliflower-like papillary solid excrescence at the floor of the third ventricle [17]. This concept, scarcely mentioned in the literature, was substituted in the following decades by the categorization of infundibulo-tuberal CPs as retrochiasmatic lesions in classical classification schemes based on the position of the tumour with respect to the optic chiasm [67, 110]. In fact, these lesions were hardly visible through the standard basal approaches—subfrontal/pterional—which led to a high rate of failed surgical procedures following these surgical routes (Table 2). William Sweet was the pioneer neurosurgeon who advocated the use of the translamina terminalis approach (TLT) to remove most adult CPs with an infundibulotuberal or predominant intraventricular location [142, 143]. He observed that the breach left in the floor of the third ventricle after total removal of these lesions did not necessarily mean that injury to the hypothalamus occurred but rather the logical consequence of removing the circumferential plane of reactive gliosis developed around the lesion’s origin at the base of the third ventricle.
In the most conclusive pathological investigation on the microscopic topographical relationships of CPs performed on necropsy specimens, Juraj Steno confirmed the whole replacement of the third ventricle floor by a majority of CPs, which were classified as extra-intraventricular lesions and presented a tight band of adherence to the hypothalamus around their equators [136]. According to Steno’s observations, the central area of the tuber cinereum is usually absent and the infundibulum is usually substituted by this kind of CP [137]. Alexander Konovalov confirmed the findings reported by J. Steno and W. Sweet to further highlight the differentiation of intraventricular CPs developed from the infundibulo-tuberal area—whose total removal usually left a wide opening at the floor of the ventricle—from the much rare type of purely intraventricular lesions [78]. The category of infundibulo-tuberal or intra-third ventricle floor CPs has been confirmed in the recent CP surgical series published by Tomita et al. [147], Shi et al. [131] and Qi et al. [115]. In light of this astounding evidence, it is surprising that the classical view of CPs as lesions located in the sellar/suprasellar regions has dominated the literature.
Topographical classification of CPs involving the third ventricle: the infundibulo-tuberal or not strictly intraventricular category
A majority of CPs involve the third ventricle at the time of diagnosis; however, different manners of third ventricle involvement must be distinguished. Of special importance is differentiating between the morphological distortions of the hypothalamus caused by compression by an extra-axial lesion and the intrinsic damage to the hypothalamus caused by invasion of this region by an intraparenchymal lesion (Fig. 1) [6]. In 2004, a topographical classification scheme for CPs with a predominant or whole intraventricular position was designed according to the tumour-third ventricle relationships described in individual case reports since 1955 [106]. The objective of such a classification was to highlight the existence of two categories of CPs within the ill-defined term “intraventricular”, the strictly and the not strictly intraventricular topographies, the latter associated with a lower total excision rate and a higher poor postoperative outcome rate because of its closer anatomical relationship to the hypothalamus. Although the anatomical criteria for segregation in these two categories rested primarily on the intactness or disruption of the third ventricle floor, the essential fact supporting the usefulness of such a differentiation is the tumour’s tighter attachment to the neural tissue and worse patient outcomes observable in the group of not strictly intraventricular CPs, which represents approximately 75% of lesions developing within the third ventricle. The tight tumoural adherence is related to the lack of a leptomeningeal layer separating the neural tissue from the tumour wall, because of the subpial expansion of the lesion within the neural layer of the third ventricle floor [104, 106, 115]. The progressive growth of a CP originating within the floor of the third ventricle would cause the stretching and atrophy of this neural tissue as the infundibulo-tuberal area is progressively replaced by the mass, as has been very elegantly proved in the histological studies by Qi et al. [115] and Pan et al. [106]. At the same time, a reactive gliotic layer will develop between the mass and the hypothalamic nuclei.
The concepts of not-strictly intraventricular and infundibulo-tuberal topographical categories for CPs with a major third ventricle component can be used as equivalent, interchangeable terms. Strictly intraventricular lesions, whether pathologically or intraoperatively proven, should be considered as the special and rare subgroup of infundibulo-tuberal lesions presenting a dissectable attachment to the inner surface of the third ventricle floor [104, 106, 136]. Numerous surgical series of CPs reported in the literature consider a topographical category of lesions involving specifically the infundibulo-tuberal area of the hypothalamus (Table 1). Multiple examples of lesions disclosed at autopsies of non-operated patients are displayed in many other studies even when their authors did not consider such specific topography and defined a retrochiasmatic or a general suprasellar position for these CPs (Table 2) [66, 110]. The infundibulo-tuberal category is predominant in the most recent series of CPs diagnosed at autopsy [60, 76, 83, 136, 165]. On the basis of such a body of evidence the differentiation of a category of infundibulo-tuberal or not strictly intraventricular CP seems to be conclusive.
Embryological basis for the development of infundibulo-tuberal CPs
CP was initially considered a benign, epithelial type of lesion developed from embryologic remnants of the hypophyseal duct [49, 97]. This concept was established by the prominent Austrian pathologist Jakob Erdheim, who observed the predominant location of CPs in the pars tuberalis, precisely the region of the pituitary gland where scattered nests of squamous-epithelial cells were known to occur with the highest frequency (Fig. 3e) [49, 133]. The pars tuberalis is the slender, tongue-like upward extension of pituitary glandular tissue covering the anterolateral surface of the pituitary stalk, the infundibulum and the tuber cinereum (Fig. 2h) [10, 95]. It is constituted by a functionally distinct population of cells which have receptors for melatonine, plus a small population of gonadotrophs and other fibrous cells [63]. In 1875, von Mihalkovics described, in seminal studies on the embryological development of the pituitary gland of dogs, how the lowest edge of Rathke’s pouch, in contact with the vanishing hypophyseal duct, underwent an antero-superior rotation to become the segment of the pars tuberalis that spreads beneath the infundibulum and the tuber cinereum (Fig. 2f) [150]. According to Erdheim, this embryological rotation would account for the presence of epithelial nests within the pars tuberalis, as well as for the development of epithelial growths with a structure similar to the oral mucosa within the tubero-infundibular area, as a consequence of tumoral transformation of these epithelial cell remnants [49]. Modern embryological studies on hypophyseal development have shown that the pars tuberalis joins the diencephalic floor during the 7th week of embryo development (Carnegie stage 20, 18-22 mm) and that the hypophyseal duct is vanished at the end of the 8th week (Carnegie stages 21–23, 20-25 mm) [101, 102, 134]. For its part, the undifferentiated mesenchyma situated between the hollow vesicle derived from rotation of the Rathke’s pouch and the floor of the diencephalon, undergoes transformation into the leptomeninges surrounding the pars tuberalis and the infundibulum at later stages of development, in embryos from about 25 mm on (Fig. 3 a1-a3) [156]. Therefore, epithelial cells derived from the craniopharyngeal duct or transferred from the primitive mouth with the rotation of the Rathke’s pouch may become embedded within the neural tissue of the diencephalic floor and give rise to the development of infundibulo-tuberal CPs [27].
The identification of epithelial cell nests at the pars tuberalis of fetus and infants by Goldberg et al. [57] and of some cases of CPs developed during the embrionary period [157], besides the high rate of CPs occurring in the infundibulo-tuberal area in the studies by Tomita and Bowman [147], Shi et al. [131] and Qi et al. [115], constitute additional sources of evidence supporting the embryological theory for the origin of infundibular-tuberal lesions (Table 1). Alternatively, a metaplasic squamous transformation of glandular cells of the pars tubelis has been considered as a plausible process for the development of CPs at adult age, given the increasing number of squamous cell nests found in the pars tuberalis with age, and the predominant histological papillary structure among adult CPs involving the infundibulum and the third ventricle [23, 33, 68, 92]. Further support for this theory came from immunochemical studies demonstrating the double-labelling of CP cells for keratin and hormones [8, 139] and the presence of glandular cells within CPs [144, 145].
Ciric and Cozzens [28] proposed that the development of CPs within the tuberal area and the third ventricle could be explained if cell remnants of the craniopharyngeal duct become embedded within the neural tissue of the third ventricle floor before the formation of the leptomeningeal layer of arachnoid-pia mater (Fig. 3a). In fact, the accurate relationships of the pia mater and the pars tuberalis remains as one of the most intringuing issues not well addressed in embryological studies focused on the development of meningeal layers around the pituitary gland. Attwell [10] considered that the pars tuberalis was ensheated between two layers of pia mater: (1) the inner layer, interposed between the dorsal aspect of the pars tuberalis and the tuber cinereum, which is derived from the primitive pia mater covering the neural tube, and (2) the outer layer, which, together with the arachnoid, covers the ventral aspect of the pars tuberalis (Fig. 3b). This concept of a double pia mater covering for the pars tuberalis was initially proposed by Roussy and Mosinger in 1917 [121], then it was confirmed by Suderland [140] on human fetuses. Ultrastructural studies of the pia mater have shown that it does not represent a continuous water-tight layer isolating the cerebral surface, a role played by the interdigitated processes of the glia limitans [91]. On the other hand, the pars tuberalis is perforated by subarachnoid channels, allowing the tuberal cells to be bathed by soluble factors in the cerebrospinal fluid such as melatonine [2]. These structural properties favour the subpial expansion of epithelial nests located at the dorsal surface of the pars tuberalis into the neural tissue of the third ventricle floor.
Growing evidence for the existence of overlapping histological features within epithelial lesions of the pituitary-hypothalamic axis, supports the concept of a common origin of CPs, Rathke’s pouch cysts and supresellar epidermoid/dermoid lesions, from remnants of the primitive mouth incorporated into Rathke’s pouch [62, 159]. Depending on the original position and type of cell that gives origin to an epithelial parasellar lesion, either from embrionary cells of the dental ridge/primitive oral mucosa or from metaplasic transformation of cells of Rathke’s pouch, different types of lesions may develop [159]. Independently of the mechanism involved, CPs developing at the floor of the third ventricle should be considered as lesions of definite origin from primitive or metaplasic squamous cells placed within the pars tuberalis.
Hypothalamus impairment by infundibulo-tuberal CPs
CPs involving the third ventricle and the hypothalamus frequently associate symptoms of obesity or emaciation, sexual dystrophy and diabetes insipidus (DI). In 1929, Jonh F. Fulton and Percival Bailey described a specific clinical picture consisting of drowsiness, mental disturbances and reduced awareness caused by tumours affecting the hypothalamus and the third ventricle [54]. As early as 1930, Cushing noticed that those CPs situated at the tuber cinereum and expanding into the third ventricle caused specific metabolic and behavioural disturbances such as hypersomnia, hyperphagia with marked adiposity, personality changes and polyuria (Fig. 1e) [37–39]. By analysis of human patients and experimental hypothalamic lesions induced in animals, it was shown that many of these symptoms had a hypothalamic origin [4, 17, 21, 46, 100, 154]. For example, the isolated bilateral destruction of the ventromedial nuclear complex of the hypothalamus led to marked hyperphagia, obesity, and associated rage behaviour [117]. Other symptoms of hypothalamic damage are severe hyperthermia, marked peripheral vasoconstriction, sleep-like coma and electrolyte imbalances [22, 29]. In addition, remarkable evidence of a Korsakoff’s dementia-like disorder associated with intraventricular CPs was accumulated. Mental impairment was recorded in one-third of tuberal CPs reported by Northfield, with variable symptomatology from dullness and moderate impaired memory to frank dementia [99, 100]. Williams and Pennybacker [154] drew attention to the memory defect found in 75% of cases of CPs involving the third ventricle. In the surgical series reported by Aiba et al. [3], CPs with a tuberal involvement were also characterized by apparition of mental disturbances associated with DI after surgery. The existence of psychiatric symptoms was significantly correlated with death or a poor postoperative outcome in the clinical study by Kobayashi et al. [76]. In the series of 45 papillary CPs by Crotty et al. [33], most of them involving the third ventricle and its floor, changes in mentation occurred in 22% of patients.
In the long term, the development of progressive obesity, Froelich syndrome or dystrophia adiposogenitalis are the principal syndromes associated with radical excision of infundibulo-tuberal CPs [100, 113]. In contrast, lateral hypothalamic lesions cause syndromes characterized by hypophagia and emaciation [22]. A positive correlation between a greater hypothalamic damage on postoperative MRI studies after surgical removal of CPs and a superior postoperative patient’s body mass index (BMI) has been demonstrated by DeVile et al. [43]. Severe postoperative obesity occurs predominantly in patients who have sustained bilateral hypothalamic damage. In particular, all the subjects with marked postoperative obesity in the study by DeVile et al. [43], showed evidence of significant disruption of the normal hypothalamus anatomy, with either a complete deficiency or extensive destruction of the third ventricle floor [42, 43]. In a recent clinical study, Meuric et al. [94] observed significant behaviour disturbances, including uncontrollable hyperphagia, in the group of CPs in which the hypothalamus became unrecognizable on the preoperative MRI.
Histopathological relationships between infundibulo-tuberal CPs and the hypothalamus
The major difference between CPs developing within the third ventricle floor in comparison with other topographies is the presence, in the former category, of a layer of neural tissue and reactive gliosis around the tumour [76, 83, 99, 106, 115, 143]. In 1926, Critchley and Ironside [32] were the first authors to describe the “glial reaction in the structures surrounding the tumor” as a consistent third element found adjacent to CPs, along with the epithelial lesion and the connective tissue. This glial proliferation forms a sort of capsule composed externally of neuroglia and internally of fibrous tissue. Bailey et al. [14] emphasized that the extreme adhesiveness of the glial layer was a sign indicating that the plane of cleavage was positioned in the normal hypothalamus. Such a statement correlated well with the fact that CPs expanding within the hypothalamus usually present a wide, circumferential band of attachment to the neural structures of the base of the third ventricle—the optic chiasm, the infundibulum and the hypothalamic walls—contrary to strictly intraventricular CPs that usually show a pedicular attachment to the third ventricle floor, without accompanying reactive gliosis [46, 106]. This histological difference was demonstrated by Steno [136] in his superb autopsy study that included 14 lesions showing disruption of the third ventricle floor, and very recently it has been confirmed intraoperatively by Pan et al. [104] in a large series of intraventricular CPs. The histological arrangement of the interface between the CP and the viable hypothalamus is a feature of paramount importance for neurosurgeons, because it predicts that the cleavage gliotic plane of dissection will be circumferential, just between the equator/lower half of the lesion and the walls of the third ventricle [104, 106, 136].
Theoretical discussions about the functionality of the neural layer covering the dome of CPs growing within the third ventricle were present in the works by Pertuiset et al. [109] and Van den Bergh et al. [148], with contradictory views between those authors warning about the risks of trespassing this still viable layer through a transventricular or a translamina terminalis (TLT) approach [6], and those affirming the non-functional gliotic nature of such tissue [78, 142]. Poor outcomes in patients with CPs implanted in the floor of the third ventricle who were operated upon by Long and Chou [90] and others through a transventricular approach seemed to validate such a warning [99]. Observation of isolated islands of epithelium at the border of infundibulo-tuberal CPs, which give rise to an intense gliosis within the proper hypothalamus, corroborated Northfield’s opinion that a true fusion of the tumour and the brain render impracticable a safe, complete surgical removal of infundibulo-tuberal CPs [99]. Against that opinion was Sweet’s claim that the finger-like epithelial streamers described by some pathologists were embedded within a gliotic non-functional layer which provides a safe cleavage plane for surgical dissection of the lesion (Fig. 6a8-a9) [142, 143]. Nearly every researcher analysing the boundaries of infundibulo-tuberal CPs have observed “fingers” or “islands” of the tumour invading the brain that were considered the cause of early recurrence after gross total resection [1, 16, 71, 76, 83, 89, 111, 132, 142]. Some authors have regarded these epithelial elements penetrating the neural tissue as the major obstacle to total surgical removal [6, 55, 71, 78, 132, 158]. Others considered that the areas of apparent CP invasion into adjacent brain probably did not represent a true tumor invasion but rather the original site of CP growth within the nervous substance of the third ventricle floor [78].
In an attempt to answer the question of brain invasiveness by CPs, thorough microscopic studies of the tumor-brain interface of CPs involving the third ventricle were performed by Kubota et al. [83] and Kobayashi et al. [76]. Kubota et al. [83] described a double-layer structure in the capsule of the intraventricular, cystic component of retrochiasmatic CPs, formed by a thin inner layer of adamantinomatous or squamous epithelium and an outer layer of reactive gliosis. Although the solid, basal portion of these lesions situated at the tubero-infundibular area was usually surrounded by a thick layer of gliosis that separated the tumor 0.5–2.5 mm from the hypothalamus, at some areas no effective distance for a safe dissection occurred between the CP and the viable hypothalamic nuclei (Fig. 4g). In addition, a hypervascular layer of brain parenchyma was observed around the peritumoural gliotic tissue, with vessels of 20–200 µm in diameter that increased the risk of hypothalamic bleeding with surgical dissection. The histological analysis by Kobayashi et al. [76] of the brain-tumor interface of predominantly intraventricular CPs confirmed that the basal portion of such lesions, located within the tissue of the third ventricle floor, showed finger-shaped protrusions of solid epithelial cords invading the adjacent brain. The electron microscopy study performed by Landolt [84] on the boundaries of CPs found that in general the basal membranes of epithelial cells and reactive glia were separated by a few collagen fibres, this interface helping the surgeon to find the safe cleavage plane of dissection. However, the gliotic boundary zone was not present in all cases. Therefore, two not mutually exclusive possibilities can occur, the presence of a functionless interface of reactive gliosis or a direct contact between the tumor and the hypothalamic nuclei, even in the same lesion. Overall, these findings highlight the high risk associated with the indiscriminate attempt of total removal of tubero-infundibular CPs.
Neuroradiological differentiation of infundibulo-tuberal CPs
Limitations of neuroradiological methods used before the introduction of MRI, led authors to use the term intraventricular for many CPs actually developed within the infundibulo-tuberal area of the third ventricle floor [108]. Definition of the infundibulo-tuberal topography for CPs on the basis of the tumour-third ventricle relationships observed on MRI scans was first proposed in the classification by Raybaud et al. [116] This location has also been considered as a separate category in the recent seminal neuroradiological work made at the Burdenko Neurosurgery Institute in Moscow [82]. Rossi et al. [118] proposed a similar MRI-based vertical classification system differentiating the classical suprasellar type of CP, originating at the pituitary stalk, and the suprasellar type of CP originating in the tuber cinereum, which typically extend upwards into the hypothalamus and the third ventricle. In the latter scheme, as well as in other topographical classification systems, pure intraventricular lesions are mistakenly considered as a separate ectopic category of CPs [118, 162], but in view of the previous body of evidence, the strictly intraventricular location represents the upper position across the spectrum of anatomical locations of CPs along the hypothalamic-pituitary axis [99, 106, 136].
An accurate preoperative definition of the degree of hypothalamic involvement by a CP is of crucial importance to determine whether the lesion is amenable to surgical excision. In recent years there has been a renewed interest in the assessment of the degree of hypothalamic impairment by CPs and several clinico-radiological studies have been conducted showing a positive correlation between a greater morphological distortion of the hypothalamus on preoperative MRI and worse patient outcomes after surgery [43, 125]. Saint Rose et al. [125] differentiated between suprasellar lesions pushing the floor of the third ventricle upward without gross invasion of the hypothalamus and suprasellar/intraventricular lesions which replaced the third ventricle floor, making the hypothalamic area no longer recognizable. A breach or anatomical defect in the floor of the third ventricle was observed after complete removal of CPs in the latter group, corresponding to 42% of CPs (Figs. 5a4, b4 and 6a4, b4, c4) [125]. This defect, which is invariably present after total excision of infundibulo-tuberal CPs tightly attached to the third ventricle floor, is not usually related to iatrogenic injury caused by the neurosurgeon but rather to the elimination of the tumoural mass which had replaced the floor of the third ventricle (Fig. 6). De Vile et al. [43] have proposed a postoperative MRI-based five-grade scoring system for the degree of hypothalamic involvement by CPs, from the third ventricle floor occupation with tumour remnants (grade I) to a thinned or distorted intact floor (grade II) or a breached floor with a small (grade III), large (grade IV) or wholly deficient third ventricle floor (grade V). The major conclusion of these works has been that patients with neuroradiological signs of hypothalamic involvement by the CP, and especially those with preoperative symptoms of hypothalamus dysfunction are at high risk of suffering from long-term hypothalamic sequelae such as progressive obesity due to intractable hyperphagia, and emotional and behavioural changes [114].
Surgical approach and complications associated with infundibulo-tuberal CPs
Surgery of intraventricular CPs associates morbidity and mortality rates three-times higher than for endosellar or suprasellar CPs [106]. Direct surgical injury to the hypothalamus remains the major cause of long-term morbidity and mortality after radical excision of infundibulo-tuberal CPs, as revealed by post-mortem necropsy findings [70, 100]. Invasion of the hypothalamus represents the main limiting factor against total removal of the lesion because of the lack of an identifiable safe, non-functional cleavage plane of dissection. According to Frank et al. [50], this fact is related to the absence of a meningeal layer interposed between the lesion and the diencephalic neural structures. Only extrapial CPs would be amenable to safe and complete surgical removal in most cases [27, 28]. Unfortunately, preoperative MRI is unable to accurately define the extrapial or subpial involvement of the third ventricle floor by the CP. When the CP causes a severe distortion of the hypothalamus—type 2 and 3 in Saint Rose and Meuric MRI classification schemes—subtotal tumor resection combined with local radiation therapy seems to represent the best therapeutic option [94, 125].
Prevention of hypothalamic injury remains as the neurosurgeon’s principal concern during removal of infundibulo-tuberal CPs. Preservation of the anteroinferior walls of the third ventricle as well as the tiny hypothalamic perforating arteries is critical to avoid delayed fatal diencephalic disturbances [42, 81, 131, 137, 143, 147]. Apart from direct mechanical injury to the hypothalamus due to inadequate surgical manipulation, disturbances of regional blood flow in the hypothalamus are one of the major causes of postoperative morbidity and mortality [81]. It is likely that traumatic and/or ischemic hypothalamic damage results from the “blind” pulling of infundibulo-tuberal, tightly adhered portions of the tumour [90]. This problem can be overcome only when the infundibulo-tuberal and intraventricular portions of a CP are sufficiently exposed to be manipulated under direct view during all phases of the surgical procedure. No single supratentorial surgical approach provides a good view of the whole hypothalamus. In the case of infundibulo-tuberal CPs extending into the third ventricle the classical pterional approach would provide insufficient exposure of the anteroinferolateral walls of the third ventricle and the perforant vessels of the hypothalamus surrounding the tumour capsule [161]. This setback led Yasargil [158] and Steno et al. [137] to employ successfully the combined transcallosal-pterional approach to have direct control of the areas of tumour adhesion to the hypothalamus during the whole procedure. Alternatively, William Sweet was the pioneer author advocating the use of the TLT approach for removal of tubero-infundibular and purely intraventricular CPs [142, 143]. This approach provides a good access to the area of hypothalamic attachment of the lesion and yields excellent results after total removal of large infundibulo-tuberal CPs [75, 104, 106, 115, 131, 137, 143, 147]. A small hole, corresponding to the area of infiltration of the lesion, is left behind the chiasm at the floor of the third ventricle, as the surgical confirmation of the intratuberal location of the CP [143]. The major disadvantage of the TLT approach is the fact that tumour remnants attached at the undersurface of optic chiasm can hardly be seen and reached.
The combination of extended transsphenoidal approaches to the suprasellar region with the use of endoscopically assisted techniques has allowed a much more reliable assessment of the anatomical relationships between infundibulo-tuberal CPs and the diencephalic structures (Fig. 6b,c) [41, 50, 72, 74, 86]. Probably this kind of approach is the only suitable procedure to perform a sharp dissection of the lesion from the hypothalamus with direct control of the perforating vessels from the beginning to the end of tumour excision. Recent series of CPs including retrochiasmatic and intraventricular cases operated upon using this approach have demonstrated the replacement of the floor of the third ventricle by CPs with an infundibulo-tuberal or not strictly intraventricular topography (Fig. 6) [41, 50, 72, 74, 86]. A wide breach at the floor of the third ventricle was left after total removal of such lesions, allowing for the direct inspection of the third ventricle with an endoscope, confirming the not-strictly intraventricular location of the mass as well as its original position within the neural tissue of the tuber cinereum [41, 72, 74, 86].
Conclusions
The concept of a general suprasellar position for craniopharyngiomas is insufficient to define the accurate relationships between the vital diencephalic structures and the tumour, and to plan the most appropriate surgical route accordingly. A large body of evidence supports the differentiation of the primary infundibulo-tuberal or not strictly intraventricular type of CP which is characterized by its primary subpial development above an identifiable pituitary stalk and a predominant expansion into the third ventricle. This topographical category of CPs is highly prevalent in both the adult and child populations, although it can hardly be differentiated from the strictly or purely intraventricular topography on preoperative MRI studies. Infundibulo-tuberal CPs usually present a basal, circumferential band of tight adhesion to the hypothalamus and remnants of the third ventricle floor. This band is formed by a non-functional layer of reactive gliosis without leptomeningeal or conjunctive tissue and can be used in many cases as a safe plane of dissection, although in some lesions finger-like epithelial protrusions extending directly into the viable hypothalamus preclude any attempt of radical excision. After total removal of the lesion, a defective or breached third ventricle floor can be observed both intraoperatively and on postoperative MRI brain scans. A deficient third ventricle floor does not invariably mean an iatrogenic injury, but rather a consequence of removal of a lesion which replaced the floor itself. The endoscopic extended transsphenoidal approach allows an accurate anatomical definition of infundibulo-tuberal CPs and a non-traumatic, sharp dissection of these lesions off the hypothalamus.
References
Adamson TE, Wiestler OD, Kleihues P, Yargil MG (1990) Correlation of clinical and pathological features in surgically treated craniopharyngiomas. J Neurosurg 73:12–17
Aguado LI, Schoebitz K, Rodriguez EM (1981) Intercellular channels in the pars tuberalis of the rat hypophysis and their relationship to the subarachnoid space. Cell Tissue Res 218:345–354
Aiba T, Yamada S (1980) Surgery of craniopharyngiomas in adults. Neurol Med Chir (Tokyo) 20:439–451
Alpers BJ (1940) Personality and emotional disorders associated with hypothalamic lesions. Psychosomatic Med 2:286–303
Allegranza A (1995) Neuropathology of craniopharyngioma. In: Broggi G (ed) Craniopharyngioma. Surgical treatment. Milano, Italy, Springer, pp 1–5
Apuzzo MLJ, Zee CS, Breeze RE (1987) Anterior and mid-thirdventricular lesions: surgical overview. In: Apuzzo MLJ (ed) Surgery of the third ventricle. Williams and Wilkins, Baltimore, pp 495–542
Armstrong CN (1924) Three cases of supra-pituitary tumour presenting Frölich syndrome. Brain 45:113–125
Asa SL, Kovacs K, Bilbao JM (1983) The pars tuberalis of the human pituitary. A histologic, immunohistochemical, ultrastructural and immunoelectron microscopic analysis. Virchows Arch 399:49–59
Attwell WJ (1918) The development of the hypophysis cerebri of the rabbit (Lepus Cunilicus L.). Am J Anat 24:271–337
Attwell WJ (1926) The development of the hypophysis cerebri in man, with special reference to the pars tuberalis. Am J Anat 37:159–193
Babinsky MJ (1900) Tumeur du corps pituitaire sans acromégalie et avec arrèt de développement des organs génitaux. Rev Neurol 8:531–533
Bailey P (1924) Concerning the cerebellar symptoms produced by suprasellar tumors. Arch Neurol Psychiatr 11:137–150
Bailey P (1933) Craniopharyngiomas. Syndrome of the Hypothalamus. In: Bailey P (ed) Intracranial Tumors. Charles H Thomas, Springfield, pp 113–137
Bailey P, Buchanan DN, Bucy PC (1939) Intracranial tumors of infancy and childhood. University of Chicago Press, Chicago, pp 349–375
Beckmann JW, Kubie LS (1929) A clinical study of twenty-one cases of tumors of the hypophyseal stalk. Brain 52:127–170
Biggart JH, Dott NM (1936) Pituitary tumors: their classification and treatment. Br Med J 2(3961):1153–1155; 2(3962):1206–1208
Biggart JH (1961) Pathology of the nervous system. A student’s introduction, 3rd edn. Williams and Wilkins, Baltimore, pp 322–327
Blackburn IW (1903) Intracranial tumors among the insane. A study of twenty-nine intracranial tumors found in sixteen hundred and forty-two autopsies in cases of mental disease. Government Printing Office, Washington
Burger PC (1994) Craniopharyngiomas. In: Atlas of tumor pathology, 3rd series, fascicle 10. Tumors of the central nervous system. Armed Forces Institute of Pathology,Washington, pp 349–354
Burger PC (2002) Craniopharyngiomas. In: Burger PC, Scheithauer B, Vogel FS (eds) Surgical pathology of the nervous system and its coverings, 4th edn. Churchill Livingstone, New York, pp 475–482
Cairns H (1952) Disturbances of consciousness with lesions of the brain-stem and diencephalon. Brain 75:109–146
Carmel W (1980) Surgical syndromes of the hypothalamus. Clin Neurosurg 27:133–159
Carmichael HT (1931) Squamous epithelial rests in the hypophysis cerebri. Arch Neurol Psychiatr 26:966–975
Choux M, Lena G, Genitori L (1991) Le craniopharyngiome de l’enfant. Neurochirurgie 37(Suppl 1):7–10
Choux M, Lena G (1998) Craniopharyngioma. In: Apuzzo MLJ (ed) Surgery of the third ventricle, 2nd edn. Williams and Wilkins, Baltimore, pp 1143–1181
Chung W-Y, Pan DH-C, Shiau C-Y, Guo W-Y, Wang L-W (2000) Gamma Knife radiosurgery for craniopharyngiomas. J Neurosurg 93(Suppl 3):47–56
Ciric IS (1987) Regional embryology. In: Apuzzo MLJ (ed) Surgery of the third ventricle. Williams and Wilkins, Baltimore, pp 167–174
Ciric IS, Cozzens JW (1980) Craniopharyngiomas: transsphenoidal method of approach—for the virtuoso only? Clin Neurosurg 27:169–187
Clar HE (1985) Disturbances of the hypothalamic thermoregulation. Acta Neurochir 75:106–112
Cohen-Gadol AA, Spencer DD (2007) The legacy of Harvey Cushing: profiles of patient care. New York, Thieme, pp 1–93
Cohen-Gadol AA, Geryk B, Binder DK, Tubbs RS (2009) Conquering the third ventricular chamber. J Neurosurg 111:590–599
Critchley M, Ironside RN (1926) The pituitary adamantinomata. Brain 49:437–481
Crotty TB, Scheithauer BW, Young WF Jr, Davis DH, Shaw EG, Miller GM et al (1995) Papillary craniopharyngioma: a clinicopathological study of 48 cases. J Neurosurg 83:206–214
Cuneo HM, Rand CW (1952) Brain tumors of childhood. Charles C Thomas Springfield, pp 43–57
Cushing H (1912) The pituitary body and its disorders. JB Lippincott, Philadelphia, pp 289–291
Cushing H (1922) Les syndromes hypophysaires au point de vue chirurgical. Rev Neurol 38:779–808
Cushing H (1930) The chiasmal syndrome of primary optic atrophy and bitemporal field defects in adults with a normal sella turcica. Arch Ophthalmol 3:505–551
Cushing H (1932) Intracranial tumors. Notes upon a series of two thousand verified cases with surgical mortality percentages pertaining thereto. Charles C. Thomas, Springfield, pp 93–98
Cushing H (1932) Papers relating to the pituitary body. Hypothalamus and parasympathetic nervous system. Charles C Thomas, Springfield, pp 43–56
Davison C, Demuth EL (1946) Disturbances in sleep mechanism. A clinicopathologic study Arch Neurol Psychiatry 55:111–125
de Divitiis E, Cappabianca P, Cavallo LM, Esposito F, de Divitiis O, Messina A (2007) Extended endoscopic transsphenoidal approach for extrasellar craniopharyngiomas. Neurosurgery 61 (ONS Suppl 2):ONS219–ONS228
De Vile CJ, Grant DB, Kendall BE, Neville BGR, Stanhope R, Watkins KE, Hayward RD (1996) Management of childhood craniopharyngioma: can the morbidity of radical surgery be predicted? J Neurosurg 85:73–81
De Vile CJ, Grant DB, Hayward RD, Kendall BE, Neville BGR, Stanhope R (1996) Obesity in childhood craniopharyngioma: relation to post-operative hypothalamic damage shown by magnetic resonance imaging. J Clin Endocrinol 81:2734–2737
Del Vivo RE, Armenise B, Regli F (1962) Varietà formali e problema istogenetici del craniofaringioma. Studio istopatologico su 30 observazioni. Arch de Vecchi Anat Pathol 38:1–79
Dialti G (1910) Patologia e Chirurgia della Ipofisi. Siena, Tipografia Editrice S. Bernardino, pp 20–24
Dott NM (1938) Surgical aspects of the hypothalamus. In: Clarke WE, Beattie J, Riddoch G, Dott NM (eds) the hypothalamus. Oliver and Boyd, Edinburgh, pp 131–199
Duffy WC (1920) Hypophyseal duct tumors. Ann Surg 72(537–555):725–757
Eldevik OP, Blaivas M, Gabrielsen TO, Hald JK, Chandler WF (1996) Craniopharyngioma: radiologic and histologic findings and recurrence. AJNR Am J Neuroradiol 17:1427–1439
Erdheim J (1904) (1904) Über Hypophysengangsgeschwulste und Hirmcholesteatome. Sitzungsb Kais Akad Wissen Math Naturw Klin 113:537–726
Frank G, Pasquini E, Doglietto F, Mazzatenta D, Sciaretta V, Farneti G, Calbucci F (2006) The endoscopic extended transsphenoidal approach for craniopharyngiomas. Oper Neurosurg 59:75–83
Frazier CH, Alpers BJ (1931) Adamantinoma of the craniopharyngeal duct. Arch Neurol Psychiatr 26:905–965
Frazier CH (1936) A review, clinical and pathological, of parahypophyseal lesions. Surg Gynec Obst 62:1–33
Fukushima T, Yamakawa K (1989) Surgical management of craniopharyngiomas. Comparison of results by different operative approaches. In: Holtzman RNN, Stein BM (eds) Surgery of the diencephalon. Plenum, New York, pp 259–269
Fulton JF, Bailey P (1929) Tumors in the region of the third ventricle: their diagnosis and relation to pathological sleep. J Nerv Mental Dis 69:1–25, 145–164, 261–277
Ghatak NR, Hirano A, Zimmerman HM (1978) Ultrastructure of a craniopharyngioma. Cancer 27:425–430
Globus JH, Gang KM (1945) Craniopharyngeoma and suprasellar adamantinoma. J Mt Sinai Hosp 12:220–276
Goldberg GM, Eshbaugh DE (1960) Squamous cell nests of the pituitary gland as related to the origin of craniopharyngiomas. Arch Pathol 70:293–299
Goldstein K (1928) Kasuistiche Mitteilungen zur Klinik und pathologischen anatomie der nervenkrankheiten. I. Cohn H, Goldstein K: Zur klinik und pathologie hypophysärer erkrankungen, im besonderen der tumoren der hypophysengegend. Deutsch Zeitschr Nervenheilkund 103:225–274
Grekhov VV (1959) Topography of craniopharyngioma. Vopr Neirokhir 23:12–17
Grekhov VV (1959) Structure of parenchyma of craniopharyngiomas. Vopr Neirokhir 23:1–5
Harbitz F (1935) Tumors arising from the hypophysial duct, and other neoplasms related thereto. Chordomas Acta Pathol Microbiol Scand 12:38–78
Harrison MJ, Morgello S, Post KD (1994) Epithelial cystic lesions of the sellar and parasellar region: a continuum of ectodermal derivatives? J Neurosurg 80:1018–1025
Hazlerigg DG (2001) What is the role of melatonin within the anterior pituitary? J Endocrinol 170:493–501
Henke F, Lubarsch O (1926) Handbuch der Speziellen Pathologischen Anatomie und Histologie, Bd 8: Drusen mit innerer secretion. Springer, Berlin
Hirsch O, Hamlin H (1959) Symptomatology and treatment of the hypophyseal duct tumors (craniopharyngiomas). Confin Neurol 19:153–219
Hoffman HJ, Hendrick EB, Humphreys RP, Buncic JR, Armstrong DL, Jenkin RDT (1977) Management of craniopharyngioma in children. J Neurosurg 47:218–227
Hoffman HJ, De Silva M, Humphreys RP, Drake JM, Smith ML, Blaser SI (1992) Agressive surgical management of craniopharyngiomas in children. J Neurosurg 76:47–52
Hunter IJ (1955) Squamous metaplasia of cells of the anterior pituitary gland. J Path Bact 69:141–145
Ingraham FD, Scott HW (1946) Craniopharyngiomas in children. J Pediatrics 29:95–116
Kahn EA (1955) Tumors of the sellar region. In: Kahn EA, Bassett RC, Schneider RC, Crosby EC (eds) Correlative neurosurgery. Charles C Thomas, Springfield, pp 174–181
Kahn EA, Gosch HH, Seeger J, Hicks JF (1973) Forty-five years experience with craniopharyngiomas. Surg Neurol 1:5–12
Kassam AB, Gardner PA, Snyderman CH, Carrau RL, Mintz AH, Prevedello DM (2008) Expanded endonasal approach, a fully endoscopic transnasal approach for the resection of midline suprasellar craniopharyngiomas: a new classification based on the infundibulum. J Neurosurg 108:715–728
Kaufmann E (1929) Lehrbuch der Pathologischen Anatomie. Translated by SP Reinmann SP, vol III. Blackiston, Philadelphia
Kim EH, Ahn JY, Kim SH (2011) (2011) Technique and outcome of endoscopy-assisted microscopic extended transsphenoidal surgery for suprasellar craniopharyngiomas. J Neurosurg 114:1338–1349
King TT (1979) Removal of intraventricular craniopharyngiomas through the lamina terminalis. Acta Neurochir (Wien) 45:277–286
Kobayashi T, Kageyama N, Yoshida J, Shibuya N, Yonezawa T (1981) Pathological and clinical basis of the indications for treatment of craniopharyngiomas. Neurol Med Chir (Tokyo) 21:39–47
Kobayashi T, Tanaka T, Kida Y (1994) Stereotactic gamma radiosurgery of craniopharyngiomas. Pediatr Neurosurg 21(Suppl 1):69–74
Konovalov AN (1981) Operative management of craniopharyngiomas. Adv Tech Stand Neurosurg 8:281–318
Konovalov AN, Goralyshev SK (1992) Surgical treatment of anterior third ventricle tumours. Acta Neurochir 118:33–39
Konovalov AN (1993) Craniopharyngioma: complications and their avoidance. In: Apuzzo MLJ (ed) Brain surgery: complications avoidance and management. Churchill Livingstone, New York, pp 362–368
Konovalov AN (1998) Technique and strategies of direct surgical management of craniopharyngiomas. In: Apuzzo MLJ (ed) Surgery of the third ventricle, 2nd edn. Williams and Wilkins, Baltimore, pp 1133–1142
Kornienko VN, Pronin IN (2009) Diagnostic neuroradiology. Springer, Berlin, pp 558–573
Kubota T, Yamamoto S, Kohno H, Ito H, Hayashi M (1980) Operative procedures of craniopharyngiomas estimated by autopsy findings. Neurol Med Chir (Tokyo) 20:341–354
Landolt A (1972) Die ultrastruktur des kraniopharyngeoms. Schweiz Arch Neurol Neurochir Psychiatry 111:313–329
Langer F (1892) Über cystiche tumoren im bereiche des infundibulum cerebri. Zeitschr f Heilkunde 13:57–68
Laufer I, Anand VK, Schwartz TH (2007) Endoscopic, endonasal extended transsphenoidal, transplanum transtuberculum approach for resection of suprasellar lesions. J Neurosurg 106:400–406
Lee Y-Y, Wong T-T, Fang Y-T, Chang K-P, Chen Y-W, Niu D-M (2008) Comparison of hypothalamopituitary axis of dysfunction of intrasellar and third ventricular craniopharyngiomas in children. Brain & Development 30:189–194
Lena G, Paredes AP, Scavarda D, Giusiano B (2005) Craniopharyngioma in children: Marseille experience. Childs Nerv Syst 21:778–784
Liszczak T, Richardson EP, Phillips JP, Jacobson S, Kornblith PL (1978) Morphological, biochemical, ultrastructural, tissue culture and clinical observations of typical and aggressive craniopharyngiomas. Acta Neuropathol (Berl) 43:191–203
Long DM, Chou SN (1973) Transcallosal removal of craniopharyngiomas within the third ventricle. J Neurosurg 39:563–567
Lopes CAS, Mair WGP (1974) Ultrastructure of the outer cortex and the pia mater in man. Acta Neuropathol (Berl) 28:79–86
Luse SA, Kernohan JW (1955) Squamous-cell nests of the pituitary gland. Cancer 8:623–628
Malamud N (1967) Psychiatric disorder with intracranial tumors of limbic system. Arch Neurol 17:113–123
Meuric S, Brauner R, Trivin C, Souberbielle J-C, Zerah M, Sainte-Rose C (2005) Influence of tumor location on the presentation and evolution of craniopharyngiomas. J Neurosurg 103(5 Suppl Pediatr):421–426
Morgan PJ, Williams LM (1996) The pars tuberalis of the pituitary: a Gateway for neuroendocrine output. Rev Reprod 1:153–161
Mortini P, Losa M, Pozzobon G, Barzaghi R, Riva M, Acerno S, Angius D, Weber G, Chiumello G, Giovanelli M (2011) Neurosurgical treatment of craniopharyngioma in adults and children: early and long-term results in a large case series. J Neurosurg 114:1350–1359
Mott FW, Barratt JOW (1899) Three cases of tumor of the third ventricle. Arch Neurol Psychiatry 1:417–439
Newmark L (1917) A case of infundibular tumor in a child. Arch Int Med 19:550–563
Northfield DWC (1957) Rathke-pouch tumours. Brain 80:293–312
Northfield DWC (1973) The surgery of the central nervous system: a textbook for postgraduate students. Blackwell, London, pp 280–330
O’Rahilly R, Müller F (1986) The meninges in human development. J Neuropathol Exp Neurol 45:588–608
O’Rahilly RR, Miller F (2001) Human embryology and teratology. Wiley-Liss, New York
Orthner H, Rettinger E (1965) An intraventricular craniopharyngioma, with a contribution to a hypothalamically induced Korsakoff syndrome. Comparative review of the hypothalamically inducible disorders of the memory and instinctual life in humans and animals Fortschr Neurol Psychiatr Grenzgeb 33:299–331
Pan J, Qi S, Lu Y, Fan J, Zhang X, Zhou J, Pen J (2011) Intraventricular craniopharyngioma: morphological analysis and outcome evaluation of 17 cases. Acta Neurochir (Wien) 153:773–784
Pang D (2005) Craniopharyngiomas. In: Rengachary SS, Ellenbogen RG (eds) Principles of neurosurgery, 2nd edn. Elsevier Mosby, Edinburgh, pp 621–646
Pascual JM, González-Llanos F, Barrios L, Roda JM (2004) Intraventricular craniopharyngiomas: topographical classification and surgical approach selection based on an extensive overview. Acta Neurochir (Wien) 146:785–802
Pascual JM, Carrasco R, Prieto R, Gonzalez-Llanos F, Alvarez F, Roda JM (2008) Craniopharyngioma classification. J Neurosurg 109:1180–1183, Letter
Pascual JM, Prieto R, Navas M, Carrasco R (2010) Conquest of third ventricle craniopharyngiomas. J Neurosurg 112:1156–1161, Letter
Pertuiset B, Janny P, Allègre G, Olivier L (1962) Cranio-pharyngiomes simulant une tumeur antérieure du III ventricule. Presse Med 70:1846–1848
Pertuiset B (1975) Craniopharyngiomas. In: Vinken PJ, Bruin GW (eds) Handbook of clinical neurology, vol 19. Part II: Tumors of the brain and skull. North Holland, Amsterdam, pp 531–572
Petito CK, DeGirolami U, Earle KM (1976) Craniopharyngiomas: a clinical and pathological review. Cancer 37:1944–1952
Pierre-Kahn A, Brauner R, Renier D, Saint-Rose C, Gangemi MA, Rappaport R, Hirsch JF (1988) Traitement des craniopharyngiomes de l’enfant. Arch Fr Pediatr 45:163–167
Poretti A, Grotzer MA, Ribi K, Schonle E, Boltshauser E (2004) Outcome of craniopharyngioma in children: long term complications and quality of life. Dev Med Child Neurol 46:220–229
Puget S, Garnett M, Wray A, Grill J, Habrand JL, Bodaert N, Zerah M, Bezerra M, Renier D, Pierre-Kahn A, Sainte-Rose C (2007) Pediatric craniopharyngiomas: classification and treatment according to the degree of hypothalamic involvement. J Neurosurg 106(1 Suppl):3–12
Qi S, Lu Y, Pan J, Zhang X, Long H, Fan J (2011) Anatomic relations of the arachnoidea around the pituitary stalk: relevance for surgical removal of craniopharyngiomas Acta Neurochir (Wien) 153:785–96
Raybaud C, Rabehanta P, Girard N (1991) Aspects radiologiques des craniopharyngiomas. Neurochirurgie 37(Suppl 1):44–58
Reeves AG, Plum F (1969) Hyperphagia, rage and dementia accompanying a ventromedial hypothalamic neoplasm. Arch Neurol 20:616–624
Rossi A, Cama A, Consales A, Gandolfo C, Garrè ML, Milanaccio C, Pavanello M, Piatelli G, Ravegnani M, Tortori Donati P (2006) Neuroimaging of pediatric craniopharyngiomas: a pictorial essay. J Pediatr Endocrinol Metab 19(Suppl 1):299–319
Rougerie J, Fardeau M (1962) Les Cranio-pharyngiomes. Masson, Paris
Rougerie J (1979) What can be expected from surgical treatment of cranioparyngiomas in children? Childs Brain 5:433–449
Roussy G, Mossinger M (1934) Les méninges périhypophysaires; leurs rapports avec l’hypophyse. Rev Neurol 1:568–570
Rouvière H, Giroud A, Desclaux P, Roux P (1947) Le développement du lobe antérieur de l’hypophyse et ses consequences physio-pathologiques. Ann Anat Path 17:113–124
Rushing EJ, Giangaspero F, Paulus W, Burger PC (2007) Craniopharyngioma. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK (eds) WHO classification of tumors of the central nervous system, 4th edn. International Agency for Research of Cancer, Lyon, pp 238–240
Saeki N, Murai H, Kubota M, Fujimoto N, Iuchi T, Yamaura A, Sunami K (2002) Heavily T2 weighted MR images of anterior optic pathways in patients with sellar and parasellar tumours-prediction of surgical anatomy. Acta Neurochir 144: 25–35
Saint Rose C, Puget S, Wray A, Zerah M, Grill J, Brauner R, Boddaert N, Pierre-Khan A (2005) Craniopharyngioma: the pendulum of surgical management. Childs Nerv Syst 21:691–695
Samii M, Tatagiba M (1995) Craniopharyngioma. In: Kaye AH, Laws ER Jr (eds) Brain tumors. An encyclopedic approach. Churchill Livingstone, New York
Sartoretti-Schefer S, Wichmann W, Aguzzi A, Valavanis A (1997) MR Differentiation of adamantinomatous and squamous-papillary craniopharyngiomas. AJNR 18:77–87
Schäfer EA (1922) Discussion on disease of the pituitary body. The structure and functions of the pituitary. In: Biedl A Physiologie und Pathologie der hypophyse. von JF Bergmann, München Wiesbaden, pp 1–20
Schilder P, Weissmann M (1927) Amente psychose bei hypophysengangtumor. Ztschr Neurol Psychiatr 110:767–778
Schmidt B, Gherardi R, Poirier J, Caron JP (1984) Craniopharyngiome pediculè du troisième ventricule. Rev Neurol 140:281–283
Shi XE, Wu B, Zhou ZG, Fan T, Zhang YL (2006) Microsurgical treatment of craniopharyngiomas: report of 284 patients. Chin Med J 119:1653–1663
Shillito J (1980) Craniopharyngiomas: the subfrontal approach or none at all? Clin Neurosurg 27:188–205
Simonds JP (1922) The pathological anatomy and histology of the hypophysis. In: Barker LF (ed): Endocrinology and metabolism, vol 1. D. Appleton, New York, p 799
Solov’ev GS, Bogdanov AV, Panteleev SM, Yanin VL (2008) Embryonic morphogenesis of the human pituitary. Neurosci Behav Physiol 38:829–833
Steiner L, Payne BR, Prasad D, Lindquist C, Steiner M (2000) Gamma surgery in cerebral vascular lesions, malformations, tumors, and functional disorders of the nervous system. In: Schmidek HH, Sweet WH (eds) Operative neurosurgical techniques, indications, methods and results, 4th edn. WB Saunders, Philadelphia, pp 670–706
Steno J (1985) Microsurgical topography of craniopharyngiomas. Acta Neurochir (Suppl) 35:94–100
Steno J, Malácek M, Bízik I (2004) Tumor-third ventricular relationships in supradiaphragmatic craniopharyngiomas: correlation on morphological, magnetic resonance imaging, and operative findings. Neurosurgery 54:1051–1060
Strada F (1911) Beitrage zur Kenntniss der Gerschwulste der hypophyse und der hypophysengegend. Virchows Arch f Path Anat 203:1–65
Sumi T, Stefeneanu L, Kovacs K (1993) Squamous-cell nests in the pars tuberalis of the human pituitary: immunocytochemical and in situ hybridization studies. Endocrine Pathol 4:155–161
Sunderland S (1945) The meningeal relations of the human hypophysis cerebri. J Anat 79:33–37
Svolos DG (1969) Craniopharyngiomas. A study based on 108 verified cases. Acta Chir Scand Suppl 403:8–44
Sweet WH (1976) Radical surgical treatment of craniopharyngioma. Clin Neurosurg 23:52–79
Sweet WH (1988) Craniopharyngiomas, with a note on Rathke’s cleft or epithelial cyst and on suprasellar cysts. In: Schmideck HH, Sweet WH (eds) Operative neurosurgical techniques, indications, methods and results, 2nd edn. Grune and Stratton, New York, pp 349–379
Szeifert GT, Pasztor E (1993) Could craniopharyngiomas produce pituitary hormones? Neurol Res 15:68–69
Tachibana O, Yamashima T, Yamashita J, Takabatake Y (1994) Immunohistochemical expression of human chorionic gonadotropin and P-glycoprotein in human pituitary glands and craniopharyngiomas. J Neurosurg 80:79–84
Thapar K, Kovacs K (1998) Neoplasms of the sellar region. In: Bigner DD, McLendon RE, Bruner JM (eds): Russell and Rubenstein’s Pathology of tumors of the nervous system, 6th edn, vol 2. Edward Arnold, London, pp 561–680
Tomita T, Bowman RM (2005) Craniopharyngiomas in children: surgical experience at children’s memorial hospital. Childs Nerv Syst 21:729–746
Van Den Bergh R, Brucher JM (1970) (1970) L’abord transventriculaire dans les craniopharyngiomes du troisième ventricule. Aspects neuro-chirurgicaux et neuropathologiques Neurochirurgie 16:51–65
Van Effenterre R, Boch AL (2002) Craniopharyngioma in adults and children: a study of 122 surgical cases. J Neurosurg 97:3–11
Von Mihalkovics V (1875) Wirbelsaite und Hirnanhang. Arch f Mikr Anat 11:389–441
Wang K-C, Kim S-K, Choe G, Chi JG, Cho B-K (2002) Growth patterns of craniopharyngioma in children: role of the diaphragm sellae and its surgical implication. Surg Neurol 57:25–33
Weiner HL, Wisoff JH, Rosenberg ME, Kupersmith MJ, Cohen H, Zagzag D, Shiminski-Maher T, Flamm ES, Epstein FJ, Miller DC (1994) Craniopharyngiomas: a clinicopathological analysis of factors predictive of recurrence and functional outcome. Neurosurgery 35:1001–1011
Wheatley T, Clark JDA, Stewart S (1986) Craniopharyngioma with hyperprolactinaemia due to a prolactinoma. J Neurol Neurosurg Psychiatr 49:1305–1307
Williams M, Pennybacker J (1954) Memory disturbances in third ventricle tumours. J Neurol Neurosurg Psychiat 17:115–123
Wislocki GB (1936) The meningeal relations of the hypophysis cerebri. I The relations in adult mammals Anat Rec 67:273–293
Wislocki GB (1937) The meningeal relationships of the hypophysis cerebri. II An embryological study of the meninges and blood vessels of the human hypophysis Am J Anat 61:95–125
Yamada H, Haratake J, Narasaki T, Oda T (1995) Embryonal craniopharyngioma. Case report of the morphogenesis of a craniopharyngioma Cancer 75:2971–2977
Yasargil MG (1996) Craniopharyngioma. In: Yasargil MG (ed) Microneurosurgery, vol IV-B: microneurosurgery of CNS tumors. Georg Thieme, Stuttgart New York, pp 205–223
Zada G, Lin N, Ojerholm E, Ramkissoon S, Laws ER (2010) Craniopharyngioma and other cystic epithelial lesions of the sellar region: a review of clinical, imaging, and histopathological relationships. Neurosurg Focus 28:E4
Zhang YQ, Wang CC, Ma ZY (2002) Pediatric craniopharyngiomas: clinicomorphological study of 189 cases. Pediatr Neurosurg 36:80–84
Zhang YQ, Ma ZY, Wu ZB, Luo SQ, Wang ZC (2008) Radical resection of 202 pediatric craniopharyngiomas with special reference to the surgical approaches and hypothalamic protection. Pediatr Neurosurg 44:435–443
Zhou L, You C (2009) Craniopharyngioma classification. J Neurosurg 111:197–199, Letter
Ziegler E (1902) Lehrbuch der Speziellen Pathologische Anatomie. Von Gustav Fischer, Jena, p 403
Zuccaro G (2005) Radical resection of craniopharyngioma. Childs Nerv Syst 21:679–690
Zülch KJ (1975) Atlas of gross neurosurgical pathology. Springer, New York, pp 155–160
Zülch KJ (1986) Brain tumors. Their biology and pathology, 3rd edn. Springer, Berlin, pp 426–432
Acknowledgements
We especially thank Crystal Smith, Reference Librarian of the History of Medicine Department of the National Library of Medicine (NLM) at the National Intitutes of Health, Bethesda, MD, USA for her kind guidance and assistance at the National Library of Medicine during our process of search and review of the articles and monographs used in this study. We are also indebted to Lucretia MacLure and all the staff at The Francis Countway Medical Library at Harvard Medical School, Boston, MA, USA for their invaluable help in obtaining a considerable amount of the original research material used for this study. Finally, we want to thank George Hamilton for his critical review of language and style of the manuscript.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Comment
This substantial piece of work makes a compelling case for a new classification of craniopharyngiomas. Whereas the “Not strictly intraventricular” name does not quite slip off the tongue, the concept will give those working in the field, particularly those adept in the extended endoscopic transsphenoidal procedure, some help in deciding on the radicality of their resection. Also to add a word of caution, as things stand, radiation techniques do continue to have a very major place in the management of these tumours. There is little mention of the role of this therapy here.
Michael Powell
London, UK
Rights and permissions
About this article
Cite this article
Pascual, J.M., Prieto, R. & Carrasco, R. Infundibulo-tuberal or not strictly intraventricular craniopharyngioma: evidence for a major topographical category. Acta Neurochir 153, 2403–2426 (2011). https://doi.org/10.1007/s00701-011-1149-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-011-1149-4