Abstract
Background
Specific microanatomical characteristics of the trigeminal nerve root (TNR) blood supply and close neurovascular relationships with surrounding vessels as well as their possible clinical significance were the main reasons for this study.
Method
The vasculature of 25 adult and four fetal TNRs were microdissected and examined under the stereoscopic microscope, after injecting their arteries with India ink.
Results
The trigeminal vessels, which varied between two and five in number, arose from two or three of the following arteries: the superolateral pontine (92%), anterior inferior cerebellar (AICA) (88%), inferolateral pontine (72%), and superior cerebellar (SCA) (12%). The trigeminal vascular twigs had a mean diameter of 0.215 mm. A single vessel may supply either the motor portion of the nerve root or the sensory portion or both. The trigeminal vasculature formed the proximal and distal rings. The proximal ring was located at the trigeminal root entry zone. Its central branches extended along the TNR to the principal sensory and motor trigeminal nuclei while its peripheral longitudinal twigs followed the TNR fascicles. The incomplete distal arterial ring embraced the middle portion of the TNR before the level of its entrance into the arachnoid sleeve. The most frequent contact of the TNR was noticed with the SCA (20%), the petrosal or Dandy’s vein (24%), and the AICA (12%).
Conclusions
The observed characteristics of the TNR vasculature could be the anatomical basis for decompressive neurovascular surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Only a few articles have been devoted to the blood supply and neurovascular relations of the trigeminal nerve root (TNR) itself [5–7, 12, 15]. We still do not know the precise vascular pattern of the TNR, blood supply, and neurovascular relationships, which are important for neurologists, neuroradiologists, and neurosurgeons. Insufficiency of relevant anatomic data and great neurological and neurosurgical significance were the reasons for this study with scientific and practical implications. Therefore, the purpose of this research was to obtain the data about the microanatomical characteristics of the supply and vascular relations of the TNR that also could have a role in the pathophysiology of trigeminal neuralgia.
Materials and methods
For this microanatomical study, we used 13 adult human brains (aged 46–74) of individuals in which the cause of death lay outside the head. After perfusion of the vertebrobasilar and carotid arterial systems with isotonic saline solution, 10% mixture of India ink and gelatin was injected. The brain specimens were then fixed in 4% formaldehyde solution for 3 weeks. Only one TNR was not injected properly and finally the 25 well-prepared specimens were examined. The trigeminal blood vessels were microdissected and examined under the stereoscopic microscope, and precise drawings and measurements of the vessels were made by the ocular micrometer.
Longitudinal sections of one TNR, together with the adjacent part of the transversely sectioned pons (1 mm thick), were cleared by the Spalteholtz technique and observed under transmitted light. One TNR was sectioned longitudinally, serially in 5-μm thick slices, to show the central myelin–peripheral myelin transitional zone following the immune reaction against the glial fibrillary acidic protein in the astrocytes, and against the myelin basic protein in both the central and the peripheral myelin. In this study, we also used two heads of nine-lunar-month-old fetuses, injected with India ink and gelatin and then fixed in 4% formalin solution, in order to perform the finest microdissection of the cerebellopontine cistern elements in situ. The main advantage of this technique is that it enables an easy distinction between the vessel types, a clear definition of the anastomoses and, finally, the following of each vessel to its smallest rami, thus permitting a detailed description of its territory of distribution.
Results
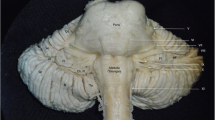
The TNR extends from the ventrolateral surface of the pons (Fig. 1) to the dural entrance into the Meckel’s cave where the trigeminal ganglion is situated. The TNR, which is located in the cerebellopontine cistern, is composed of a large sensory and a small motor root. Both roots are surrounded and supplied by the trigeminal arteries, which may originate from the basilar artery (BA) and its side branches: the superolateral and inferolateral pontine arteries (i.e., the two long circumferential pontine arteries), the anterior inferior cerebellar artery (AICA), and the superior cerebellar artery (SCA) (Figs. 1 and 2).
Ventral (inferior) view of the pons and arteries that may supply the trigeminal nerve root—TNR (1, left and 1’, right). 2, left SCA (arrow, the large loop of the lateral branch of the SCA); 2’, right SCA; 3, left superolateral pontine artery (small arrows, the distal, incomplete arterial ring); 3’, right superolateral pontine artery (small arrows, the distal arterial ring); 4, left and 4’, right inferolateral pontine artery; 5, left AICA; 5’, two right AICAs; 6, left petrosal vein; 6’, right petrosal vein; 7, left and 7’, right vertebral artery; 8, basilar artery
The superolateral pontine artery (SPA) was the most frequent source of the trigeminal root supply (Figs. 1, 2, and 3). Originating as a separate side branch of the BA and only in one case by a common stem with the inferolateral pontine artery (Fig. 4), it approached from above the ventral side of the TNR. It took part in the supplying of 23 (92%) of the nerve roots, always as a single one, by giving off one to three trigeminal arteries with their diameters ranging from 110 to 320 μm (mean, 230 μm) (Table 1). In rare cases, i.e., in two (8%), we found an unusual position of the terminal part of the SPA. Instead of ending on the TNR, it continued between the motor and sensory root fascicles and encircled the nerve root from below, toward the middle cerebellar peduncle (Fig. 5; Table 2).
Left cerebellopontine angle, after dissection and removal of the pons and cerebellum, with a posterosuperior view of the TNR (1) entering the Meckel’s cave. 2, facial and vestibulocochlear nerves entering the internal acoustic meatus; 3, abducent nerve; 4, superolateral pontine artery; 5, inferolateral pontine artery; 6, trigeminal branch of the SCA (partially removed); 7, AICA giving off labyrinthine artery (8), and trigeminal branch (arrows), forming a complete arterial ring of the TNR
The inferolateral pontine artery (IPA), which existed in 18 (72%) of the cases, arose from the BA just caudal to the previous vessel. It was a singular artery while only in one case it originated by a common stem with the superolateral pontine artery. The IPA approached from below the ventral side of the TNR (Figs. 1, 2, 3, 4, and 5). It sent to the TNR 1–2 trigeminal vascular twigs measuring from 120 to 240 μm (mean, 180 μm) in diameter (Table 1).
The AICA, which was found in all cases except in two specimens, originated as a collateral branch of the proximal part of the BA. It ran laterally inferior to the TNR to supply the anterolateral part of the inferior cerebellar surface (Fig. 1). It usually coursed ventral to the abducent, facial, and vestibulocochlear nerves and often formed a loop which entered the internal acoustic meatus commonly below these nerves (Fig. 3). Usually, one trigeminal vessel, measuring from 140 to 340 μm (mean, 240 μm) in diameter, arose from the AICA’s peduncular cerebellar branch which supplies the middle cerebellar peduncle. In 22 (88%) of the cases, the trigeminal vessel was distributed to the caudal, lower part of the TNR (Figs. 3 and 4; Table 1). In our series, the neurovascular contact between the loop of a rostral terminal trunk of the AICA and the TNR was seen in three (12%) of the specimens (Table 2).
The SCA, as the last collateral branch of the BA (Figs. 1 and 2), winds laterally, around the cerebral peduncle and reaches the superior surface of the cerebellar hemisphere. Only in three (12%) of the cases, its small branch, measuring on average 220 μm in diameter, was distributed to the dorsal side of the TNR (Figs. 1, 2, and 5; Table 1).
The SCA was the most frequent vessel to be in a direct relation with the TNR. The main trunk of this artery, before its terminal bifurcation, was closely above the TNR in eight (32%) of the hemispheres and in direct contact with a TNR in two (8%) of the specimens (Table 2). The lateral terminal branch of the SCA looped inferiorly between the pons and the nerve in 15 (60%) cases, cross compressing the upper surface of the nerve in three (12%) cases (Fig. 1; Table 2).
The trigeminal vessels coming from these parent arteries varied in number from two to five (mean, 3.6) per nerve, and in diameter from 110 to 340 μm (mean, 215 μm). A single branch may supply either the motor portion of the nerve root or sensory portion or both. The motor portion and the upper part of the sensory TNR were usually perfused by the SPA (92%). The middle (maxillary) part was most often supplied by the SPA and IPA (72%) and the lower part by the AICA (88%). Each TNR received blood supply from two or more of the parent vessels (Figs. 1, 2, 3, 4 and 5; Table 1).
In patients with trigeminal neuralgia there is usually a cross compression of the TNR close to its entry zone into the pons. Because of that, we made a longitudinal section of one TNR to demonstrate immunohistochemically the transitional region between the central and peripheral type of myelin within the nerve root (Fig. 6). Exactly here, a vascular ring is located.
A TNR specimen following the immune reaction against the myelin basic protein (MBP) shows central myelin–peripheral myelin transitional zone (large arrows), and the difference between the central and peripheral myelin. Small arrows point to vessels around the root entry zone (original magnification ×20)
The anastomoses among the trigeminal arteries were found around the root entry zone in all of the examined TNRs in the form of the encircling arterial ring (Figs. 3 and 6). This proximal vascular ring, which surrounded the pontine attachment of the TNR, gave off twigs that entered among the nerve fascicles. The ring was the main source of the centrifugal and centripetal feeding arteries to the nerve itself. On the one hand, small vessels originated from the ring at the root entry zone, which coursed longitudinally along the TNR fascicles. On the other hand, another group of small branches had a different direction, i.e., they ran down and accompanied the TNR fibers into the pontine tissue, reaching its nuclei (Figs. 7 and 8). As regards their nourishing, the motor trigeminal nucleus is mainly supplied by the superolateral pontine artery, but the principal sensory trigeminal nucleus is mainly perfused by the inferolateral pontine artery, together with branches of the AICA.
Superior view of the right cerebellopontine angle, after removal of the pons, with the TNR (1) entering the Meckel’s cave. 2, proximal, complete arterial ring sending longitudinal extrapontine vessels (large arrows), and intrapontine twigs (small arrows); 3, distal arterial ring; 4, oculomotor nerve (displaced posteriorly); 5, superior margin of the right petrous part of temporal bone
In 19 (76%) of the trigeminal nerves, there existed an incomplete distal arterial ring. The latter ring was formed by the trigeminal branch of the superolateral pontine artery, whose two terminal vessels embraced the middle portion of the TNR at its superomedial side before the level of the TNR entrance into the arachnoid sleeve (Figs. 1, 2, 5, 7).
The petrosal vein (PV), i.e., Dandy’s vein or the superior petrosal vein, which is a tributary of the superior petrosal sinus, received veins from the superior and middle cerebellar peduncles, and anterior part of the cerebellum. The veins from the pons, the medulla, and the fourth ventricle also contributed to the formation of this vein. The confluence of these veins most often (64%) took place immediately dorsal to the point of entry of the sensory portion of the TNR (Fig. 1). The PV ran in anterolateral direction, posterior and slightly dorsal to the TNR, to enter the superior petrosal sinus between the internal acoustic meatus and Meckel’s cave. We noticed the neurovascular contact of this vein with the TNR in three specimens (12%) (Fig. 4). In another three hemispheres (12%), the described initial point of the PV was positioned ventral and medial to the TNR. The petrosal vein, before entering the superior petrosal sinus, surrounded the TNR along its medial side, and in two cases (8%) it ran between the motor and sensory root fascicles (Fig. 9; Table 2).
Discussion
The anatomy and histology of the trigeminal nerve root (TNR), as well as certain neurovascular relations, have been examined in detail [6, 11, 18]. On the other hand, only some aspects of the TNR blood supply have been reported so far [5, 12]. Our microanatomical investigation emphasized the origin, size, branching patterns, and distribution areas of the trigeminal arteries, as well as the neurovascular relationships of the TNR. This study of the normal anatomical vascular relations of the TNR in the posterior cranial fossa, using the injected specimens and the stereoscopic microscope, could provide useful information on the incidence of trigeminal contact and compression in normal persons for comparison with the clinical cases. Precise assessment of the complex nerve–vessel relationship close to the TNR entry zone is useful for planning microvascular decompression in patients with trigeminal neuralgia [10, 16].
Dandy and Jannetta have proposed that trigeminal neuralgia is associated with vascular compression of the TNR by branches of the SCA [7]. The vascular contact or compression of the TNR in trigeminal neuralgia patients is more than 90% in most reports [7, 8, 15, 17]. The arterial compression is the most frequent one (mean, 84%) while the venous compression is rarely present (14% on average) [7, 8]. The majority of the vascular compressions on the TNR are due to the superior cerebellar artery and less frequently to the anterior inferior cerebellar artery [7, 9, 15]. In addition to a major vessel, there are often small pontine arteries or veins in the vicinity of the root entry zone. Multiple vessel involvement in compression neuropathies of the posterior fossa is not uncommon [3].
We have shown vascular contacts, without grooving or distortion of the TNR, in 56% of our cases, either by an artery or a vein, comparing with reported incidences of 58% [9] and 35% [7]. The contact was most often located close to the root entry zone, i.e., at the level of the transition between the central and peripheral type of myelin sheath around the TNR axons, as noticed in patients with neuralgia [3, 4, 8, 13]. Yet, there are no records on trigeminal neuralgia in the former individuals, probably because the contact itself is usually not sufficient to trigger the underlying pathophysiological mechanisms. On the other hand, there are some patients with trigeminal neuralgia without a neurovascular contact [2, 14].
In rare cases, the TNR could be penetrated by the corresponding cerebral vessels [7, 15]. Penetrations of the TNR that we described could have certain consequences for both nerve and vessel. The penetrating artery or vein can compress or distort the TNR. The nature of a resulting neurologic deficit depends on the site of penetration. The vessels that we found ran between the motor and sensory root fascicles, what might be an etiological factor for paresis of the masticatory muscles and hypaesthesia in the territory of the ophthalmic division of the trigeminal nerve [1]. Such a relationship between the nerve fibers and blood vessels would also be a challenge to neurosurgeons because classical vascular decompression is impossible in such cases.
The trigeminal vasculature formed two arterial rings around the TNR. The proximal arterial ring, which encircled the TNR around its entry zone and supplied the pontine and cisternal parts of the TNR, has been reported in only a few articles [5, 12]. The proximal ring gives rise to the intrapontine and extrapontine trigeminal vessels which supply both the TNR and its main nuclei. Because of that, its occlusion could cause the lateral midpontine syndrome [1].
The microanatomical characteristics of the distal arterial ring seem to have escaped the attention of many investigators [12]. The trigeminal branch of the SPA, which forms that circle around the middle portion of the TNR, should be spared during the microvascular decompression, and especially when opening the arachnoid sleeve of the TNR during surgery.
In conclusion, we have presented a detailed analysis of the small arteries that supply the TNR, which most often arose from the superolateral and inferolateral pontine vessels, as well as from the anterior inferior cerebellar artery. The trigeminal twigs formed a proximal arterial circle around the trigeminal root entry zone at the lateral part of the pons, as well as a distal arterial ring encircling the middle part of the TNR. Twigs of the former circle nourish both the TNR and its nuclei while branches of the latter ring supply the remaining part of the TNR. Our findings could help the neurosurgeons to avoid damage to the trigeminal vessels during microvascular decompression of the TNR.
References
Adams RD, Victor M (1989) Cerebrovascular diseases. In: Principles of neurology. McGraw-Hill, New York, pp 617–692
Baechli H, Gratzi O (2007) Microvascular decompression in trigeminal neuralgia with no vascular compression. Eur Surg Res 39(1):51–57
Choudhari K (2007) Quadruple vessel involvement at root entry zone in trigeminal neuralgia. Clin Neurol Neurosurg 109:203–205
Devor M, Govrin-Lippmann R, Rappaport HZ (2002) Mechanism of trigeminal neuralgia: an ultrastructural analysis of trigeminal root specimens obtained during microvascular decompression surgery. J Neurosurg 96:532–543
Duvernoy HM (1978) Arteries and veins of the brainstem. In: Human Brainstem Vessels. Springer-Verlag, Berlin, pp 5–25
Gudmundsson K, Rhoton AL, Rushton JG (1971) Detailed anatomy of the intracranial portion of the trigeminal nerve. J Neurosurg 35:592–600
Haines SJ, Jannetta PJ, Zorub DS (1980) Microvascular relations of the trigeminal nerve. An anatomical study with clinical correlation. J Neurosurg 42:381–386
Hamlyn PJ (1997) Neurovascular relationships in the posterior cranial fossa, with special reference to trigeminal neuralgia. Clin Anat 10:371–379
Hardy DG, Rhoton AL (1978) Microsurgical relationships of the superior cerebellar artery and the trigeminal nerve. J Neurosurg 49:669–678
Kabatas S, Karasu A, Civelek E, Sabanci A, Hepgul K, Teng Y (2009) Microvascular decompression as a surgical management for trigeminal neuralgia: long-term follow-up and review of the literature. Neurosurg Rev 32:87–94
Lang J (1991) Clinical anatomy of the posterior cranial fossa and its foramina. Georg Thieme Verlag, Stuttgart, pp 83–92
Marinković S, Gibo H (1995) The blood supply of the trigeminal nerve root, with special reference to the trigeminocerebellar artery. Neurosurgery 37(2):309–317
Peker S, Kurtkaya O, Uzun I, Pamir MN (2006) Microanatomy of the central myelin-peripheral myelin transition zone of the trigeminal nerve. Neurosurgery 59(2):354–359
Revuelta-Gutierrez R, Lopez-Gonzalez MA, Soto-Hernandez JL (2006) Surgical treatment of trigeminal neuralgia without vascular compression: 20 years of experience. Surg Neurol 66(1):32–36
Rusu MC, Ivascu RV, Cergan R, Paduraru D, Podoleanu L (2009) Typical and atypical neurovascular relations of the trigeminal nerve in the cerebellopontine angle: an anatomical study. Surg Radiol Anat 31(7):507–516
Satoh T, Onoda K, Date I (2007) Preoperative simulation for microvascular decompression in patients with idiopathic trigeminal neuralgia: visualization with three-dimensional magnetic resonance cisternogram and angiogram fusion imaging. Neurosurgery 60(1):104–113
Sindou M, Howeidy T, Acevedo G (2002) Anatomical observations during microvascular decompression for idiopathic trigeminal neuralgia (with correlations between topography of pain and site of the neurovascular conflict). Prospective study in a series of 579 patients. Acta Neurochir (Wien) 144:1–13
Ziyal IM, Sekhar LN, Ozgen T, Soylemeyoglu F, Alpter M, Beser M (2004) The trigeminal nerve and ganglion: an anatomical, histological, and radiological study addressing the transtrigeminal approach. Surg Neurol 61:564–573
Acknowledgements
This work was supported by grant no. 175061 from the Ministry of Science and Environmental Protection, Serbia.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Comment
This is an interesting anatomical research, well-written, elegantly illustrated, and convincingly discussed. There is no really new information, but the anatomy of these so clinically relevant neurovascular structures is worth to be recalled to Acta readers.
Domenico d’Avella
Padova, Italy
Rights and permissions
About this article
Cite this article
Ćetković, M., Antunović, V., Marinković, S. et al. Vasculature and neurovascular relationships of the trigeminal nerve root. Acta Neurochir 153, 1051–1057 (2011). https://doi.org/10.1007/s00701-010-0913-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-010-0913-1