Abstract
Purpose
There is much debate regarding the optimal strategy for extracranial–intracranial (EC–IC) bypass for complex aneurysms. We introduce the concept of a flow replacement bypass which aims to compensate for loss of flow in the efferent vessels of the aneurysm. The strategy to achieve this utilizes direct intraoperative flow measurements to guide optimal revascularization by matching graft flow to demand.
Methods
We reviewed all EC–IC bypass cases performed over a 6-year period. We identified cases in which intraoperative flow measurements using an ultrasonic flow probe were utilized to determine the revascularization strategy and analyzed the decision-making paradigm.
Results
Twenty-three cases were analyzed. For terminal aneurysms, flow measurement in the affected vessel at baseline predicted the flow required for full replacement: middle cerebral artery (MCA), 50 ± 25 cc/min (n = 9); posterior inferior cerebellar artery (PICA), 13 ± 7 cc/min (n = 4); posterior cerebral artery (PCA), 33 cc/min (n = 1); and superior cerebellar artery (SCA), 10 cc/min (n = 1). For proximal internal carotid artery (ICA) aneurysms (n = 8), the flow deficit from baseline during carotid temporary occlusion was measured (26 ± 18 cc/min, an average of 44% drop from baseline). The adequacy of flow from the superficial temporal artery (STA) or occipital artery (OA), when available, was assessed prior to bypass, and STA, OA, or vein interposition grafts were used accordingly. Measurement of bypass flow following anastomosis confirmed not only patency but sufficient flow in all cases: MCA 50 ± 25 cc/min, PICA 18 ± 9 cc/min, PCA 64 cc/min, SCA 12 cc/min, ICA 36 ± 25 cc/min (STA), and >200 cc/min (vein).
Conclusions
Direct intraoperative measurement of flow deficit in aneurysm surgery requiring parent vessel sacrifice can guide the choice of flow replacement graft and confirm the subsequent adequacy of bypass flow.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is much debate and variability regarding the strategy for extracranial–intracranial bypass in the setting of complex aneurysms [20, 26, 28, 30]. Fundamentally, bypass in aneurysm surgery is created for the purpose of flow replacement, whereby a conduit is established to compensate for loss of flow in the efferent vessels of the aneurysm. Options for donor grafts range from in situ pedicles, such as the superficial temporal artery (STA) or occipital artery (OA), to interposition grafts using radial artery or saphenous vein as conduits. Terms such as “high flow”, “intermediate flow”, and “low flow” have been variably used to imply vein, radial artery, and in situ pedicles, respectively. Methods for selection of such graft have been primarily based upon speculation using expected territory flows, or preoperative assessments such as balloon occlusion testing (BOT), to deduce the anticipated flow needing replacement. However, the optimal revascularization strategy should directly match graft flow to demand on a case by case basis, preventing the higher morbidity of an interposition vein or arterial graft if unnecessary and avoiding inadequate replacement if an in situ pedicle is insufficient.
In this paper, we propose the concept of a flow replacement bypass implemented using intraoperative flow measurements for graft selection and verification of bypass success.
Methods
Patient population and selection criteria
We performed a retrospective review of bypass surgeries (n = 125) performed at the University of Illinois Medical Center at Chicago over a 6-year period. The cases in which bypass was performed for the purposes of flow replacement in the treatment of anterior or posterior circulation aneurysms were selected (n = 42). Of these, we identified the cases in which detailed records of intraoperative flow measurements were available and were utilized in the decision-making for revascularization strategy; the flow measurements and outcomes in these cases were analyzed. Cases of bypass surgery performed for flow augmentation in anterior or posterior circulation ischemia were excluded. Patients with aneurysms undergoing primary vessel sacrifice without bypass were also excluded.
Preoperative assessment
Patients were admitted for either elective surgery or emergently following hemorrhage or following onset of acute symptoms or signs related to the aneurysm. Digital subtraction angiography (DSA) was performed in all patients preoperatively to define the location and anatomy of the aneurysm, evaluate the size and adequacy of in situ donors such as the STA or OA, and assess the status of the anterior and posterior communicating arteries when relevant.
For carotid aneurysms felt not to be amenable to direct surgical or endovascular treatment, BOT was performed to assess tolerance to carotid sacrifice, unless the patient’s clinical condition did not allow reliable monitoring for neurological changes, or heparinization, during the test (as with neurologically impaired patients, and/or subarachnoid hemorrhage). At our institution, the BOT protocol consists of temporary balloon occlusion of the internal carotid artery (ICA) for 15 min with continuous neurological and electroencephalographic (EEG) monitoring [11], followed by hypotensive challenge, with assessment of cerebral blood flow using 99mTc-HMPAO single photon emission computed tomography (SPECT) [17, 31]. SPECT images are analyzed for asymmetrical distribution of the tracer and hypoperfusion ipsilateral to BOT and compared to SPECT images obtained at baseline.
Intraoperative blood flow measurements
Intraoperatively, blood flow was measured quantitatively in cubic centimeter per minute using an ultrasonic flow probe (Charbel Micro-Flowprobe; Transonics Systems Inc., Ithaca, NY, USA). The probe utilizes the principle of ultrasonic transit-time to sense flow in vessels independent of the flow velocity profile, turbulence, or hematocrit [9, 21]. The flow probe is manufactured in a variety of sizes to accommodate intracranial or extracranial vessels ranging from 1 to 3 mm in width. Direct vessel contact is not required as space between the probe and vessel can be filled with an ultrasonic couplant, typically saline. In vitro and in vivo studies have established the accuracy of flow measurements using this device [21].
Flows through the vessels of interest associated with the aneurysm, in situ donor vessel (when available), and the bypass conduit after completion of the anastomosis, and after proximal occlusion or trapping of the aneurysm were recorded. Blood pressure and end-tidal carbon dioxide (CO2) are kept constant during all flow measurements. In addition, EEG burst suppression is routinely induced by inhalational agents during aneurysm surgery, and the flow measurements are performed once a steady state of EEG burst suppression has been achieved.
Intraoperative management
For terminal aneurysms (Fig. 1), i.e., those located beyond the anastomotic connections of the Circle of Willis such as the middle cerebral artery (MCA), posterior cerebral artery (PCA), posterior inferior cerebellar artery (PICA), and ICA terminus, the standard cranial approaches are utilized. The STA for anterior circulation aneurysms, or the OA for PICA/vertebral aneurysms, are carefully preserved. Once the aneurysm and associated vessels are exposed using standard microdissection, flow measurements are obtained in all distal vessels which could be compromised by trapping of the aneurysm. For example, the M2 branches would represent the distal vessels in the case of an M1 aneurysm, or the PICA trunk would represent the distal vessel in a PICA origin aneurysm. The cumulative flow in the distal vasculature reflects the distal territory flow requiring replacement by the bypass and is used to guide the choice of appropriate bypass (in situ STA or OA vs. interposition vein or artery graft) and the subsequent adequacy of the bypass.
Steps for using intraoperative flow measurements to implement a flow replacement bypass for terminal aneurysms. a A fusiform M1 aneurysm is demonstrated. Flow is measured in both M2 branches, totaling 65 cc/min. The cut flow in the STA is found to closely approximate this distal territory flow at 60 cc/min. b Anastomosis is performed between the STA and an M3 branch. Patency of the bypass is confirmed by measuring the flow in the bypass, although bypass flow is expected to be low given that the parent vessel is still open. c The final step in aneurysm obliteration is trapping of the aneurysmal M1 segment. The patency and adequacy of the bypass is then confirmed by measuring graft flow, which at 65 cc/min provides complete flow replacement to the distal MCA territory
For ICA aneurysms (Fig. 2), including cavernous segment, ophthalmic, and paraclinoid aneurysms, the operation is performed with simultaneous exposure of the cervical ICA in the neck, while performing a standard ipsilateral pterional craniotomy. The STA when available is preserved, using Doppler to map the course of the anterior and posterior branches in relation to the planned craniotomy incision prior to the opening. Following craniotomy, the sylvian fissure is routinely opened with microdissection, exposing the M1 and A1 segments. Flow in the M1 is measured and recorded at baseline, and then repeated following temporary occlusion of the ICA. Temporary occlusion is performed on the cervical ICA when the planned surgical intervention is to be proximal occlusion, or on the supraclinoid ICA distal to the aneurysm if the surgical plan is trapping of the lesion. The decrement in M1 flow during temporary vessel occlusion is designated as the flow deficit and used to determine the appropriate donor vessel (in situ pedicle STA graft vs. interposition graft) and to ensure adequate bypass flow following anastomosis.
Steps for using intraoperative flow measurements to implement a flow replacement bypass for proximal ICA aneurysms. a A cavernous ICA aneurysm is demonstrated. Following exposure of the cervical ICA, and the intracranial vessels, baseline flow in the M1 is measured. b With a temporary clip on the cervical ICA, flow in the M1 is re-measured and falls from 70 to 20 cc/min. This represents a flow deficit of 50 cc/min. If the STA cut flow is inadequate, an interposition graft is placed between the ECA and MCA. c Following the bypass, permanent proximal occlusion of the cervical ICA is performed, and graft patency is confirmed by flow measurement. Due to the large size of the conduit, the interposition graft flow can exceed even the previously measured flow deficit
The flow capacity of in situ pedicle grafts (STA or OA) is directly measured to determine their adequacy for flow replacement. This is performed by determining the cut flow of the donor graft [4], which reflects the maximal potential flow capacity of the vessel. Flow measurements in the intact STA or OA generally demonstrate low values in the 5–10-cc/min range due to the small caliber of the vessels and the high resistance of the surrounding scalp tissue. However, the full carrying capacity of the vessel can be determined once the vessel is dissected, and the distal end is cut allowing the blood to flow freely (termed the cut flow) [4, 25]. Once the vessel has been dissected free from surrounding tissues, it is wrapped in papaverine-soaked cottonoids for 20–30 min during the time that the craniotomy is performed, to relieve any spasm induced by vessel manipulation. The flow is then measured shortly prior to planned bypass. If the cut flow approaches or exceeds the flow required for adequate flow replacement, the in situ graft is utilized for the bypass. Existing data regarding blood flow and perfusion assessment during vessel occlusion indicate that a 20–25% or greater reduction in distal flow is correlated with ischemia [11, 12, 15]. Therefore, to avoid risk of ischemia, we accept a cut flow only within 20% of the recipient territory flow as adequate. The cut flow is generally measured prior to cutting the graft to its final length in order to allow heparin flushing of the vessel distal to the ultimate anastomosis site (in order to prevent risk of endothelial injury from the flushing needle). For vessels of small diameter such as the STA, the reduction in graft length with final trimming will inevitably result in some increase in carrying capacity. If the in situ vessel is inadequate, an interposition graft is used.
Bypass technique
The technique for STA bypass has been previously described in detail [10]. In brief, the STA is dissected, transected and fish-mouthed following heparin irrigation, and sutured to the appropriate recipient branch using interrupted suture technique with 10-0 nylon suture. The same technique is utilized for OA bypass. Running suture technique is occasionally employed if the donor and recipient are of larger size (>2 mm).
For interposition grafts, we utilize saphenous vein grafts preferentially to radial artery due to the ease of harvesting and preferred tissue handling properties. The vein is harvested in the calf or thigh following preoperative mapping to determine the suitability (size and length) of the vein. The vein is subjected to pressure distention with heparinized saline utilizing the Shiley balloon distention kit and sutured to the appropriate recipient branch with 9-0 or 8-0 nylon using running suture technique for the distal anastomosis. The graft is tunneled pre-auricular through a 28-French chest tube to the neck. The proximal anastomosis is created to the external carotid artery in either an end-to-end fashion or end-to-side fashion after creating an arteriotomy with an appropriately sized aortic punch device. Running suture technique using 7-0 prolene is utilized. Typically, 2,000 units of intravenous heparin is administered prior to temporary occlusion for the distal anastomosis and an additional 1,000 units prior the proximal anastomosis. The proximal anastomosis is occasionally created in an end-to-end fashion to the stump of the STA if the donor vessel is suitable.
Postoperative management
Patients with both in situ and vein grafts are routinely maintained on 325 mg of aspirin daily beginning immediately postoperatively (administered per rectum if oral administration is not feasible). Postoperatively, the success of the bypass is judged by angiographic patency, in addition to quantitative measurements of blood flow through the graft using quantitative magnetic resonance angiography [6].
Analysis
Absolute values of flow measurements, or averages ± standard deviations (if multiple cases of the same aneurysm type), are provided. Individual territory flows and subsequent replacement bypass flows are presented.
Results
Patient population
During the study period, we identified 23 bypass surgeries which used intraoperative flow measurements to guide the revascularization strategy. The age of these patients at the time of bypass ranged from 22 to 77 years old, with an average age of 56 years old. Female patients comprised the majority, 78% (n = 18) of the group.
Aneurysm characteristics and operative procedures
The aneurysms treated with bypass and flow replacement strategy consisted of eight proximal ICA, two ICA terminus, seven MCA, two vertebral artery (VA), two PICA, one basilar artery (BA) terminus, and one PCA aneurysm (Tables 1 and 2). Bypass using an in situ pedicle donor vessel, either STA or OA, was performed in 16 of the 23 (70%) cases. In some cases, the option for use of STA was not present as the patient had undergone prior craniotomy with disruption of the STA. The remainder of the cases consisted of saphenous vein interposition grafts between the cervical carotid or STA stump and MCA.
In the majority of cases, the bypass was performed as a permanent conduit for replacement of flow once the aneurysm had been excluded from the circulation. However, in a small number of cases (n = 4), the bypass was performed as protection against ischemia when prolonged temporary vessel occlusion was anticipated or required; in these cases, the aneurysm was ultimately amenable to direct clipping without parent vessel sacrifice. The adequacy of the bypass during the temporary vessel occlusion was assessed in the same fashion as in the patients who underwent permanent vessel sacrifice.
Flow replacement strategy
Terminal aneurysms
For the MCA aneurysms (n = 7) and the ICA bifurcation aneurysm (n = 2), the distal territory flow calculated from the branches in the MCA averaged 50 ± 25 cc/min. For the VA/PICA aneurysms, flow in the PICA distal to the aneurysm (n = 4) averaged 13 ± 7 cc/min. For a trapped BA terminus aneurysm (n = 1), flow in the isolated superior cerebellar artery (SCA) was 10 cc/min. For the PCA aneurysm (n = 1), the distal PCA flow was 33 cc/min. These aneurysms were comprised of fusiform aneurysms or complex aneurysms which incorporated branch vessels. Four patients were treated for aneurysms which had recurred after previous clipping or endovascular interventions performed at other institutions (Table 1).
In four cases, the STA could not be assessed as an in situ donor due to prior craniotomy, vessel injury, or diminutive size. In the remaining cases, the adequacy of flow from in situ donors was assessed prior to bypass. Cut flows in the STA and OA varied from 8 to 82 cc/min and approached or exceeded the distal territory flow which required replacement in all but one case (case #2, illustrative case). Consequently, in cases where the in situ pedicle was deemed adequate, it was utilized for revascularization without use of interposition vein or arterial grafts.
Subsequent to the bypass and aneurysm trapping/parent vessel sacrifice, bypass flow was measured to confirm adequate flow replacement, whether a vein graft or in situ donor had been utilized. Measurement of bypass flow following anastomosis was able to confirm not only graft patency but sufficient territorial flow in all cases: MCA territory, 50 ± 25 cc/min; PICA territory, 18 ± 9 cc/min; SCA territory, 12 cc/min; and PCA territory, 63 cc/min.
Proximal ICA aneurysms
For proximal ICA aneurysms, the flow deficit from baseline in the MCA averaged 26 ± 18 cc/min, an approximate 40% drop from baseline. The eight cases included four patients who had failed BOT due to evidence of hypoperfusion on the SPECT portion of the BOT. An additional three patients were not good candidates for preoperative BOT, having presented with Hunt and Hess grade 3 or 4 subarachnoid hemorrhage; in these cases, angiography suggested that carotid occlusion would be poorly tolerated due to small or absent collaterals. In one additional patient, BOT was not performed as angiography demonstrated the posterior communicating artery (PcoA) to be arising from the base of the aneurysm; proximal BOT was felt not to be useful in accurately reflecting tolerance to ischemia if the aneurysm were to require trapping with occlusion of the PcoA origin (Table 2).
The flow in the STA ranged from 10 to 55 cc/min in seven cases; in one case, the STA was diminutive and could not be utilized. In the patient with the low STA branch cut flow of 10 cc/min (case #18), the STA was truncated down to its stump [3], which demonstrated an excellent flow of 90 cc/min. The stump was utilized with an interposition vein graft, carrying a final bypass flow of 85 cc/min. Following anastomosis, the flow in the in situ STA grafts (n = 6) averaged 28 ± 12 cc/min, replacing the 19 ± 5 cc/min flow deficit in those cases. In the remaining case, the flow in the external carotid artery-vein graft bypass was 220 cc/min during carotid temporary occlusion, replacing the full hemispheric flow rather than merely the flow deficit due to the large size of the conduit. In this case, the bypass allowed prolonged temporary clipping with suction decompression of the giant ophthalmic aneurysm; this ultimately permitted direct clipping of the aneurysm, without carotid sacrifice.
Illustrative cases
ICA terminus aneurysm (case #2)
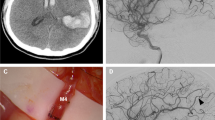
A 44-year-old female presented with a 1-week history of severe headache. A head CT scan showed hyperdensity over the right carotid terminus region. Cerebral angiogram showed a dissecting pseudoaneurysm extending from the right supraclinoid ICA into the ICA terminus and occluding the origin of the anterior cerebral artery (ACA; Fig. 3a, b). This aneurysm represents a terminal aneurysm with the outflow from the ICA terminus only supplying the MCA territory. During surgery, flows were measured in the MCA branches and totaled 81 cc/min. The STA was dissected for the required length, and the cut flow was found to be 28 cc/min, which was inadequate for flow replacement; therefore, an interposition graft was felt to be necessary. Although the STA branch carried inadequate flow, the STA was truncated to its stump [3], which had a cut flow of 100 cc/min. Therefore, the interposition saphenous vein was placed as a short jump graft between the STA stump and an M2 branch. Following the bypass, the pseudoaneurysm was surgically trapped. Final bypass flow was measured to be 95 cc/min, and postoperative angiogram revealed filling of the full MCA territory (Fig. 3c).
A 44-year-old female with severe headache and acute dissecting pseudoaneurysm of the ICA terminus. a Anteroposterior view of right ICA angiogram demonstrating the dissecting aneurysm. b Anteroposterior view of the left ICA angiogram demonstrating occlusion of the origin of the A1 and cross-filling of the right ACA. c Anteroposterior view of postoperative angiogram demonstrating the vein interposition graft between the STA stump and the MCA
Proximal ICA aneurysm (case #16)
A 73-year-old female presented with a 2-week history of progressive left ophthalmoplegia. Magnetic resonance imaging (MRI) demonstrated a giant partially thrombosed cavernous carotid aneurysm, confirmed on DSA (Fig. 4). The patient underwent BOT, which she passed clinically. However, the SPECT demonstrated hypoperfusion in the left hemisphere during hypotensive challenge. Intraoperatively, a flow deficit of 21 cc/min was measured in the MCA trunk. The STA cut flow was 55 cc/min, adequate to replace the flow deficit. The patient underwent STA–MCA bypass using the anterior temporal artery as a recipient vessel. Patency of the bypass was confirmed with flow measurement and the aneurysm was trapped. The final bypass flow following trapping was 51 cc/min.
A 73-year-old female with a symptomatic partially thrombosed giant cavernous ICA aneurysm. a Coronal T1-weighted post contrast MRI demonstrating the partially thrombosed aneurysm. b Anteroposterior view of angiogram demonstrating the giant aneurysm with partial filling of the aneurysmal sac. c Anteroposterior view of postoperative angiogram demonstrating the filling of the MCA branches via the STA (arrows)
Outcomes
None of the 23 patients developed stroke (radiological or clinical) as a consequence of inadequate blood flow to the revascularized territory. Twelve patients remained neurologically intact postoperatively. Seven patients with pre-existing neurological deficits had no new deficits following surgery and showed subsequent improvements. Two patients with subarachnoid hemorrhage developed new neurological deficits and strokes in the postoperative period secondary to severe vasospasm. One patient (case #10) experienced a transient hemiparesis after STA–PCA bypass due to ischemia from temporary clipping of a P2 segment perforator. Another patient suffered a vocal cord paresis following VA aneurysm trapping (case #13) likely due to cranial nerve manipulation during the approach.
Discussion
Definitive management of giant or fusiform aneurysms may require sacrifice of the parent vessel, with proximal occlusion or trapping. This paper illustrates the concept of a flow replacement bypass, utilizing a flow-assisted technique aimed at matching supply to demand in order to maintain adequate flow in the parent and/or distal branches. The flow demand is measured directly intraoperatively: for proximal ICA aneurysms, this consists of the flow deficit measured in the major distal outflow, the M1 trunk; for terminal aneurysms, this consists of the distal territory flow in the efferent branches of the aneurysm. The supply from in situ pedicle grafts such as the STA and OA is determined by measuring the cut flow, and the suitability of these vessels for matching the demand determined directly. Lastly, once the bypass has been performed, the success in achieving full flow replacement is verified intraoperatively by measuring the bypass graft flow.
Bypass graft options
Using this flow-based strategy, the traditional decision between “high flow” and “low flow” bypass becomes less relevant, as the primary goal is to determine the “appropriate flow” bypass. Using in situ pedicle grafts such as the STA has the advantage of greater patency rates, better longevity, and need for only one anastomosis [1, 28], but risks inadequate revascularization if the STA cannot supply the needed flow; significant stroke complications have been noted in patients with apparently intact STA bypasses [2, 13]. Indiscriminate use of interposition radial artery or vein grafts creates the need for an additional anastomosis and results in overall lower patency and higher morbidity rates [14, 20, 24, 26, 27]. Additionally, for a small distal territory, the low flow requirement may actually result in low demand and graft occlusion. Vein grafts placed in the arterial circulation appear to require flow rates of 40 cc/min to maintain good patency rates [29].
Graft selection strategies
In the management of unclippable proximal ICA aneurysms, the information from BOT itself may be a guide to the choice of flow replacement graft [16]: failure based upon clinical criteria indicates a more profound lack of collaterals compared to failure on blood flow imaging or provocative testing and would indicate the need for a higher flow bypass. Intraoperative flow measurements, however, are useful to confirm the results of the BOT and verify the choice of bypass graft. Additionally, in cases when BOT cannot be performed, such as immediately following subarachnoid hemorrhage, the intraoperative flow measurements can provide the necessary information regarding the need for revascularization. Another intraoperative strategy described by Kubo et al. advocates the use of intraoperative cortical blood flow measurements over the frontal and temporal lobes with a thermal diffusion flow probe [18], to determine the need for interposition vein graft. Although the authors achieved good results, the technique measures only blood flow in cortical regions beneath the probe and may underestimate changes in perfusion to the entire territory at risk.
Fusiform or complex aneurysms of distal vessels, such as the MCA or its branches, typically require revascularization as collaterals to such terminal vessels are generally inadequate in the acute setting. Intraoperative measurements provide a reliable mechanism to measure distal territory flow and assess adequacy of in situ donor vessels. Although angiographic size of the STA or OA does correlate with its flow capacity, the correlation is not absolute; in situ pedicled arterial donors can occasionally carry flows >100 cc/min [4]. The final bypass flow in some of our cases exceeded the measured distal territory flow. This may reflect a transient post-ischemic hyperperfusion, following the temporary vessel occlusion associated with performing the bypass, a phenomenon which we have also observed during aneurysm surgery following temporary vessel occlusion [5].
Assessment of bypass patency
Intraoperative evaluation of bypass patency can be performed in a variety of ways beyond simple visual inspection and palpation of pulse. These include routine intraoperative angiography, microvascular Doppler assessment [7], or fluorescent dye (indocyanine green) angiography [23, 32]. However, these methods do not provide a quantitative assessment of the flow in the bypass and therefore are less definitive in confirming the adequacy of the revascularization strategy. An electromagnetic flow meter can provide the same quantitative information as the ultrasonic flow probe [22], but because of the requirement for intimate contact with the vessel, such a device can result in vessel constriction and is less practical for routine intraoperative use.
Limitations of flow replacement technique
It is important to ensure that systemic factors which can alter blood flow intraoperatively are kept constant during baseline and subsequent measurements, such as blood pressure, end tidal CO2, and EEG burst suppression. For proximal ICA aneurysms, with additional territory at risk other than the MCA (such as the ACA territory, if absent anterior communicating artery, or the PCA territory, if fetal PCA), the flow deficit in the M1 will not reflect the true overall flow deficit to the hemisphere. In such circumstances, flow in the ICA itself would optimally be measured, but the mass of the aneurysm itself often precludes this strategy. Alternatively, the deficit of flow in the A1 and/or posterior communicating artery in addition to the M1 needs to be measured and presents a drawback of the technique as additional dissection and manipulation of the relevant vessels is required. Another potential limitation in the accuracy of the proposed technique of graft selection is that the major vessel flows may not reflect the only potential route of flow to the brain, given that leptomeningeal collaterals can also contribute to perfusion to some degree [8]. Furthermore, in situ grafts have the potential to “mature” over time, albeit months, increasing their size and flow capacity [19]. As such, replacing the full flow measured in the major vessels with the flow replacement techniques may be unnecessary if leptomeningeals or graft maturation can compensate for a portion of the flow in the distal tissue bed.
Limitations of this study also include the retrospective nature of the data collection and review and the potential for selection bypass given that all bypasses for aneurysms during the period were not managed with this flow-based strategy. The latter is due to the evolution of the technique over time rather than specific selection of cases for this approach.
Conclusion
Challenges in decision-making for revascularization of complex aneurysms fall into two major categories: graft selection and verification of bypass success. In regard to graft selection, the primary goal is to adequately match supply to demand, which requires both determining the flow demand and assessing the adequacy of potential in situ donors. A nonselective approach carries the potential for an inadequate bypass if an in situ pedicle graft is utilized or subjecting patients unnecessarily to the additional morbidity of vein or arterial interposition grafts. Direct intraoperative measurement of flow can guide the choice of the flow replacement bypass graft, helping to ensure an optimal revascularization strategy. Furthermore, the adequacy and patency of the graft can be immediately and quantitatively verified intraoperatively.
References
[No authors listed] (1985) Failure of extracranial–intracranial arterial bypass to reduce the risk of ischemic stroke. Results of an international randomized trial. The EC/IC Bypass Study Group. N Engl J Med 313:1191–1200
Abruzzo T, Joseph GJ, Owens DS, Dawson RC 3rd, Reid J, Barrow DL (2000) Prevention of complications resulting from endovascular carotid sacrifice: a retrospective assessment. Neurosurgery 46:910–916, discussion 916–917
Alaraj A, Ashley WW Jr, Charbel FT, Amin-Hanjani S (2008) The superficial temporal artery trunk as a donor vessel in cerebral revascularization: benefits and pitfalls. Neurosurg Focus 24:E7
Amin-Hanjani S, Du X, Mlinarevich N, Meglio G, Zhao M, Charbel FT (2005) The cut flow index: an intraoperative predictor of the success of extracranial–intracranial bypass for occlusive cerebrovascular disease. Neurosurgery 56:75–85
Amin-Hanjani S, Meglio G, Gatto R, Bauer A, Charbel FT (2006) The utility of intraoperative blood flow measurement during aneurysm surgery using an ultrasonic perivascular flow probe. Neurosurgery 58:305–312, discussion ONS-312
Amin-Hanjani S, Shin J, Zhao M, Du X, Charbel FT (2007) Evaluation of extracranial–intracranial bypass using quantitative magnetic resonance angiography. J Neurosurg 106:291–298
Badie B, Lee FT Jr, Pozniak MA, Strother CM (2000) Intraoperative sonographic assessment of graft patency during extracranial–intracranial bypass. AJNR: Am J Neuroradiol 21:1457–1459
Brozici M, van der Zwan A, Hillen B (2003) Anatomy and functionality of leptomeningeal anastomoses: a review. Stroke 34:2750–2762
Charbel FT, Hoffman WE, Misra M, Ostergren L (1998) Ultrasonic perivascular flow probe: technique and application in neurosurgery. Neurol Res 20:439–442
Charbel FT, Meglio G, Amin-Hanjani S (2005) Superficial temporal artery-to-middle cerebral artery bypass. Neurosurgery 56:186–190, discussion 186–190
Charbel FT, Zhao M, Amin-Hanjani S, Hoffman W, Du X, Clark ME (2004) A patient-specific computer model to predict outcomes of the balloon occlusion test. J Neurosurg 101:977–988
Eckert B, Thie A, Carvajal M, Groden C, Zeumer H (1998) Predicting hemodynamic ischemia by transcranial Doppler monitoring during therapeutic balloon occlusion of the internal carotid artery. AJNR: Am J Neuroradiol 19:577–582
Fox AJ, Vinuela F, Pelz DM, Peerless SJ, Ferguson GG, Drake CG, Debrun G (1987) Use of detachable balloons for proximal artery occlusion in the treatment of unclippable cerebral aneurysms. J Neurosurg 66:40–46
Jafar JJ, Russell SM, Woo HH (2002) Treatment of giant intracranial aneurysms with saphenous vein extracranial-to-intracranial bypass grafting: indications, operative technique, and results in 29 patients. Neurosurgery 51:138–144, discussion 144–136
Jawad K, Miller D, Wyper DJ, Rowan JO (1977) Measurement of CBF and carotid artery pressure compared with cerebral angiography in assessing collateral blood supply after carotid ligation. J Neurosurg 46:185–196
Kai Y, Hamada J, Morioka M, Yano S, Mizuno T, Kuroda J, Todaka T, Takeshima H, Kuratsu J (2007) Treatment strategy for giant aneurysms in the cavernous portion of the internal carotid artery. Surg Neurol 67:148–155, discussion 155
Komiyama M, Khosla VK, Tamura K, Nagata Y, Baba M (1992) A provocative internal carotid artery balloon occlusion test with 99mTc-HM-PAO CBF mapping—report of three cases. Neurol Med Chir (Tokyo) 32:747–752
Kubo Y, Ogasawara K, Tomitsuka N, Otawara Y, Kakino S, Ogawa A (2006) Revascularization and parent artery occlusion for giant internal carotid artery aneurysms in the intracavernous portion using intraoperative monitoring of cerebral hemodynamics. Neurosurgery 58:43–50, discussion 43–50
Latchaw RE, Ausman JI, Lee MC (1979) Superficial temporal–middle cerebral artery bypass. A detailed analysis of multiple pre- and postoperative angiograms in 40 consecutive patients. J Neurosurg 51:455–465
Lawton MT, Hamilton MG, Morcos JJ, Spetzler RF (1996) Revascularization and aneurysm surgery: current techniques, indications, and outcome. Neurosurgery 38:83–92, discussion 92–84
Lundell A, Bergqvist D, Mattsson E, Nilsson B (1993) Volume blood flow measurements with a transit time flowmeter: an in vivo and in vitro variability and validation study. Clin Physiol 13:547–557
Nornes H, Wikeby P (1977) Cerebral arterial blood flow and aneurysm surgery. Part 1: local arterial flow dynamics. J Neurosurg 47:810–818
Raabe A, Beck J, Gerlach R, Zimmermann M, Seifert V (2003) Near-infrared indocyanine green video angiography: a new method for intraoperative assessment of vascular flow. Neurosurgery 52:132–139, discussion 139
Regli L, Piepgras DG, Hansen KK (1995) Late patency of long saphenous vein bypass grafts to the anterior and posterior cerebral circulation. J Neurosurg 83:806–811
Samson DS, Neuwelt EA, Beyer CW, Ditmore QM (1980) Failure of extracranial–intracranial arterial bypass in acute middle cerebral artery occlusion: case report. Neurosurgery 6:185–188
Sekhar LN, Kalavakonda C (2002) Cerebral revascularization for aneurysms and tumors. Neurosurgery 50:321–331
Sen C, Sekhar LN (1992) Direct vein graft reconstruction of the cavernous, petrous, and upper cervical internal carotid artery: lessons learned from 30 cases. Neurosurgery 30:732–742, discussion 742–733
Spetzler RF, Schuster H, Roski RA (1980) Elective extracranial–intracranial arterial bypass in the treatment of inoperable giant aneurysms of the internal carotid artery. J Neurosurg 53:22–27
Sundt TM 3rd, Sundt TM Jr (1987) Principles of preparation of vein bypass grafts to maximize patency. J Neurosurg 66:172–180
Sundt TM Jr, Piepgras DG, Marsh WR, Fode NC (1986) Saphenous vein bypass grafts for giant aneurysms and intracranial occlusive disease. J Neurosurg 65:439–450
Tanaka F, Nishizawa S, Yonekura Y, Sadato N, Ishizu K, Okazawa H, Tamaki N, Nakahara I, Taki W, Konishi J (1995) Changes in cerebral blood flow induced by balloon test occlusion of the internal carotid artery under hypotension. Eur J Nucl Med 22:1268–1273
Woitzik J, Horn P, Vajkoczy P, Schmiedek P (2005) Intraoperative control of extracranial–intracranial bypass patency by near-infrared indocyanine green videoangiography. J Neurosurg 102:692–698
Acknowledgment
We would like to acknowledge Christa Wellman for the illustrations and figure preparation.
Disclosure
FTC is a consultant for Transonic Systems, Inc.
Author information
Authors and Affiliations
Corresponding author
Additional information
Comments
Dr. Amin-Hanjani et al. are to be congratulated for the thoughtful paper with the concept of “Flow replacement bypass for aneurysm” and excellent clinical results. As the authors mention, considerable numbers of large or giant unclippable aneurysms cannot be put to the BOT preoperatively, so that the method can contribute to select an appropriate bypass method also in a quasi-emergency situation. The same might be performed also with the use of electromagnetic flow meter, but the apparatus proposed by the authors is reported to be simpler to handle. With the combination of such apparatus with Peltier stack, one might know more delicate change of the cortical flow reflecting physiological neurovascular response such as non-reflow phenomenon, reactive hyperemia during the construction course of flow replacement bypass.
Reference
1. Ogata N, Fournier JY, Imhof HG, Yonekawa Y: Thermal diffusion blood flow monitoring during aneurysm surgery. Acta Neurochir (Wien) 138: 726–731, 1996
Yasuhiro YONEKAWA M.D.
Zürich
In this nice and well-written paper, the importance of how intraoperative flow measurements guide optimal revascularization by matching graft flow to demand is studied as definite management of some aneurysms may require sacrifice of the parent vessel. They reviewed all EC–IC bypasses performed over a 6-year period and identified 23 patients in whom US flow probe was utilized to determine the strategy. The techniques used and strategies chosen are clearly described providing the reader with sound and evidence-based advice on graft selection and how to confirm bypass success with only the retrospective nature of the study as the limitation. In the future, most likely, the number of cerebral bypasses will increase both in the treatment of impractical aneurysms but also in ischemic conditions and potentially in some skull-base tumors, and therefore, we need techniques that will help us not only in choosing those who would benefit from bypasses but also ultimately in making surgery safer with optimal results to provide adequate cerebral blood flow in every situation.
Mika Niemelä
Juha Hernesniemi
Helsinki, Finland
Rights and permissions
About this article
Cite this article
Amin-Hanjani, S., Alaraj, A. & Charbel, F.T. Flow replacement bypass for aneurysms: decision-making using intraoperative blood flow measurements. Acta Neurochir 152, 1021–1032 (2010). https://doi.org/10.1007/s00701-010-0635-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-010-0635-4