Abstract
Purpose
We propose the combined neurosurgical-endovascular treatment with the balloon occlusion of parent artery during surgery of giant paraclinoid and vertebrobasilar aneurysms, which are unsuitable for a pure endovascular or surgical approach.
Methods
Between January 2003 and December 2007, we treated surgically 15 giant aneurysms (11 paraclinoid and four vertebrobasilar) with the combined approach of surgery and endovascular intraoperative technique.
Findings
Complete aneurysm occlusion was achieved in all 15 aneurysms, as confirmed by intraoperative angiographic control. In one paraclinoid aneurysm, a small recurrence became evident 1 year after surgery and needed coil embolisation.
Conclusions
The temporary balloon occlusion technique is useful and improves the safety of the unavoidable exposure of the parent artery in the surgical treatment of giant paraclinoid and vertebrobasilar aneurysms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An essential step in cerebral vascular surgery is the exposure of the proximal part of parent vessels. We need to both check their patency and have them well exposed for temporary clipping. Among the restricted group of cerebral giant aneurysms, it is more difficult to obtain vascular proximal control of those belonging to the clinoid-ophthalmic portion of internal carotid artery (ICA) or posterior circulation [42]. This is principally because of the size of these aneurysms but also their contiguity to the skull base and tight relationship with the surrounding nervous structures. The manipulation of such aneurysms is associated with a higher risk of rupture and direct injury of contiguous neural structures. To gain a better vascular control during dissection of the aneurysmal sac, we employed a simple technique of keeping a balloon close to the beginning of the neck of the aneurysm. This allows the temporary occlusion of the parent artery and, consequently, safer surgical manoeuvres regarding the clipping of the sac.
Materials and methods
From January 2003 to September 2007, we treated surgically 15 patients (12 females and three males) with giant aneurysms: 11 with the paraclinoid segment of ICA and four with the vertebrobasilar tract. The average age was 51.7 years (37–68 years). Table 1 summarises the patients' clinical characteristics at admission. We evaluated the specific symptoms and the functional health of our patients, the latter according the modified Rankin Scale (mRS) [52]. Fourteen patients were referred to our centre for unruptured-symptomatic intracranial aneurysms with clinical presentation related to the mass effect of the sac (headache, seizures, cranial nerve compression or other neurologic disorders) and all could care for themselves (mRS of 1–2). We also treated a patient (mRS of 4) with a giant partially thrombosed aneurysm of posterior circulation, which had previously been treated for slight bleeding by surgery at another institution.
The surgical procedure was always performed with the intraoperative aid of a non-detachable flow-dependent balloon catheter (Balt B1, Balt Extrusion, 95160 Montmorency, France).
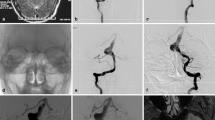
All patients were studied by magnetic resonance tomography of the brain and angiography (Figs. 1a and 2a). A diagnostic angiogram was always completed with a temporary balloon test occlusion (BTO) using a flow-dependent catheter (Fig. 1b). This test has many variants, as reported in literature [7, 8, 12, 14, 24, 27, 33, 43, 45, 51]. In our experience during this exam, the balloon was placed below the neck of all the aneurysms, but in those of posterior circulation, another was pushed in the dominant posterior cerebral artery (PCA). Particular attention was paid to collateral circulation, venous drainage and angiographic fillings of the cortical veins in the tested vascular area (Fig. 1c, d). According to van Rooij et al. [51], we agree that simultaneous filling of the cortical veins in both vascular territories reflects an equal circulation time, and this haemodynamic phenomenon apparently predicts a long-term tolerance to carotid occlusion.
a Left carotid angiography showing a paraclinoid aneurysm. b A non-detachable flow-directed balloon catheter (black arrow) was positioned just below the left OphA to perform the BTO. c, d After the right carotid injection, a good collateral arterial perfusion in the left hemisphere and a simultaneous filling of the cortical veins in both vascular territories were noted during the BTO. BTO balloon test occlusion, OphA ophthalmic artery
a Vertebrobasilar artery injection cerebral angiogram demonstrated a partially thrombosed giant basilar tip aneurysm. b Balloon occlusion (black arrow) of the basilar artery (BA) during left carotid angiogram. c–e Balloon occlusion (black arrow) of the BA during right carotid angiogram. Note the progressive and partial aneurysmal refill only via the right PcomA. f Post-surgery angiogram showing the successful clipping of the basilar aneurysm. ACA anterior cerebral artery, PCA posterior cerebral artery, PcomA posterior communicating artery, MCA middle cerebral artery
On the day of surgery under general anaesthesia in the radiology suite, a 7-French sheath was placed in the femoral artery, and a 6-French guiding catheter was positioned in the first part of the ICA or vertebral artery (VA) to avoid vasospasm. In paraclinoid aneurysms, the balloon catheter was then pushed into the intracavernous segment or immediately proximal to the origin of ophthalmic artery (OphA), whereas in vertebrobasilar aneurysms, it was placed below the neck (Fig. 2b–e). To treat the giant aneurysms of posterior circulation, another balloon catheter was located in the PCA to stop the retrograde blood flow from the dominant ICA. We used flow-directed catheters because of the lower thromboembolic risk compared with over-the-wire balloon catheters, even though the latter have a better positioning accuracy. The exact position and the inflation and deflation volume of the balloon were carefully checked several times. A 3,000 unit of heparin bolus was injected at the beginning of the procedure. The catheter had been successively washed with heparinised saline solution (5,000 units/l). When we were sure of the correct position and the adequate volume of balloon inflation, we moved patients with the complete arterial femoral system to the neurosurgical theatre. They were placed on a radiolucent operating table and the head secured with a standard Mayfield headholder. An intraoperative angiogram was always executed in the operating room to ensure the right position of the balloon in the parent artery before beginning surgery. No different positions of the balloon in the proximal vessels compared with those checked previously in the department of radiology were discovered after this exam. All patients with paraclinoid aneurysms and two with apex basilar aneurysms underwent an extended pterional approach with the interfascial dissection of scalp and temporal muscle [53, 55]. For two aneurysms of vertebrobasilar junction, a suboccipital approach was performed.
During surgical manoeuvres, the balloon was inflated when requested by the neurosurgeon. In this way, besides further guaranteeing the temporary occlusion of parent artery in the case of aneurysmal sac rupture, the dome became more manoeuvrable, and this permitted better vision of the parent artery, contiguous perforating branches and the correct placement of permanent clips.
Intraoperative angiogram control with a portable fluoroscope was performed at the end of the procedure to verify the adequacy of clipping and patency of the parent vessels.
Neurological status was evaluated after the procedure at hospital discharge, at 30 days after treatment and then at yearly intervals. In our report, we have considered and compared the neurologic assessment at discharge and after 1 year. This latter period of follow-up can be retained enough long to judge the definitive neurologic conditions of patients.
An angiography was undertaken before discharge (Fig. 2f) and successively 1 year after surgery.
Results
Table 2 summarises the patients' cumulative time of parent artery occlusion, number of balloon inflations, outcome at discharge and after 1 year and occurrence of aneurysmal recurrence.
The average time of cumulative temporary occlusion was 17 min. Inflation time was no longer than 5 min. Generally, no more than three or four balloon inflations were necessary for a total of 15–20 min occlusion time. This was enough time to complete the dissection of the aneurysm, obtain proximal vascular control and exclude the sac. Balloon rupture occurred during the last inflation in one patient, fortunately at the end of the dissection with no significant incidence on the surgical result. No embolic complications related to this procedure were observed. All 15 cases treated with this technique were excluded from parent vessel patency as confirmed by an intraoperative angiography.
Neurological status evaluated at discharge showed complete recovery of symptoms in one patient, improvement in 11 patients and unchanged symptoms in three patients. Furthermore, the mRS score was 0 in case 13; 1 in cases 1, 3, 4, 5, 6, 8, 9, 10, 11, 12, 14 and 15; 2 in case 2 and 4 in case 7. The complete recovery of symptomatology at 1 year occurred in ten patients, a further improvement in comparison with the neurologic outcome at the discharge in three patients and unchanged symptomatology in two patients. The mRS score was 0 in cases 3, 4, 5, 6, 8, 10, 11, 12, 13 and 15; 1 in cases 1, 9 and 14; 2 in case 2 and 4 in case 7 1 year after surgery.
In one paraclinoid aneurysm, a slight recurrence 1 year after surgery required a coil embolisation without clinical changes.
Discussion
Giant paraclinoid aneurysms are a rare pathology and represent a surgical challenge, even for experienced neurosurgeons. Because of their broad-based necks and close relation to the cavernous sinus, skull base and optic system, it is difficult to achieve safely proximal vascular control. In the literature, the extra/intra-dural resection of the anterior clinoid has been widely reported. Unroofing the orbital canal and opening the falciform fold of the optic nerve and the distal ICA ring to display a large clinoidal segment of ICA allow the latero-medial mobilisation of the optic nerve and its dissection from the aneurysmal sac [3–5, 9, 10, 13, 15, 16, 18–21, 29, 37, 39, 41, 42, 49, 50, 54]. This surgical strategy is exposed to the risk of accidental rupture of the aneurysm without a safe control of the proximal part of the artery. Simple occlusion or trapping of the arterial segment can be inadequate to soften the aneurysm and achieve the desired proximal vascular control, because of the retrograde blood flow through the OphA and cavernous branches. The literature is poor on this aspect. To solve this problem, Batjer and Samson [9] and Tamaki et al. [49] described a retrograde suction decompression through the cervical ICA cannulation after the temporary trapping of the aneurysm; variations on this technique were successively reported by others [19, 40]. Shucart et al. [41] proposed the intraoperative temporary balloon occlusion of the parent vessel via a transfemoral route, whereas Scott et al. [40] combined this method with a retrograde suction using a double-lumen catheter. In all previous reports, the authors utilised a single-lumen [41] or double-lumen balloon [5, 18, 29, 35, 37, 39, 40] temporarily delivered into the proximal parent artery, whereas in those of Thorell et al. [50] and Steiger et al. [44], the occlusion was performed intracranially below the aneurysmal orifice or neck. In our experience, the balloon was always situated across the intracavernous segment or just before the origin of OphA. This technique prevented partial aneurysmal refill through the carotid cavernous branches, whereas that through the OphA persisted. It was enough to stop the aneurysm perfusion via the cavernous branches to achieve an adequate deflation and dissection of the aneurysmal sac. We never employed the retrograde suction procedure with the potential risk of intravasal aspiration of air.
Of all cerebral aneurysms, those arising from posterior circulation have traditionally been the most difficult to treat [36]. The anatomic localisation of the vertebrobasilar tract with its branches near the brain stem, skull base and cranial nerves increases the difficulty of excluding aneurysms situated there, especially if they are giant ones. Few reports with anecdotal experience about the use of temporary balloon occlusion during the surgery of vertebrobasilar aneurysms have previously been published in the literature [6, 30, 39, 41]. We operated on four giant posterior circulation aneurysms using simultaneously two balloon catheters: one situated in the VA or basilar artery (BA) below the aneurysmal orifice, and the second placed in the PCA to prevent retrograde blood flow from the dominant ICA.
In our opinion, the ideal treatment of giant symptomatic aneurysms remains the complete exclusion from the circulation by employing multiple clips-assisted reconstructions. Furthermore, during the surgical procedure, the puncture or resection of the aneurysmal dome, the suction of a possible thrombus and coagulation of the sac can be performed to relieve the space-occupying effect.
Some authors emphasise the use of deep hypothermia and artificial cardiac arrest in selected cases [1, 23, 25, 46] and the selective cerebral revascularisation in aneurysms believed “unclippable” [11, 17, 22, 31, 34, 47]. Both these strategies seem to relieve the dangerous occurrence of temporary cerebral blood flow hypoperfusion. Nevertheless, in our series, the patients could tolerate a period time of temporary ICA much longer than that performed during the dissection and clipping of the aneurysms. The cerebral revascularisation was always taken in consideration as a possible therapeutic option in case the exclusion of the aneurysmal sac failed for insuperable problems during the surgical procedure.
Anyway, deep hypothermia and cardiac arrest or cerebral revascularisation is not free from risk.
Deep hypothermic circulatory arrest in some published series of giant aneurysms has been especially evaluated for posterior circulation aneurysms [23, 46], and in others also for anterior circulation [1, 25]. Circulatory arrest collapses the aneurysm, which can be easily manipulated together with its adjacent perforating arteries, whereas hypothermia confers cerebroprotective effects, lowering the metabolic demands of neurons and enhancing the brain's tolerance for the absence of cerebral blood [23]. However, it has many disadvantages: the increasing risk of arterial occlusion or dissection after femoral cannulation, the increasing risk of intraoperative and postoperative haemorrhage after intraoperative heparinisation, the traumatism of red blood cells and platelets because of a cardiopulmonary bypass pump, the worsening of hypocoagulable state, the increasing risk of stroke after circulatory arrest, the need for a multidisciplinary team of surgeons and technologists, which can be a difficult logistical endeavour, and the high cost of this procedure [23]. In Mack et al. [25] the total 30-day perioperative mortality, neurologic and medical complications were 12%, 14% and 17%. In Lawton et al. [23], the overall surgical mortality and morbidity were 8.3% and 13.3%.
A cerebral bypass is indicated when the occlusion of the parent vessel or one of the major branches is mandatory to treating complex giant aneurysms, and a preoperative BTO has shown inadequate collateral blood flow. The BTO was obviously executed in all cases of our series. A neurological follow-up and a haemodynamic assessment of collateral circulation, venous drainage and angiographic fillings of the cortical veins during for a period of 30 min were evaluated. Based on the testing results, all patients treated with the intraoperative balloon occlusion passed neuroclinically and showed the venous phase of the tested hemisphere simultaneous with the venous filling of the controlateral carotid artery or VA. Moreover, it has to be considered that in all the cases, the giant aneurysm had determined a narrowing of the parent vessel, giving time to establish an arterial collateral flow already evident before surgery. Electrophysiological monitoring with motor evoked potentials (MEPs), somatosensory evoked potentials (SSEPs) and electroencephalogram (EEG) during the preoperative angiography and BTO has been performed only in four cases with posterior circulation aneurysms, which added no information about the clinical response of these patients. Furthermore, in all our cases, the intraoperative cumulative time of temporary occlusion was always much less than the 30-min period assessment performed during the BTO. The temporary occlusion of the parent artery allowed the aneurysmal sac to soften enough to obtain a good view of the parent vessel and perforating branches before placing the clips. It is generally accepted that the complete obliteration of the aneurysmal sac with the reconstruction of the parent artery using multiple clips and the newer endovascular strategies represent the preferable treatment for giant complex aneurysms before employing the cerebral bypass technique [11, 17, 22, 31, 34, 47]. Moreover, as reported in O'Shaughnessy et al. [34], the bypass procedure has a significant morbidity, even in the best hands.
Even if the intraoperative electrophysiological monitoring with MEPs, SSEPs, EEG and microvascular Doppler ultrasonography are applicable to the surgical treatment of aneurysms [26, 32, 38, 48], it was not retained for utility in our series. A well-tolerated BTO and the adequate softening of the aneurysmal sac during the balloon inflation helped to achieve a safe temporary occlusion of the parent vessel and a good observation of perforators, thereby avoiding the risk of damaging them with consequently low blood perfusion in crucial territories.
In Alexander et al. [2], the intraoperative angiography showed that about 12% of craniotomies for intracranial aneurysms were associated with unexpected residual aneurysms or arterial occlusions. After the final obliteration of the aneurysm, we always performed an intraoperative angiography. In one patient, a residuum of the aneurysmal sac of the apex BA was detected, permitting the immediate repositioning of the clips. Furthermore, we found a slight recurrence of a paraclinoid aneurysm in one case a year after surgery.
Dangerous complications of all catheter techniques concerning the thromboembolic events have occasionally been reported [5, 18, 29, 37, 39]. Micro-embolism did not affect patients of our series. To avoid this, we utilised flow-directed catheters irrigated with heparinised saline flush. These catheters are smaller and safer than over-the-wire catheters. In our patients, after 5 min of temporary occlusion, the balloon was deflated and then re-inflated if necessary. In our opinion, this brief inflation time decreases the risk of developing thrombotic material that could spread after the deflation of the balloon. This aspect was also emphasised in the work of Fan et al. [19]. Even if their surgical procedure is different to ours, employing a double-lumen catheter placed in the ICA and a clip distal to the aneurysm, they crucially minimised the time taken for temporary clipping [19]. One of the major risks of developing micro-embolism seems to be related to the reopening of the temporary clipping and the spreading of the thrombotic material formed in the sac.
In the literature, the overall reported complication rate related to vessel injury or cerebral infarction for temporary ICA occlusion varies from 0% to 8.3% [28]. Nevertheless, in the vast experience of Meyers et al. [28], no carotid artery injury or complication has been shown after temporary endovascular occlusion of ICA, and this technique was considered extremely safe and cost-effective because of the low rate of complications.
In the end, we have to consider new endovascular stenting material and techniques, which will surely reduce the necessity for temporary balloon occlusion, leaving such a modality for selected cases. The recent use of new endovascular stents cases in our institute has confirmed this trend.
Conclusion
In our experience, combined balloon occlusion and surgical technique might be a helpful adjuvant strategy to increase the safety of the surgery of selected giant paraclinoid and vertebrobasilar aneurysms. The temporary blood flow interruption represents a major aid to the neurosurgeon in achieving vascular control and performing the usual surgical manoeuvres. In these cases, cooperation between the interventional neuroradiologist and the neurosurgeon is mandatory, but the recent new endovascular stenting technique will surely limit this approach to selected cases.
Abbreviations
- BA:
-
Basilar artery
- BTO:
-
Balloon test occlusion
- EEG:
-
Electroencephalogram
- ICA:
-
Internal carotid artery
- mRS:
-
Modified Rankin scale
- MEPs:
-
Motor evoked potentials
- OphA:
-
Ophthalmic artery
- PCA:
-
Posterior cerebral artery
- SSEPs:
-
Somatosensory evoked potentials
- VA:
-
Vertebral artery
References
Aebert H, Brawanski A, Philipp A, Behr R, Ullrich OW, Keyl C, Birnbaum DE (1998) Deep hypothermia and circulatory arrest for surgery of complex intracranial aneurysms. Eur J Cardiothorac Surg 13:223–229. doi:10.1016/S1010-7940(98)00018-9
Alexander TD, Macdonald RL, Weir B, Kowalczuk A (1996) Intraoperative angiography in cerebral aneurysm surgery: a prospective study of 100 craniotomies. Neurosurgery 39:10–18
Al-Mefty O (1992) The cranio-orbital zygomatic approach for intracranial lesions. Contemp Neurosurg 14:1–6
Al-Mefty O, Smith RR (1990) Tailoring the cranio-orbital approach. Keio J Med 39:217–224
Arnautovic KI, Al-Mefty O, Angtuaco E (1998) A combined microsurgical skull base and endovascular approach to giant and large paraclinoid aneurysms. Surg Neurol 50:504–520
Bailes J, Deeb Z, Wilson JA, Jungreis CA, Horton JA (1992) Intraoperative angiography and temporary balloon occlusion of the basilar artery as an adjunct to surgical clipping: technical note. Neurosurgery 30:949–953
Barker DW, Jungreis CA, Horton JA, Pentheny S, Lemley T (1993) Balloon test occlusion of the internal carotid artery: change in stump pressure over 15 minutes and its correlation with xenon CT cerebral blood flow. AJNR Am J Neuroradiol 14:587–590
Barr J, Lemley TJ, McCann RM (1998) Carotid artery balloon test occlusion: combined clinical evaluation and xenon-enhanced computed tomographic cerebral blood flow evaluation without patient transfer or balloon reinflation: technical note. Neurosurgery 43:634–638
Batjer HH, Samson DS (1990) Retrograde suction decompression of giant paraclinoid aneurysms: technical note. J Neurosurg 73:305–306. doi:10.3171/jns.1990.73.2.0305
Batjer HH, Kopitnik TA, Giller CA, Samson DS (1994) Surgery for paraclinoid carotid artery aneurysms. J Neurosurg 80:650–658. doi:10.3171/jns.1994.80.4.0650
Cantore G, Santoro A, Guidetti G, Delfinis CP, Colonnese C, Passacantilli E (2008) Surgical treatment of giant intracranial aneurysms: current viewpoint. Neurosurgery 63(4 Suppl 2):279–290. doi:10.1227/01.NEU.0000313122.58694.91
Dare AO, Chaloupka JC, Putman CM, Fayad PB, Awad IA (1998) Failure of the hypotensive provocative test during temporary balloon test occlusion of the internal carotid artery to predict delayed hemodynamic ischemia after therapeutic carotid occlusion. Surg Neurol 50:147–156
Day AL (1990) Aneurysms of the ophthalmic segment. A clinical anatomical analysis. J Neurosurg 72:677–691. doi:10.3171/jns.1990.72.5.0677
do Souto AA, Domingues FS, Espinosa G, Wajnberg E, Chagas H, Tragante R, Altino M, André C, de Souza JM (2006) Complex paraclinoidal and giant cavernous aneurysms: importance of preoperative evaluation with temporary balloon occlusion test and SPECT. Arq Neuropsiquiatr 64:768–773. doi:10.1590/S0004-282X2006000500013
Dolenc VV (1985) A combined epi- and subdural direct approach to carotid-ophthalmic artery aneurysms. J Neurosurg 62:667–672. doi:10.3171/jns.1985.62.5.0667
Dolenc VV (1990) Surgery of vascular lesions of the cavernous sinus. Clin Neurosurg 36:240–255
Ewald CH, Kuhne D, Hassler WE (2000) Bypass-surgery and coil-embolisation in the treatment of cerebral giant aneurysms. Acta Neurochir 142:731–738. doi:10.1007/s007010070087
Fahlbusch R, Nimsky C, Huk W (1997) Open surgery of giant paraclinoid aneurysms improved by intraoperative angiography and endovascular retrograde suction decompression. Acta Neurochir 139:1026–1032
Fan YW, Chan KH, Lui WM, Hung KN (1999) Retrograde suction decompression of paraclinoid aneurysms—a revised technique. Surg Neurol 51:129–131
Heros RC, Nelson PB, Ojemann RG, Crowell RM, DeBrun G (1983) Large and giant paraclinoid aneurysms: surgical techniques, complications, and results. Neurosurgery 12:153–163
Kobayashi S, Kyoshima K, Gibo H, Hegde SA, Takemae T, Sugita K (1989) Carotid cave aneurysms of the internal carotid artery. J Neurosurg 70:216–221. doi:10.3171/jns.1989.71.2.0302
Langer DJ, Van der Zwan A, Vajkoczy P, Kivipelto L, Van Doormaal TP, Tulleken CA (2008) Exicimer laser-assisted non occlusive anastomosis. An emerging technology for use in the creation of intracranial-intracranial and extracranial-intracranial cerebral bypass. Neurosurg Focus. doi:10.3171./FOC/2008/24/2/E6
Lawton MT, Raudzens PA, Zabramski JM, Spetzler RF (1998) Hypothermic circulatory arrest in neurovascular surgery: evolving indications and predictors of patient outcome. Neurosurgery 43:10–20
Linskey ME, Jungreis CA, Yonas H, Hirsch WL Jr, Sekhar LN, Horton JA, Janosky JE (1994) Stroke risk after abrupt internal carotid artery sacrifice: accuracy of preoperative assessment with balloon test occlusion and stable xenon-enhanced CT. AJNR Am J Neuroradiol 15:829–843
Mack WJ, Ducruet AF, Angevine PD, Komotar RJ, Shrebnick DB, Edwards NM, Smith CR, Heyer EJ, Monyero L, Connolly ES Jr, Solomon RA (2007) Deep hypothermic circulatory arrest for complex cerebral aneurysms: lessons learned. Neurosurgery 60:815–827. doi:10.1227/01.NEU.0000255452.20602.C9
Martin CJ, Sinson G, Patterson T, Zager EL, Stecker MM (2002) Sensitivity of scalp EEG, cortical EEG, and somatosensory evoked responses during surgery for intracranial aneurysms. Surg Neurol 58:317–321
Mathis JM, Barr JD, Jungreis CA, Yonas H, Sekhar LN, Vincent D, Pentheny SL, Horton JA (1995) Temporary balloon test occlusion of the internal carotid artery: experience in 500 cases. AJNR Am J Neuroradiol 16:749–754
Meyers PM, Thakur GA, Tomsick TA (1999) Temporary endovascular balloon occlusion of the internal carotid artery with a non-detachable silicon balloon catheter: analysis of technique and cost. AJNR Am J Neuroradiol 20:559–564
Mizoi K, Takahashi A, Yoshimoto T (1993) Combined endovascular and neurosurgical approach for paraclinoid internal carotid artery aneurysms. Neurosurgery 33:986–992
Mizoi K, Yoshimoto T, Takahashi A, Ogawa A (1994) Direct clipping of basilar trunk aneurysms using temporary balloon occlusion. J Neurosurg 80:230–236. doi:10.3171/jns.1994.80.2.0230
Mohit AA, Sekhar LN, Natarajan SK, Britz GW, Ghodke B (2007) High-flow bypass grafts in the management of complex intracranial aneurysms. Neurosurgery 60(Suppl):105–123. doi:10.1227/01.NEU.0000249243.25429.EE
Neuloh G, Schramm J (2004) Monitoring of motor evoked potentials compared with somatosensory evoked potentials and microvascular Doppler ultrasonography in cerebral aneurysm surgery. J Neurosurg 100:389–399. doi:10.3171/jns.2004.100.3.0389
Origitano TC, Al-Mefty O, Leonetti JP, DeMonte F, Reichman OH (1994) Vascular considerations and complications in cranial base surgery. Neurosurgery 35:351–363
O'Shaughnessy BA, Salehi SA, Mindea SA, Batjer HH (2003) Selective cerebral revascularization as an adjunct in the treatment of giant anterior circulation aneurysms. Neurosurg Focus 14:e4. doi:10.3171/foc.2003.14.3.5
Parkinson RJ, Bendok BR, Getch CC, Yashar P, Shaibani A, Ankenbrandt W, Awad IA, Batjer HH (2006) Retrograde suction decompression of giant paraclinoid aneurysms using a No. French balloon-containing guide catheter: technical note. J Neurosurg 105:479–481. doi:10.3171/jns.2006.105.3.479
Peerless SJ, Drake CG (1990) Management of aneurysms of the posterior circulation. In: Youmans JR (ed) Neurological surgery. Philadelphia, WB Saunders Co, pp 1764–1806
Puay-Yong NG, Huddle D, Gunel M, Awad IA (2000) Intraoperative endovascular treatment as an adjunct to microsurgical clipping of paraclinoid aneurysms. J Neurosurg 93:554–560. doi:10.3171/jns.2000.93.4.0554
Quinones-Hinojosa A, Alam M, Lyon R, Yingling CD, Lawton MT (2004) Transcranial motor evoked potentials during basilar artery aneurysm surgery: technique application for 30 consecutive patients. Neurosurgery 54:916–924. doi:10.1227/01.NEU.0000114511.33035.AF
Ricci G, Ricci M, Gallucci M, Zotta D, Scogna A, Costagliola C, Galzio RJ (2005) Combined endovascular and microsurgical approach in the treatment of giant paraclinoid and vertebrobasilar aneurysms. J Neurosurg Sci 49:1–6
Scott JA, Terry GH, Leipzig TJ (1991) Retrograde suction decompression of an ophthalmic artery aneurysm using balloon occlusion: technical note. J Neurosurg 75:146–147. doi:10.3171/jns.1991.75.1.0146
Shucart WA, Kwan ES, Heilman CB (1990) Temporary balloon occlusion of a proximal vessel as an aid to clipping aneurysms of the basilar and paraclinoid internal carotid arteries: technical note. Neurosurgery 27:116–119
Spetzler RF, Rijna A, Lemole GM (2001) Giant aneurysms. Neurosurgery 49:902–908
Standard SC, Ahuja A, Guterman LR, Chavis TD, Gibbons KJ, Barth AP, Hopkins LN (1995) Balloon test occlusion of the internal carotid artery with hypotensive challenge. AJNR Am J Neuroradiol 16:1453–1458
Steiger HJ, Lins F, Mayer T, Schmid-Elsaesser R, Stummer W, Turowski B (2005) Temporary aneurysm orifice balloon occlusion as an alternative to retrograde suction decompression for giant paraclinoid internal carotid artery aneurysm: technical note. Neurosurgery 56(2 Suppl):E 442. doi:10.1227/01.neu.0000157102.01803.8c
Sudhakar KV, Sawlani V, Phadke RV, Kumar S, Ahmed S, Gujral RB (2000) Temporary balloon occlusion of internal carotid artery: a simple and reliable clinical test. Neurol India 48:140–143
Sullivan BJ, Sekhar LN, Duong DH, Mergner G, Alyano D (1999) Profound hypothermia and circulatory arrest with skull base approaches for treatment of complex posterior circulation aneurysms. Acta Neurochir 141:1–12. doi:10.1007/s007010050259
Surdell DL, Hage ZA, Eddleman CS, Gupta DK, Bendok BR, Batjer HH (2008) Revascularization for complex intracranial aneurysms. Neurosurg Focus. doi:10.3171/FOC.2008.25.2.E21
Szelenyi A, Langer D, Beck J, Raabe A, Flamm ES, Seifert V, Deletis V (2007) Transcranial and direct cortical stimulation for motor evoked potential monitoring in intracerebral aneurysm surgery. Neurophysiol Clin 37:391–398. doi:10.1016/j.neucli.2007.09.006
Tamaki N, Kim S, Ehara K, Asada M, Fujita K, Taomoto K, Matsumoto S (1991) Giant carotid-ophthalmic artery aneurysms: direct clipping utilizing the “trapping-evacuation” technique. J Neurosurg 74:567–572. doi:10.3171/jns.1991.74.4.0567
Thorell W, Rasmussen P, Perl J, Masarik T, Mayberg M (2004) Balloon-assisted microvascular clipping of paraclinoid aneurysms. J Neurosurg 100:713–716. doi:10.3171/jns.2004.100.4.0713
van Rooij WJ, Sluzewski M, Metz NH, Nijssen PC, Wijnalda D, Rinkel GJ, Tulleken CA (2000) Carotid balloon occlusion for large and giant aneurysms: evaluation of a new test occlusion protocol. Neurosurgery 47:116–121
van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J (1988) Interobserver agreement for the assesment of handicap in stroke patients. Stroke 19:604–607
Yasargil MG (1984) Interfascial pterional (frontotemporosphenoidal) craniotomy. In: Yasargil MG (ed) Microneurosurgery vol 1. Georg Thieme Verlag, Stuttgart, pp 217–220
Yasargil MG (1984) Microneurosurgery. Vol 2. Clinical considerations, surgery of the intracranial aneurysms and results. Georg Thieme Verlag, Stuttgart
Yasargil MG, Reichman MV, Kubik S (1987) Preservation of the fronto-temporal branch of the facial nerve using the interfascial temporalis flap for pterional craniotomy. J Neurosurg 67:463–466. doi:10.3171/jns.1987.67.3.0463
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Skrap, M., Petralia, B. & Toniato, G. Temporary balloon occlusion during the surgical treatment of giant paraclinoid and vertebrobasilar aneurysms. Acta Neurochir 152, 435–442 (2010). https://doi.org/10.1007/s00701-009-0566-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-009-0566-0