Abstract
Purpose
With the frequent use of magnetic resonance imaging (MRI), patients with subtle and diffuse symptoms due to small syrinx cavities increasingly present to neurosurgical care. In this respect, a dilated central canal, hydromyelia, must be separated from patients with true syringomyelia with an underlying disorder, as they do not share clinical and radiological features. We hypothesize that a differentiation of these two entities with distinct diagnostic tools is possible.
Methods
To describe the entity of hydromyelia, we excluded all patients from the syringomyelia database (n = 142) with any obvious cause of a syringomyelia, any objective neurological deficits on clinical examination, pathological results on electrophysiological monitoring (SSEP, MEP, silent periods) or a widening of the spinal cord cavity of more than 6 mm on MRI [routine acquisitions with FLAIR, T1/T2-weighted images, Cine and CISS (constructive interference in steady-state) studies]. Life quality was assessed through SF-36 questionnaires and an individualized questionnaire for the clinical history, pain and alternative therapies.
Results
Forty patients (15 males/25 females) matched the criteria of a hydromyelia. With a mean age of 36.7 years (range 11–62), they almost all presented with pain (79%) or dysaesthesia of the limbs, with some having been an incidental finding (10%). Over a follow-up time of 36.9 months (range 6–93) there was no neurological or radiological deterioration.
Conclusions
Patients with a hydromyelia do not share clinical or radiological characteristics with patients harbouring a true syringomyelia. As hydromyelia does not represent a disease with an underlying pathology, no clinical or radiological progression has been seen. With sophisticated diagnostic tools to rule out any pathology this subset of patients can be identified.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A centromedullary syndrome with predominant symptoms of dissociated pain and thermal sensory impairment clinically characterizes syringomyelia. Later in the course of the disease with distension of important pathways, segmental weakness, atrophy, upper motor neuron syndrome and autonomic dysfunction may occur.
However, with the more frequent use of magnetic resonance imaging (MRI) in the last few decades, patients with subtle and diffuse symptoms or incidentally detected small syrinx cavities increasingly present to neurosurgical care. Some authors named these cavities “central canal syrinx”, “syringohydromyelia”, “idiopathic localized hydromyelia” or “slit-like syrinx cavities” [11–13, 20, 27]. A consistent definition of non-syringomyelia cavities has not been given up to today as some authors included in their studies various expansive forms of central canal syringes [19], diameters up to 5 mm [11] or only non-dilatating cavities at all [13].
Based on a workflow for syringomyelia patients, we aimed to define the term “hydromyelia” in contrast to true syringomyelia with an underlying disorder. With the help of specific MRI sequences, electrophysiological studies and clinical follow-up results, we want to articulate that a differentiation between hydromyelia and syringomyelia is possible.
Materials and methods
Inpatients and outpatients undergoing treatment for syringomyelia at the Department of Neurosurgery, University of Tübingen, Germany voluntarily participated in the present study, which was conducted from July 2005 to July 2008. A total of 142 patients were examined, 88 were female and 54 were male (sex ratio 1.63:1), with a mean age of 50.4 years (age range: 14–79 years).
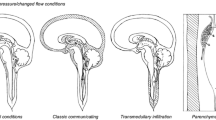
To eliminate patients with syringomyelia from the database, exclusion criteria for patients incorporated in the “hydromyelia study group” were neuroradiological detection of any obvious cause of a syringomyelia: malformations of the cranio-cervical junction, e.g. Chiari malformation, tethered cord, spina bifida occulta or aperta, subarachnoid cysts, intraspinal tumours, severe scoliosis, spinal trauma in the medical history, status post meningitis, any previous spinal surgery (ventral fusions, dorsal instrumentations) as well as previous peridural anaesthesia (Fig. 1).
To detect even subtle pathological changes of the spinal subarachnoid space, all patients had MRI examinations on a Magnetom Sonata 1.5 T (Siemens, Erlangen, Germany) with a circular polar phased-array spinal coil. In addition to routine acquisition of T1-weighted spin-echo (SE) and T2-weighted fast SE (FSE) sagittal and axial images with and without contrast enhancement, constructive interference in steady-state (CISS) sequences, as well as cardiac-gated cine-MRI studies were obtained [28]. Neurophysiological investigation was performed with standard electrodiagnostic equipment (Viking IV P, Nicolet Company). Conduction studies included SSEP and MEP monitoring for all extremities, as well as detection of spino-thalamic pathway alterations with silent period studies (CSP, MNSP and CoSP) as previously described [29]. Any pathological results on electrophysiological monitoring as well as a widening of a centrally located spinal cord cavity of more than 6 mm were considered to be of different origin to the central canal and were not incorporated in the study [11].
Life quality for all patients (n = 142) was assessed through the standardized SF-36 questionnaire and a Syringomyelia Disability Index (SDI). The SDI, composed from the Neck Disability Index (NDI) and the Oswestry Disability Index is comprised of 23 questions pertaining to routine activities, pain, and physical function and was tailored to address the symptoms of syringomyelia patients [7, 34]. Statistical analysis was conducted with SPSS 15.0 software to calculate coherence, including Pearson’s correlation for metric data, two-sided tests for p value, and a t-test for group mean comparisons.
Results
Forty patients met all the inclusion criteria for the hydromyelia study group; among them there were 25 females and 15 males (mean age 36, range 11–62). The hydromyelia was located mainly over the thoracic segments (51%), the cervico-thoracic in 25% and the cervical area in 23% (Fig. 2). The mean sagittal expansion of the hydromyelic cavity was 3.5 vertebrae. Only one cavity over the length of the spinal cord was shown by 73% of patients; 23% showed two dilatations and 33% had three cavities present. The mean transversal diameter of the expansive area over the spinal cord was 2.7 mm (range 1.2–5.8; SD 1.2). The central canal as a holocord extension over the entire spinal cord was visible in over 70% of patients on high resolution CISS imaging (slices <1 mm).
Almost two-thirds of all patients in the hydromyelia study group presented with pain as the primary symptom and this was the reason for MRI examination, whereas four patients had an incidental finding on MRI (10%) (Fig. 3). Pain character varied from radicular, to burning neuropathic or diffuse musculoskeletal sensations. Subjective sensory or motor function disturbance was mentioned as well as gait instability or typical dissociative symptoms. However, none of the complained symptoms could be verified on neurological examination.
With the adjusted diagnostic protocol comprising MRI sagittal CISS- and Cine-sequences and extensive electrophysiological testing, ten patients from the former “idiopathic” syringomyelia group could be identified as harbouring a small arachnoid cyst leading to cerebrospinal fluid (CSF) flow disturbance and a concomitant alteration of spino-thalamic pathways detected with silent periods on electrophysiological testing. These lesions were then classified as a small emerging syringomyelia and no hydromyelia (Fig. 1).
The remaining patients with true hydromyelia had no radiological changes in 93% of cases over a mean follow-up period of 36.9 months (range 6–93; SD 22.1). In two patients, the expansion of the dilatation seemed to be less; however, the measurable difference was less than 10%. Concomitant to the radiological state, clinical re-evaluation showed a stable situation in all patients with no neurological deficit occurring over the observation period.
The physical (PCS) and mental (MCS) component score of the SF-36 questionnaire revealed significant differences between the mean of the German population (PCS 50.2, MCS 51.5) [5], syringomyelia and hydromyelia patients (p < 0.01), but no differences among patients with either syringomyelia (PCS 33.2, MCS 45.0) or hydromyelia (PCS 37.9, MCS 46.6) (Fig. 4). However, the SDI was significantly higher in hydromyelia patients (73%) compared with syringomyelia patients (64%) (p < 0.023). No significant changes in life quality assessment have been noticed over the follow-up period. Moreover patients’ judgment concerning their clinical restriction was improved or stable in 85%. Compared with patients with a true syringomyelia (n = 102), this is highly significant (p < 0.001) (Fig. 5).
Discussion
Whenever a hydromyelia is being detected by neuroradiological imaging, patients get confronted with the diagnosis of a syringomyelia. With the frequent use of internet-based information, patients get extremely confused and do quite often not except the fact that it might be a congenital variant. The term syringomyelia was first described by Ollivier D’Angers in 1824 [24], but in 1859 Stilling reported about hydromyelia as a pathological excavation of the spinal cord [32]. From that time onwards these terms were inconsequently used; describing similar pathologies, Leyden stated in 1876 that “Syringomyelia found in an adult is a kind of a congenital hydromyelia...” [17]. In recent decades the term syringomyelia has become established, although it again subsumes various kinds of pathologies.
The central canal of the spinal cord is a midline space opening superiorly into the fourth ventricle and extending inferiorly throughout the central spinal cord to terminate into the proximal filum terminale. The canal derives from the neural groove appearing on the 7th day of embryonic life and later becoming the neural tube. The neural tube closes on the 14th day of gestation progressively forming a canal. At birth, its diameter is only 0.05–0.1 mm [1]. In the region of the conus medullaris, the canal normally expands during embryogenesis as a fusiform terminal ventricle, the ventriculus terminalis [6, 18, 30]. Before it undergoes developmental obliteration, the central canal contains small amounts of CSF and is lined by a columnar ciliated ependymal cell ephitelium [25, 36]. Anatomical studies suggest that the sharply delineated central canal is seen only in fetal and newborn spinal cords [36], as it is widely accepted that the central canal undergoes age-related stenosis such that it is obliterated in the vast majority of adults [14, 20, 22, 37]. The results of Yasui et al. [37], who analyzed the age-related morphological changes of the central canal in 158 autopsy cases, suggest that the stenosis of the central canal influences the anatomical features of syringomyelia. On standard investigation the central canal is not visible [2], in paediatric sonography it is described as a linear echo [10, 21].
With the widespread use of MRI, more of these slit-like syrinx cavities have been diagnosed [11]. In their report on three adult patients, Jinkins et al. [13] found asymptomatic localized widening of the central canal of the spinal cord and termed this condition “idiopathic localized hydromyelia”. All of their patients were clinically and radiologically stable over a follow-up period of 3 years. Holly and Batzdorf [11] described 32 patients, 80% of whom had no further progression after 3 years. The fact that not all of the reported patients had a stable clinical course demonstrates the difficulty in defining the line between a stable patent central canal and an emerging syrinx [8, 26].
As the underlying pathology in borderline cases is difficult to assess, electrophysiological diagnostics may help to distinguish between a physiologically patent central canal en route to a syringomyelia with early alteration of the spino-thalamic tract [29]. We demonstrated that an emerging syringomyelia can be sufficiently diagnosed with a combination of several electrophysiological parameters, including the silent periods [29]. However, electrophysiological diagnosis must match the clinical and investigative findings. Patients can be re-evaluated without the risk of subsequent progression, but follow-up studies are necessary to show the predictive value of the silent periods on the long-term course of syringomyelia. Sophisticated imaging with high spatial resolution [26], stronger magnetic fields and specially designed spinal coils almost close the gap between pathological and normal images [3]. Adapted MRI sequences help to identify patients formerly diagnosed with idiopathic syringomyelia. Subtle subarachnoid adhesions might be missed on routine acquisitions, but detected on CISS sequences [28].
We and others have found the hydromyelia to be predominantly centrally located in the thoracic spine, extending over 3–5 segments and of filiforme shape [11, 26]. No neurological deficits come along with the patients, who present mainly because of diffuse pain, different from neuropathic pain in dissociative syndrome. Regular electrophysiological parameters on routine and extended conduction of different spinal cord pathways underline the benignity of hydromyelia. According to Jinkins et al. [13], the criteria for hydromyelia should also include a localized short-segment, non-enhancing centromedullary cavity, occurring in an non-enlarged or only slightly enlarged spinal cord; the cavity should not progress over time and the patient should be without progressive signs of symptoms specifically related to the spinal cord.
With the invention of modern diagnostic procedures, it has been postulated that minor spinal traumas or focal subarachnoid haemorrhages could be responsible for the development of a syringomyelia years later [15, 28]. But one of the greatest mysteries in the development of syringomyelia is why only 5% of patients with minor spinal cord trauma develop a syringomyelia years later [4, 35]. Additionally, why do not all of the patients operated on an intradural or intramedullary spinal tumour suffer from late sequelae due to a syrinx cavity? Furthermore, one of the exceptional aspects surrounding Chiari I–induced syringomyelia is that only 30% develop a syringomyelia at all and that the location of the syrinx varies from patient to patient and may begin several vertebral levels below the foramen magnum [23].
The hydromyelia might be a predisposition. If in these patients an adequate trauma occurs, the development of a syringomyelia due to pressure changes in the subarachnoid space can take place [9]. Furthermore, holocord syrinx cavities are more frequently found in children, in whom the canals are more likely to be patent [11], whereas adults are more likely to harbour focal syringes. Levy et al. [16] reported about a patient with Chiari malformation, in whom spinal cord swelling preceded by a few months the development of a syrinx in the same location. This report is consistent with the theory that patients with Chiari malformation have increased transmural flow of CSF, which causes spinal cord swelling that later coalesces into a syrinx. In this context, Takamura et al. [33] described the case of a young man with the development of a post-traumatic syringomyelia. The MRI studies clearly demonstrated that a pre-existing patent central canal became dilated as the syrinx developed.
Hydromyelia seen today might be the underlying predisposition for developing a syringomyelia if an adequate impulse takes place. It must be our responsibility to further investigate how to predict the occurrence of syringomyelia in which patients and vice versa. The diagnosis of a syringomyelia has a potentially great impact on the further life of the patient [31]. The socio-economic and psycho-social consequences for patients with the diagnosis of a syringomyelia can be significant. Financial compensation for loss of work ability, anxiety and reduced daily activities may increase disease chronicity where no underlying pathology exists. When treating hydromyelia patients, one of the goals must be not to lose track of them, as they often insist on invasive diagnostics and even therapies. Explaining the benignity of the lesion should have a positive influence on the patients, not leaving them alone and denying symptoms [31]. Pain described by almost all of these patients is managed very well with continuously conservative treatment, strengthening of the musculoskeletal axis and slowing of spinal degenerative processes.
Thus, we suggest the following definition of hydromyelia based on clinical, electrophysiological and radiological criteria in order to distinguish it from presyrinx and the syrinx state: hydromyelia is an intramedullary, centrally located, slit-like cavitation. Patients present without neurological deficits but unspecific pain syndromes; they lack electrophysiological alterations and do not inherit any intraspinal pathology potentially responsible for CSF flow disturbances on neuroradiological imaging.
References
Aboulker J (1979) Syringomyelia and intra-rachidian fluids. X. Rachidian fluid stasis. Neurochirurgie 25(Suppl 1):98–107
Beuls E, Gelan J, Vandersteen M, Adriaensens P, Vanormelingen L, Palmers Y (1993) Microanatomy of the excised human spinal cord and the cervicomedullary junction examined with high-resolution MR imaging at 9.4 Tesla. AJNR Am J Neuroradiol 14:699–707
Beuls EA, Vandersteen MA, Vanormelingen LM, Adriaensens PJ, Freling G, Herpers MJ, Gelan JM (1996) Deformation of the cervicomedullary junction and spinal cord in a surgically treated adult Chiari I hindbrain hernia associated with syringomyelia: a magnetic resonance microscopic and neuropathological study. Case report. J Neurosurg 85:701–708
Biyani A, el Masry WS (1994) Post-traumatic syringomyelia: a review of the literature. Paraplegia 32:723–731
Bullinger M, Kirchberger I (1998) SF-36 Fragebogen zum Gesundheitszustand, Handanweisung. Hogrefe, Göttingen
Coleman LT, Zimmerman RA, Rorke LB (1995) Ventriculus terminalis of the conus medullaris: MR findings in children. AJNR Am J Neuroradiol 16:1421–1426
Fairbank JC, Pynsent PB (2000) The Oswestry disability index. Spine 25:2940–2952. doi:10.1097/00007632-200011150-00017 discussion 2952
Fischbein NJ, Dillon WP, Cobbs C, Weinstein PR (1999) The “presyrinx” state: a reversible myelopathic condition that may precede syringomyelia. AJNR Am J Neuroradiol 20:7–20
Greitz D (2006) Unraveling the riddle of syringomyelia. Neurosurg Rev 29:251–264. doi:10.1007/s10143-006-0029-5
Gusnard DA, Naidich TP, Yousefzadeh DK, Haughton VM (1986) Ultrasonic anatomy of the normal neonatal and infant spine: correlation with cryomicrotome sections and CT. Neuroradiology 28:493–511. doi:10.1007/BF00344103
Holly LT, Batzdorf U (2002) Slitlike syrinx cavities: a persistent central canal. J Neurosurg Spine 97:161–165
Iskandar BJ, Hedlund GL, Grabb PA, Oakes WJ (1998) The resolution of syringohydromyelia without hindbrain herniation after posterior fossa decompression. J Neurosurg 89:212–216
Jinkins JR, Sener RN (1999) Idiopathic localized hydromyelia: dilatation of the central canal of the spinal cord of probable congenital origin. J Comput Assist Tomogr 23:351–353. doi:10.1097/00004728-199905000-00004
Kasantikul V, Netsky MG, James AE Jr (1979) Relation of age and cerebral ventricle size to central canal in man. Morphological analysis. J Neurosurg 51:85–93
Klekamp J (2002) The pathophysiology of syringomyelia—historical overview and current concept. Acta Neurochir (Wien) 144:649–664. doi:10.1007/s00701-002-0944-3
Levy EI, Heiss JD, Kent MS, Riedel CJ, Oldfield EH (2000) Spinal cord swelling preceding syrinx development. Case report. J Neurosurg Spine 92:93–97
Leyden E (1873) Ueber Hydromyelus und Syringomyelie. Virchows Arch A Pathol Anat Histol 68:1–26
Matsubayashi R, Uchino A, Kato A, Kudo S, Sakai S, Murata S (1998) Cystic dilatation of ventriculus terminalis in adults: MRI. Neuroradiology 40:45–47. doi:10.1007/s002340050537
Milhorat TH, Johnson RW, Milhorat RH, Capocelli AL Jr, Pevsner PH (1995) Clinicopathological correlations in syringomyelia using axial magnetic resonance imaging. Neurosurgery 37:206–213. doi:10.1097/00006123-199508000-00003
Milhorat TH, Kotzen RM, Anzil AP (1994) Stenosis of central canal of spinal cord in man: incidence and pathological findings in 232 autopsy cases. J Neurosurg 80:716–722
Nelson MD Jr, Sedler JA, Gilles FH (1989) Spinal cord central echo complex: histoanatomic correlation. Radiology 170:479–481
Netsky M (1953) Syringomyelia. A clinicopathologic study. Arch Neurol Psychiatry 70:741–777
Oldfield EH (2001) Syringomyelia. J Neurosurg Spine 95:153–155
Ollivier d’Angers C (1824) Traité de la moelle epinaré et de ses maladies. Chez Crevot, Paris
Parent A (1996) Carpenter’s human neuroanatomy. Williams & Wilkins, Baltimore
Petit-Lacour MC, Lasjaunias P, Iffenecker C, Benoudiba F, Hadj RM, Hurth M, Doyon D (2000) Visibility of the central canal on MRI. Neuroradiology 42:756–761. doi:10.1007/s002340000373
Rao VR, Joseph S, Mandalam KR, Jain SK, Gupta AK, Unni NM, Rao AS, Mohan PK (1991) Syringohydromyelia: radiological evaluation of 82 patients in a developing country. Clin Radiol 44:165–171. doi:10.1016/S0009-9260(05)80861-X
Roser F, Ebner FH, Danz S, Riether F, Ritz R, Dietz K, Naegele T, Tatagiba M (2008) 3D-constructive interference in steady-state (CISS) Magnetic resonance imaging in Syringomyelia: advantages to conventional imaging. J Neurosurg 8:429–435
Roser F, Ebner FH, Liebsch M, Dietz K, Tatagiba M (2008) A new concept in the electrophysiological evaluation of syringomyelia. J Neurosurg 8:256–260
Sigal R, Denys A, Halimi P, Shapeero L, Doyon D, Boudghene F (1991) Ventriculus terminalis of the conus medullaris: MR imaging in four patients with congenital dilatation. AJNR Am J Neuroradiol 12:733–737
Sixt C, Riether F, Will B, Tatagiba M, Roser F (2009) Evaluation of quality of life parameters in Syringomyelia patients. J Clin Neurosci (in press)
Stilling B (1859) Neue Untersuchungen über den Bau des Rückenmarks. Hotop Kassel
Takamura Y, Kawasaki T, Takahashi A, Nunomura K, Tiba K, Hasunuma M, Itou T (2001) A craniocervical injury-induced syringomyelia caused by central canal dilation secondary to acquired tonsillar herniation. Case report. J Neurosurg Spine 95:122–127
Vernon H (2008) The Neck Disability Index: state-of-the-art, 1991–2008. J Manipulative Physiol Ther 31:491–502. doi:10.1016/j.jmpt.2008.08.006
Wang D, Bodley R, Sett P, Gardner B, Frankel H (1996) A clinical magnetic resonance imaging study of the traumatised spinal cord more than 20 years following injury. Paraplegia 34:65–81
Williams B, Sgouros S, Nenji E (1995) Cerebrospinal fluid drainage for syringomyelia. Eur J Pediatr Surg 5(Suppl 1):27–30. doi:10.1055/s-2008-1066259
Yasui K, Hashizume Y, Yoshida M, Kameyama T, Sobue G (1999) Age-related morphologic changes of the central canal of the human spinal cord. Acta Neuropathol 97:253–259. doi:10.1007/s004010050982
Author information
Authors and Affiliations
Corresponding author
Additional information
Comment
Roser and co-workers have approached a difficult and controversial subject: hydromyelia. If all cavities in the spinal cord can be named syrinxes, it seems often difficult to be sure that we are truly dealing with a dilated central canal. If thin syrinxes can be a central canal residue or perhaps a dilated central canal (hydromyelia), they can also be an intraparenchymal cavity located truly inside the spinal cord parenchyma. On the other hand, large syrinxes with prominent related signs could also be dilated central canal or a combination of central canal and intraparenchymal cavities. Using different terminologies outside a physiopatholgical discussion can be confusing and misleading. In most cases of possible or even probable hydromyelia we remain, nonetheless, uncertain. Besides, some of these slit-syrinxes that are possibly hydromyelia, instead of being really asymptomatic could be related to a very progressive atrophic process, becoming more evident clinically and radiologically on a longer follow-up.
C. Raftopoulos
Belgium
Rights and permissions
About this article
Cite this article
Roser, F., Ebner, F.H., Sixt, C. et al. Defining the line between hydromyelia and syringomyelia. A differentiation is possible based on electrophysiological and magnetic resonance imaging studies. Acta Neurochir 152, 213–219 (2010). https://doi.org/10.1007/s00701-009-0427-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-009-0427-x