Abstract
Background
Compared with lower lumbar disc herniations, upper lumbar disc herniations at L1–L2 and L2–L3 have specific characteristics that result in different surgical outcomes after conventional open discectomy. There are no published studies on the feasibility of percutaneous endoscopic lumbar discectomy for upper lumbar disc herniation. The purpose of this study was to assess the clinical outcome, prognostic factors and the technical pitfalls of PELD for upper lumbar disc herniation.
Method
Forty-five patients with a soft disc herniation at L1–L2 or L2–L3 underwent percutaneous endoscopic discectomy. Posterolateral transforaminal endoscopic laser-assisted disc removal was performed under local anesthesia. Clinical outcomes was assessed using the Prolo scale. The prognostic factors associated with outcome were then analyzed.
Findings
The mean follow-up was 38.8 months (range, 25–52 months). The outcome of the 45 patients was excellent in 21 (46.7%), good in 14 patients (31.1%), fair in six patients (13.3%), and poor in four patients (8.9%). Four patients with a poor outcome underwent further open surgery. Mean scores on a visual analog scale decreased from 8.38 to 2.36 (P < 0.0001). Age less than 45 years and a lateral disc herniation were independently associated with an excellent outcome (P < 0.05).
Conclusions
Patient selection and an anatomically modified surgical technique promote a more successful outcome after percutaneous endoscopic discectomy for upper lumbar disc herniation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the spectrum of disc herniation, the meaning of “upper lumbar” remains controversial. Most authors consider the upper lumbar discs as L1–L2 and L2–L3 [4, 5, 8, 34], some expand this to include L3–4 [3, 6, 9, 17, 30, 37]. Some have expanded the definition to include T12–L1 [12]. Generally, compared with lower lumbar disc herniations, upper lumbar disc herniations at L1–L2 and L2–L3 have specific characteristics that result in a less favorable outcome after operation [34]. However, recent advances in endoscopic technology, have made a selective epidural discectomy for an extruded disc feasible under local anesthesia via the transforaminal approach [1, 2, 15, 21, 35, 38].
The preliminary results of several randomized trials show that percutaneous endoscopic lumbar discectomy (PELD) is effective in selected patients, and its clinical outcome is comparable to that after conventional open surgery with the added benefit of reduced invasiveness [11, 23, 24]. Nevertheless, the clinical and radiologic features of upper lumbar disc herniation are different from those of lower lumbar disc herniation, and most of this research has been limited to lower lumbar disc herniations [1, 2, 28, 35]. There are few reports of the outcome of endoscopic discectomy for upper lumbar disc herniation.
We evaluated the outcomes and characteristics of percutaneous endoscopic discectomy for upper lumbar disc herniation. We also described the prognostic factors and technical pitfalls that might be specific to performing the operation for upper lumbar disc herniations.
Materials and methods
Patients and assessment of outcome
The clinical data of 45 consecutive patients who had undergone percutaneous endoscopic discectomy at either the L1–L2 or the L2–L3 level at our hospital between January 2001 and March 2003 were reviewed. The inclusion criteria were patients with a soft disc herniation at the L1–L2 or the L2–L3 level as demonstrated by computed tomography (CT) and magnetic resonance (MR) imaging, with no segmental instability on plain radiography; unilateral radicular leg pain; and a lack of response to extensive conservative treatment. The exclusion criteria were the presence of spinal stenosis, chronic discogenic back pain, calcified fragments, painless motor weakness, and pyogenic discitis.
The features of the patients were obtained from a review of the patients’ charts and a patient-based outcome questionnaire. At each follow-up, patients completed a questionnaire that reflected their functional status and degree of pain intensity. The surgical outcomes were assessed by using the Prolo functional economic outcome rating scale [32] (Table 1). The patients’ status was classified as excellent (Prolo scale score, 9–10), good (7–8), fair (5–6), and poor (4 or less). The intensity of the pain was measured using a visual analog scale (VAS, 0–10 points). One radiologist who was not aware of the other features analyzed the radiologic findings. Relationships between the preoperative variables and outcome were analyzed using Fisher’s exact test, a Mann–Whitney U test, and an unpaired t test. Values for P less than 0.05 were considered statistically significant. Risk factors noted to be related to outcome on univariate analysis were then evaluated using a multivariate logistic regression model with SPSS 14.0 K (SPSS, Chicago, IL) statistical software.
Surgical technique
The theoretical basis for percutaneous endoscopic discectomy for upper lumbar level disc herniation can be summarized as: (1) an anatomically modified transforaminal percutaneous approach and (2) selective discectomy after annular release under direct endoscopic visualization [2, 35, 38]. The operation was performed with the patient in the prone position and under local anesthesia. A steeper needle insertion angle (35–45°) is safer, unlike the angles typically used at the lower lumbar levels. A steeper approach can guarantee adequate working space without neural damage because the upper lumbar disc is more concave. Therefore, the optimal skin entry point is more medial (6 to 9 cm from the midline) than is the case at lower lumbar levels. An 18-gauge spinal needle was gently introduced under fluoroscopic guidance until it contacted the annular surface. At this moment, the needle tip was positioned immediately lateral to the midpedicular line in the AP projection. This lateral annular puncture point was a key point for preventing dural sac damage because the dural sac may be exposed through the foraminal window in the upper lumbar level.
After inserting the needle, intraoperative discography was performed with a mixture of contrast media and indigo carmine to stain the pathological nucleus and estimate the location of the annular tear. A guide wire was then inserted through the spinal needle, and the needle was removed. A small stab incision was made at the entry site of the needle, and a tapered dilating obturator was placed over the guide wire down to the annular surface. A bevel-ended working sheath was then passed over the obturator and docked within the annulus. After the obturator was removed, an operating endoscope (5.8 × 5.1 mm ellipsoidal endoscope with an eccentrically placed 2.7-mm working channel and two irrigation channels) was inserted. The operating field was then examined via direct endoscopic visualization to determine if there was any neural tissue. After confirming that no neural tissue was impinging the endoscopic field, an initial annulotomy was performed using a small annulotomy trephine. After securing a subannular working cavity, delicate epidural exploration and selective removal of the extruded nucleus were performed.
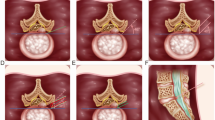
The endoscope should be positioned so that it simultaneously visualizes both the epidural and the intradiscal space in a single endoscopic frame. The blue-stained nuclear fragment, which extrudes through the inflamed annular fissure, can be easily identified (Fig. 1a). The neck of the herniated mass typically is firmly anchored to a fibrotic annular fissure and epidural inflamed adhesion. A side-firing, Holmium:YAG laser and cutting forceps were used to loosen the annular anchorage and to vaporize the herniated mass. This annular release is important for most endoscopic procedures, regardless of the level.
Intraoperative endoscopic images. a The initial view. The blue-stained hernia mass and the tenacious annular fissure are visualized. Note the side-firing laser releasing the annular anchorage. b After annular release, the hernia mass can be easily removed by the endoscopic forceps. c At the final step, decompressed dural sac and nerve root are confirmed. Note the endoscopic anatomical layers; epidural space, annular layer, and intradiscal space
After the annular fissure has been opened widely, the blue-stained herniated mass can become mobile and sometimes spontaneously squeezed out downwardly. The herniated mass was then removed as a single lump or gradually, in piecemeal fashion, using the endoscopic forceps and the side-firing laser (Fig. 1b). Once the dural sac has been exposed, special care must be taken not to directly irritate the dural surface with the surgical instruments including the laser tip. In a final step, the anatomic layers (e.g., epidural fat, dural sac, traversing root, posterior longitudinal ligament, annular fissure, and remaining normal nucleus) were confirmed (Fig. 1c). The patient was asked if the pain had decreased or disappeared, and if no complications occurred, the patient usually left the hospital within 24 h.
Results
The 45 patients who met the inclusion criteria underwent percutaneous endoscopic discectomy at 46 disc levels. One patient underwent the procedure at both the L1–L2 and the L2–L3 levels simultaneously, nine patients underwent the procedure at the L1–L2 level, and 35 patients underwent the procedure at the L2–L3 level. The mean follow-up period of 33 men and 12 women was 38.8 months (range, 25–52 months). The anatomic zones of disc herniation were central in 13, posterolateral in 26, and foraminal in 7. The mean operation time was 61.5 min (range, 25–110 min). Table 2 summarizes the clinical characteristics.
Based on the Prolo scale, the outcomes were excellent in 21 of 45 patients (46.7%), good in 14 patients (31.1%), fair in six patients (13.3%), and poor in 4 patients (8.9%). The combined rate of excellent or good outcome rate at the final follow-up was 77.8%. The mean VAS for radicular pain was 8.38 ± 1.22, and after operation decreased to 2.36 ± 1.65 (P < 0.0001). The four patients with a poor outcome subsequently had open surgery. Two patients underwent open microdiscectomy immediately because of incomplete decompression. One patient developed a recurrent herniation after 2-months free from symptoms and underwent fusion surgery at another hospital. One patient who developed a dural tear with motor weakness (ankle and quadriceps muscle power grade 2) immediately after percutaneous operation at the L2–L3 level underwent emergency open laminotomy and dural repair. With oral medication and extensive physical therapy, the patient’s motor weakness improved with residual sequelae (grade 4 quadriceps weakness and sustained dysesthesia) after 2 years. There were no instances of postoperative infection or hematoma. On the follow-up radiological studies, there was no newly developed segmental instability (Fig. 2). Three patients developed transient dysesthesia, which improved within 3 months.
Illustrated case. a A 39-year-old male patient underwent PELD for disc herniation at L2–3 (left). The extruded disc was selectively removed and the dural sac was well decompressed on postoperative MRI after 6 weeks (right). b There was no segmental instability on postoperative flexion–extension lateral lumbar radiographs at the same level after 2 years
The age of the patient and the duration of symptoms were found to be related to outcome (Table 3). Patients younger than 45 years old tended to obtain better outcomes than older patients (75% vs. 36.4%, P < 0.05). An excellent outcome was seen in 65% of patients with shorter symptom durations (less than 6 months) but was less at 32% (6 months or longer) (P < 0.05). The other clinical features, including gender, chief complaints, presence of motor weakness, presence of reverse Lasegue’s sign, and operation time, were not associated with outcome after operation. Regarding the results of the radiological analyses, the zone of disc herniation was related to outcome (Table 4). We classified the zone of disc herniation into three types: central, posterolateral, and foraminal. An excellent outcome occurred in patients with a posterolateral disc herniation (61.5%), followed by foraminal (42.9%) and central (15.4%) (P < 0.05). Nineteen (57.6%) of the 33 patients of the lateral herniation group (posterolateral and foraminal) showed an excellent outcome as compared to only two (15.4%) of the 13 patients with a central herniation (P < 0.05). The other radiological parameters, including the level of surgery, disc containment, migration of the hernia, degree of disc degeneration [31], degree of canal compromise, herniation length, segmental lordosis, and sagittal plane range of motion, had no significant relationship to the outcome.
A multivariate logistic regression model was then used to determine if the individual prognostic factors were independently associated with an excellent outcome. Age younger than 45 (OR = 16.4, 95% CI 1.69–166.83, P = 0.018) and a lateral disc herniation (OR = 12.7, 95% CI 1.24–130.35, P = 0.032) were significantly related to the outcome. After multivariate analysis, the shorter symptom duration (OR = 3.3, 95% CI 0.70–15.68, P = 0.132) was not associated with outcome because of a strong association with a lateral disc herniation. Table 5 shows the calculated predictive probabilities for different patient conditions, including the patient’s age and the zone of the disc herniation.
Discussion
The incidence of herniation in the upper lumbar region in most published surgical series is no more than 5% of all lumbar disc herniations [3, 13, 25, 34]. In four reports, the outcome of upper lumbar disc herniations is less favourable than for lower lumbar disc herniation. The definite reason remains unclear. However, it can be speculated that the standard microdiscectomy through the interlaminar window may cause neural damage and/or segmental instability. This higher probability of inflicting neural damage during the dural sac retraction or discectomy for upper lumbar level disc herniation exists because it usually consists of a smaller spinal canal and a larger dural sac, a compact neural component and conus medullaris in the dural sac, and consequently a smaller fluid barrier between the dura and the neural tissues [13, 25, 26]. Moreover, the propensity for segmental instability at the upper lumbar level may be the consequence of a more excessive removal of bony tissue including the facet joint, because of the short distance between the two pars interarticularis, smaller interlaminar space in all dimensions, and the inferior border of the lamina usually overhangs the disc space to a greater extent [25, 26, 29], requiring a wide laminectomy and facetectomy to expose the disc space and to avoid neural tissue retraction.
Percutaneous endoscopic surgery has benefits in upper lumbar soft disc herniations. First, the extruded disc can be removed without dural sac retraction. This posterolateral approach can take a bypass course through the foraminal window to reach the outer annulus, thus avoiding the dural sac. In addition, the 30° endoscope can provide a large enough visual field to include the extruded disc and neural structures according to the surgeon’s need. Moreover, the foraminal window for the transforaminal endoscopic approach is usually large enough in the upper lumbar level, and therefore, an extruded soft disc can be exposed easily. Foraminal stenosis interfering with the transforaminal approach is relatively rare in the upper lumbar level [14, 22].
The good-to-excellent outcomes of endoscopic discectomy reported in previous studies, in which most patients had lower lumbar disc herniations, are usually reported to be at least more than 78% [2, 7, 15, 18, 19, 38]. A success rate of 77.8% at the L1–L2 and the L2–L3 levels may be lower than those for lower lumbar levels. A study comparing the outcome of upper lumbar and lower lumbar disc herniation is currently underway at our institution. We believe that the unique anatomical environment of the upper lumbar level and its inherent technical difficulty may be the main reason for failure. Therefore, for satisfactory results, adequate patient selection and an anatomically modified endoscopic technique for the upper lumbar level are necessary.
This study showed some prognostic factors that related to outcome after percutaneous endoscopic operation for upper lumbar disc herniation. A younger age (less than 45 years) correlated with a higher likelihood of excellent outcome on uni- and multivariate analysis. This finding corresponds closely to the results of previous studies of conventional lumbar discectomy [10, 33, 36]. The duration of symptoms was another significant factor on univariate analysis. Patients with shorter symptom durations (less than 6 months) had a better outcome. It is generally accepted that shorter symptom duration relates to a favorable outcome for percutaneous procedures [2, 16, 18]. This might indicate that a recent soft herniation is easier to remove via an endoscope. However, the results of the multivariate analysis demonstrated that the association with the duration of symptoms was because of its close correlation with the zone of the disc herniation. A lateral herniation, including the posterolateral and foraminal zone, was independently associated with an excellent outcome. As described above, the nervous structures in the upper lumbar dural sac are more inherently vulnerable to mechanical irritation than those of the lower lumbar area. Therefore, manipulation with mechanical or thermal tools to the midline zone could increase the risk of broad dural sac irritation and neural damage. Moreover, regarding the anatomical features of upper lumbar segment, the dural convexity may be an obstacle in approaching the central zone and may lead to an incomplete decompression. In contrast, a laterally located hernia fragment might be safely removed without substantial dural sac irritation. Moreover, disc herniation involving the lateral zone could offer a more definite localization or a target for decompression than central herniation or diffuse disc bulging.
In addition to appropriate patient selection, technical considerations are important in outcome. The technical requirement for a successful discectomy is to release the annular anchorage delicately and then to remove the loose hernia fragment in both the epidural and the intradiscal space [2, 38] (Fig. 3). Without an adequate annular release, it would be difficult to mobilize or remove the tenacious hernia mass because the instruments are relatively small and weak compared with those used in open discectomy. Consequently, surgeons can remove only a partial epidural fragment (tip of the iceberg), passing over the hidden fragment (base of the iceberg), which can cause residual pain or reherniation after the procedure.
Many authors have focused on the importance of annular release, regardless of the level of the disc [2, 20, 38]. On the other hand, there are several key points specific to upper lumbar disc herniation (Fig. 4). First, the approach angle of the needle and working sheath for an upper lumbar disc should be steeper than those used for a lower level disc. Generally, a skin entry point 8 to 12 cm lateral from the midline and a 25–30° insertion angle are recommended for a standard posterolateral transforaminal discectomy. However, because the disc surface of the upper lumbar disc is more concave and has a more acute angle in the axial plane, a horizontal approach has a potential risk of dural sac damage. A 35–45° insertion angle is recommended for the upper lumbar level. Second, the annular puncture point should be more laterally for the upper lumbar level. It has been previously recommended that the needle be inserted as medial as possible to remove the fragment easily [1, 27]. However, unlike the lower lumbar level, the neural foramen is relatively large and the dural sac is readily exposed through the foraminal window in the upper lumbar level. Hence, a more lateral annular puncture is safer.
Schematic drawings demonstrating the transforaminal approach unique to upper lumbar disc. a For lower lumbar disc, the standard approach angle is 25–30° and the annular window point (closed circle) is on the medial pedicular line. b In contrast, for upper lumbar disc, a steeper needle insertion angle (35–45°) is adequate disc surface is more concave and has a more acute angle in the axial plane. The annular window (closed circle) should be laterally targeted from the mid-pedicular line to prevent dural injury
Finally, direct dural sac manipulation with forceps and a laser beam must be avoided. As described earlier, the dural sac of the upper lumbar segment is susceptible to mechanical manipulation or thermal application owing to the low buffering capacity. Thermal damage can cause prolonged dysesthesia or neurologic deficits. Severe back pain or prolonged painless muscle twitching can be a sign of dural sac irritation caused by the surgical instruments or the laser.
Conclusion
Patient selection and an anatomically modified surgical technique are important factors in a successful outcome after transforaminal percutaneous endoscopic operation for upper lumbar disc herniation. Younger patients and those with a lateral disc herniation had better outcomes.
References
Ahn Y, Lee SH, Park WM, Lee HY (2003) Posterolateral percutaneous endoscopic lumbar foraminotomy for L5-S1 foraminal or lateral exit zone stenosis. Technical note. J Neurosurg 99(3 Suppl):320–323
Ahn Y, Lee SH, Park WM, Lee HY, Shin SW, Kang HY (2004) Percutaneous endoscopic lumbar discectomy for recurrent disc herniation: surgical technique, outcome, and prognostic factors of 43 consecutive cases. Spine 29(16):E326–E332, doi:10.1097/01.BRS.0000134591.32462.98
Albert TJ, Balderston RA, Heller JG, Herkowitz HN, Garfin SR, Tomany K, An HS, Simeone FA (1993) Upper lumbar disc herniations. J Spinal Disord 6(4):351–359, doi:10.1097/00002517-199306040-00009
Bartolomei L, Carbonin C, Cagnin G, Toso V (1992) Unilateral swelling of the lower abdominal wall. Unusual clinical manifestation of an upper lumbar disc herniation. Acta Neurochir (Wien) 117(1–2):78–79, doi:10.1007/BF01400642
Bosacco SJ, Berman AT, Raisis LW, Zamarin RI (1989) High lumbar disk herniations. Case reports. Orthopedics 12(2):275–258
Dinakar I (1991) Protrusion of upper lumbar intervertebral discs (long term follow-up of operated cases). J Indian Med Assoc 89(7):199–200
Eustacchio S, Flaschka G, Trummer M, Fuchs I, Unger F (2002) Endoscopic percutaneous transforaminal treatment for herniated lumbar discs. Acta Neurochir (Wien) 144(10):997–1004
Fontanesi G, Rotini R, Pignedoli P, Giancecchi F (1987) Prolapsed intervertebral disc at the upper lumbar level. Diagnostic difficulties. A report on 12 cases. Ital J Orthop Traumatol 13(4):501–507
Gutterman P, Shenkin HA (1973) Syndromes associated with protrusion of upper lumbar intervertebral discs. Results of surgery. J Neurosurg 38(4):499–503
Hanley EN Jr, Shapiro DE (1989) The development of low-back pain after excision of a lumbar disc. J Bone Joint Surg Am 71(5):719–721
Hermantin FU, Peters T, Quartararo L, Kambin P (1999) A prospective, randomized study comparing the results of open discectomy with those of video-assisted arthroscopic microdiscectomy. J Bone Joint Surg Am 81(7):958–965
Hsu K, Zucherman J, Shea W, Kaiser J, White A, Schofferman J, Amelon C (1990) High lumbar disc degeneration. Incidence and etiology. Spine 15(7):679–682, doi:10.1097/00007632-199007000-00012
Ido K, Shimizu K, Tada H, Matsuda Y, Shikata J, Nakamura T (1998) Considerations for surgical treatment of patients with upper lumbar disc herniations. J Spinal Disord 11(1):75–79, doi:10.1097/00002517-199802000-00012
Jenis LG, An HS, Gordin R (2001) Foraminal stenosis of the lumbar spine: a review of 65 surgical cases. Am J Orthop 30(3):205–211
Kambin P, Casey K, O’Brien E, Zhou L (1996) Transforaminal arthroscopic decompression of lateral recess stenosis. J Neurosurg 84(3):462–467
Kim YS, Chin DK, Yoon DH, Jin BH, Cho YE (2002) Predictors of successful outcome for lumbar chemonucleolysis: analysis of 3000 cases during the past 14 years. Neurosurgery 51(5 suppl):123–128
Kortelainen P, Puranen J, Koivisto E, Lähde S (1985) Symptoms and signs of sciatica and their relation to the localization of the lumbar disc herniation. Spine 10(1):88–92, doi:10.1097/00007632-198501000-00014
Kotilainen E, Valtonen S (1994) Percutaneous nucleotomy in the treatment of lumbar disc herniation results after a mean follow-up of 2 years. Acta Neurochir (Wien) 128(1–4):47–52, doi:10.1007/BF01400652
Kotilainen E, Valtonen S (1998) Long-term outcome of patients who underwent percutaneous nucleotomy for lumbar disc herniation: results after a mean follow-up of 5 years. Acta Neurochir (Wien) 140(2):108–113, doi:10.1007/s007010050070
Lee S, Kim SK, Lee SH, Kim WJ, Choi WC, Choi G, Shin SW (2007) Percutaneous endoscopic lumbar discectomy for migrated disc herniation: classification of disc migration and surgical approaches. Eur Spine J 16(3):431–437, doi:10.1007/s00586-006-0219-4
Lew SM, Mehalic TF, Fagone KL (2001) Transforaminal percutaneous endoscopic discectomy in the treatment of far-lateral and foraminal lumbar disc herniations. J Neurosurg 94(2 Suppl):216–220
Maher CO, Henderson FC (1999) Lateral exit-zone stenosis and lumbar radiculopathy. J Neurosurg 90(1)(suppl):52–58
Mayer HM, Brock M (1993) Percutaneous endoscopic discectomy: surgical technique and preliminary results compared to microsurgical discectomy. J Neurosurg 78(2):216–225
Mayer HM, Brock M, Berlien HP, Weber B (1992) Percutaneous endoscopic laser discectomy (PELD). A new surgical technique for non-sequestrated lumbar discs. Acta Neurochir Suppl (Wien) 54:53–58
McCulloch JA (1999) Alternative forms of disc excision. In: Rothman R, Simeone F (eds) The spine, 4th edn. Saunders, Philadelphia, pp 691–714
McCulloch JA, Young PH (1998) Microsurgery for lumbar disc herniation. In: McCulloch JA, Young PH (eds) Essentials of spinal microsurgery. Lippincott-Raven, Philadelphia, pp 329–382
Min JH, Kang SH, Lee JB, Cho TH, Suh JK, Rhyu IJ (2005) Morphometric analysis of the working zone for endoscopic lumbar discectomy. J Spinal Disord Tech 18(2):132–135, doi:10.1097/01.bsd.0000159034.97246.4f
Mochida J, Arima T (1993) Percutaneous nucleotomy in lumbar disc herniation. A prospective study. Spine 18(4):2063–2068
Moon KH, Lee SH, Kong BJ, Shin SW, Bhanot A, Kim DY, Lee HY (2006) An oblique paraspinal approach for intracanalicular disc herniations of the upper lumbar spine: technical case report. Neurosurgery 59(4 suppl 2):ONSE 487–488
Pasztor E, Szarvas I (1981) Herniation of the upper lumbar discs. Neurosurg Rev 4(3):151–157, doi:10.1007/BF01743641
Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N (2001) Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine 26(17):1873–1878, doi:10.1097/00007632-200109010-00011
Prolo DJ, Oklund SA, Butcher M (1986) Toward uniformity in evaluating results of lumbar spine operations. A paradigm applied to posterior lumbar interbody fusion. Spine 11(6):601–606, doi:10.1097/00007632-198607000-00012
Salenius P, Laurent LE (1977) Results of operative treatment of lumbar disc herniation. A survey of 886 patients. Acta Orthop Scand 48(6):630–634
Sanderson SP, Houten J, Errico T, Forshaw D, Bauman J, Cooper PR (2004) The unique characteristics of “upper” lumbar disc herniations. Neurosurgery 55(2):385–389, discussion 389, doi:10.1227/01.NEU.0000129548.14898.9B
Tsou PM, Yeung AT (2002) Transforaminal endoscopic decompression for radiculopathy secondary to intracanal noncontained lumbar disc herniations: outcome and technique. Spine J 2(1):41–48, doi:10.1016/S1529-9430(01)00153-X
Weber H (1983) Lumbar disc herniation. A controlled, prospective study with ten years of observation. Spine 8(2):131–140, doi:10.1097/00007632-198303000-00003
Wei CP, Cheng WC, Chang CN, Lee ST, Lui TN, Wang AD (1989) Upper lumbar disc herniation. Changgeng Yi Xue Za Zhi 12(4):193–199
Yeung AT, Tsou PM (2002) Posterolateral endoscopic excision for lumbar disc herniation: Surgical technique, outcome, and complications in 307 consecutive cases. Spine 27(7):722–731, doi:10.1097/00007632-200204010-00009
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosure: This study was supported by a grant from the Wooridul Spine Foundation.
An erratum to this article can be found at http://dx.doi.org/10.1007/s00701-009-0457-4
Rights and permissions
About this article
Cite this article
Ahn, Y., Lee, SH., Lee, J.H. et al. Transforaminal percutaneous endoscopic lumbar discectomy for upper lumbar disc herniation: clinical outcome, prognostic factors, and technical consideration. Acta Neurochir (Wien) 151, 199–206 (2009). https://doi.org/10.1007/s00701-009-0204-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-009-0204-x