Abstract
Background
Trans-venous embolisation has been accepted as the preferred treatment for dural carotid–cavernous fistulae (DCCF). However, such an approach is not always feasible. In this circumstance, trans-arterial embolisation with low concentration n-butyl-cyanoacrylate glue (NBCA) may be a feasible alternative. We report our results and experience of this method for DCCF.
Materials and methods
Five patients with DCCF were treated by trans-arterial embolisation using low concentration NBCA by wedging the microcatheter into the main feeding artery. All five lesions were associated with venous drainage into the superior ophthalmic vein. The inferior petrosal sinus was patent in one patient and thrombosed in four. Additional venous drainage into the Sylvian vein and the superior petrosal sinus was observed in two patients.
Findings
The definitive NBCA injection was performed via the branches of the middle meningeal artery in three patients and accessory meningeal artery as well as ascending pharyngeal artery in two patients. Four patients showed complete obliteration of the DCCF on the post-embolisation angiogram, and follow-up studies showed clinical cure or improvement and successful obliteration of the DCCF. One patient had a residual DCCF after the procedure, but showed complete obliteration and clinical cure at 5-month follow-up. Glue penetrated into the Sylvian vein in one patient during the procedure without sequelae. Two patients had transient worsening of ocular symptoms after the procedure.
Conclusions
Trans-arterial embolisation with low concentration NBCA using a wedged microcatheter technique is still a safe and effective treatment for DCCF when the transvenous approach is not feasible. However, care must be taken to prevent inadvertent arterial and venous embolisation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dural carotid–cavernous fistulae (DCCF) are an abnormal communication between the dural branches of the internal and/or external carotid artery and the cavernous sinus (CS). The ideal goal of treatment for DCCF is to completely obliterate the pathological arterio-venous connections. Trans-arterial and/or trans-venous embolisation have been used for the treatment of DCCF. The trans-venous route is often preferred because of easier access, higher clinical and anatomical cure rates and lower complications [21]. However, trans-venous embolisation is not always feasible, especially in patients with high-grade fistulae with direct cortical venous drainage or for whom trans-venous access is limited due to venous sinus thrombosis or occlusion. For these patients, trans-arterial embolisation might be an appropriate alternative. Recently, this method using microcoils [18], n-butyl-cyanoacrylate glue (NBCA) [19, 24] and ethylene vinyl alcohol co-polymer (Onyx) [1, 27] for the treatment of dural arteriovenous fistulae have been reported with initial success. However, trans-arterial embolisation with microcoils seems doubtful because of the proximal occlusion of the feeding artery. We treated five patients with DCCF via the trans-arterial route using low concentration NBCA, and our successful results are reported in this paper.
Material and methods
Patients
Between January 2005 and June 2007, five patients with DCCF underwent trans-arterial embolisation with low concentration NBCA in our institution. Their clinical data are presented in Table 1. Three patients were female, and two patients were male. Their ages ranged from 43 to 77 years (mean 60.8 years). All patients denied a recent history of head trauma or surgery. Clinical symptoms included exophthalmos and chemosis (n = 5), visual deterioration (n = 2), headache (n = 1), and tinnitus (n = 1). The diagnosis of DCCF was confirmed in all patients by angiography including bilateral internal carotid, external carotid and vertebral arteries. According to Barrow's classification [2], four patients had type D fistulae and one patient had a type C fistula. Contralateral supply (internal and external carotid artery in two patients and internal carotid artery in one) to the fistulae was observed in three patients. All five patients had venous drainage into the superior ophthalmic vein (SOV), but an obvious distension of this vessel was not observed. Two patients had additional venous drainage into the Sylvian vein and the superior petrosal sinus (SPS), and one had additional venous drainage into the inferior petrosal sinus (IPS).
Trans-arterial embolisation technique
All procedures were performed with informed consent from the patients and their relatives. Initially, under neuroleptic sedation, bilateral common carotid and selective internal carotid artery (ICA) and external carotid artery (ECA) angiography was performed to identify the feeding arteries, the fistulous sites, the venous drainage patterns and the shunt volume. Subsequently, the patients were placed under general anaesthesia and systemically anticoagulated to an activated clotting time of 250 s. A 6F guide catheter (Envoy; Cordis Neurovascular, Miami lakes, FL, USA) was advanced into the right ECA via a 6F sheath previously placed in the right common femoral artery. Using a coaxial technique, a guidewire-directed microcatheter, such as the Prowler-10, Prowler-14, Rapid-Transit (Cordis Neurovascular, Miami Lakes, FL, USA) Excel or Renegade (Boston Scientific, Fremont, CA, USA), was placed in a proximal feeding artery. Angiography through the microcatheter was performed to identify several appropriate arterial conduits for flow-arrest delivery based on their size, length, accessibility, tortuosity, extent, type of collateralisation and flow rate. Once a feeding artery was selected, the microcatheter was advanced distally until the catheter was wedged into position. A microinjection of contrast agent was performed to confirm the flow-arrest status as well as the location and character of the targeted fistula and to identify potentially dangerous anastomoses between the internal and external carotid arteries. The injection dynamics of NBCA was predicted based on the catheter's distance from the fistula, the shunting rate, the size of the feeding artery engaged, and the extent of collateralisation present. The microcatheter and arterial territory distal to the microcatheter tip was then flushed with 5% dextrose solution. Subsequently, the prepared NBCA/Ethiodol mixture (TruFill NBCA, Cordis Neurovascular) with a concentration of 14.3% (0.5 ml NBCA: 3.0 ml Ethiodol) or 16.7% (0.5 ml NBCA: 2.5 ml Ethiodol) was injected across the pathological arterio-venous fistula and into the parent venous apparatus in a controlled manner under high resolution fluoroscopic monitoring. Once NBCA shunted into the CS by the pathological arterio-venous fistula, it was found to flow towards the SOV, IPS, SPS, cortical veins or deep veins. The injection of NBCA was paused for 2 or 3 s to control the accumulation of glue in the CS. After the stable polymerisation of NBCA in the CS, more glue NBCA was injected very slowly and with minimal pressure until the pathological CS was fully cast. If the initial selected feeding artery failed to completely cast the pathological CS, or the deterioration of the microcatheter's wedged position resulted in distal embolisation of the feeding artery, other appropriate arterial conduits for flow-arrest delivery were used to embolise the residual pathological arterio-venous fistula with glue. Post-embolisation, complete six-vessel angiography was immediately performed to assess occlusion of the fistula.
A high-flow DCCF with extensive collateralisation was noted; a preparatory embolisation via accessory pedicles with the use of 150–250 um polyvinyl alcohol (PVA) particles (Cordis Neurovascular) was performed to decrease the competing inflow into the shunt domain before the definitive injection of NBCA during the same session. After the competing inflow was diminished dramatically, the main feeding artery for flow-arrest delivery was selected; the microcatheter was then navigated distally until the catheter was wedged into position and the embolisation completed.
Post-procedure follow-up
After the procedure, all patients underwent clinical and angiographic follow-up which ranged from 5 to 17 months (9.6 months on average). Clinical follow-up outcomes were categorised as cured (complete resolution of symptoms and signs), improved (alleviation of symptoms and signs), unchanged (no change of symptoms and signs), and aggravated (deterioration of symptoms and signs or newly developed symptoms). Angiographic success was defined as complete occlusion of shunt, nearly complete occlusion with a small residual stagnant shunt that was considered likely to thrombose and incomplete occlusion with the presence of a residual shunt.
Results
All patients underwent one embolisation session. One patient (number 3) with a high-flow DCCF was treated with preparatory devascularisation of collateral inflow by using PVA particles before definitive trans-arterial NBCA deposition. The concentration of NBCA injection was 14.3% in three patients, and 16.7% in the others. In four patients, NBCA was successfully deposited across the pathological arterio-venous connection into the recipient venous structure at the initial injection attempt. One patient (number 2) required two injection attempts through two branches of the middle meningeal artery to deposit NBCA through the fistulous connection because attempts through one branch did not initially result in adequate occlusion of the recipient venous apparatus. The main feeding artery for the definitive injection of NBCA was selected from the branches of middle meningeal arteries in three patients, accessory meningeal artery in one and ascending pharyngeal artery in the other.
In four patients, NBCA permeation of the shunt with immediate occlusion of the venous apparatus was observed. No residual arterio-venous shunting was demonstrated on post-embolisation six-vessel control angiography studies. Furthermore, clinical cure or improvement was observed, and persistent occlusion of the DCCF was demonstrated on clinical and angiographic follow-up (Fig. 1). One patient (number 4) had an incomplete occlusion immediately after the procedure due to residual blood supply from dural branches of the contralateral internal and external carotid arteries, but showed complete obliteration on the 5-month follow-up angiogram. Moreover, a complete resolution of the initial presenting symptoms was also observed.
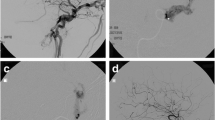
Patient 1: male, 43-year-old with a right DCCF. a and b Right internal and external carotid artery injection; lateral view, demonstrating the right DCCF (level arrow) which drains into the SOV (upright arrow) and IPS (italic arrow). c Injection of contrast agent from a microcatheter wedged in the distal middle meningeal artery (empty triangle); lateral view, visualising the fistula site and the affected CS (level arrow). d cranial lateral projection after embolisation with NBCA (14.3%) showing glue cast of cavernous sinus (level arrow). e and f Immediate right internal and external carotid artery injection after embolisation; lateral view, demonstrating occlusion of DCCF. DCCF Dural carotid–cavernous fistula, SOV superior ophthalmic vein, IPS inferior petrosal sinus, CS cavernous sinus, NBCA n-butyl cyanoacrylate
During the procedure, glue was observed to penetrate into the Sylvian vein due to fragmentation of the glue stream arising from excessive collateral inflow in one patient (number 4), but there was no identifiable neurological deficit during and after the procedure, including the follow-up period. In the remaining patients, such glue penetration into venous channels did not occur. There was no complication arising from the microcatheter in the feeding arteries. Delayed complications occurred in two patients. One patient (number 2) had an aggravated chemosis and sixth cranial nerve palsy on day 2 after embolisation. The other patient (number 4) had aggravated chemosis, sixth nerve palsies and headache on day 3 after embolisation. However, these transient worsening of symptoms completely resolved with heparin and steroid therapy after 1 week. The remaining patients were undisturbed following the procedure.
Discussion
The majority of DCCF have a benign natural history. Their course is generally indolent, with low morbidity, and a high rate of spontaneous resolution, especially after slight changes in the haemodynamics [20, 25]. Patients are usually managed conservatively by external manual carotid compression [13]. However, such management is restricted to lesions in which the venous drainage is solely via the SOV and not with other types of drainage, especially those involving the cortical veins because of the risks involved in this manoeuvre. Furthermore, conservative treatment may be unsuccessful, and the symptoms and signs may progress to decreasing vision, glaucoma, severe exophthalmos, persistent ophthalmoplegia, intolerable diplopia or bruit, or unacceptable cosmetic disfigurement. These patients with intractable or progressive ocular symptoms or with the presence of retrograde cortical venous drainage require more aggressive therapy [21].
Trans-arterial and/or trans-venous endovascular treatment is the standard therapy for DCCF that require active treatment. Trans-venous embolisation has been currently accepted as the preferred approach [21]. However, the prerequisite for successful treatment by this route is successful cannulation of the CS. There are several trans-venous paths for accessing the CS, including the IPS [3, 30], the SOV [5, 31], the SPS [23], the pterygoid plexus [12], surgical exposure or direct puncture of the angular vein or SOV [9, 26] and even the cortical veins [4, 11, 17]. However, these trans-venous routes are not always feasible. The IPS is the preferred trans-venous route for embolisation of a DCCF because of its short and straight course. Anatomically, catheterisation of the CS through the IPS is feasible in the great majority (99%) of patients [3, 30]. However, technically, cannulation of the IPS can be difficult, and the success rate ranges from 30% to 50% [30, 22]. In patients associated with a thrombosed IPS, compartmentalisation of the cavernous sinus and type III or IV anatomy of the IPS, there is little possibility of successful cannulation of the CS [6, 30, 31]. Moreover, cannulation of the IPS carries the risk of dissection of the clival dura with accidental subarachnoid haemorrhage and sixth nerve palsy [16]. When cannulation of the IPS is unsuccessful, trans-femoral or trans-jugular SOV approach has been accepted as an alternative [5, 31]. However, such a manouevre is not without difficulty. The junctions between the angular vein and the SOV and between the SOV and the naso-frontal vein around the orbital brim are usually of narrow calibre and a tortuous course, which pose a challenge to catheterisation [6, 30, 31]. Moreover, when the SOV is not significantly enlarged and very posterior in the orbital apex, trans-orbital venous access may not be possible [8, 9]. Surgical exposure or direct cannulation of the SOV can be used as an option if trans-femoral or trans-jugular SOV approaches have failed [9, 26]. However, cannulation of the SOV is still limited by the inherent anatomical course of the SOV. Additionally, this approach carries the risk of retro-ocluar haemorrhage, infection, thrombosis of the SOV, redirection of the venous drainage leading to retrograde drainage into a cortical vein, or injury of the supraorbital nerve or levator muscle [8, 25]. Other less commonly used trans-venous routes, such as a cortical vein [11, 17], is also an alternative if the frequently used trans-venous approaches have failed. However, these approaches are by no means straightforward and usually need burr hole access and thus are considered only as a last resort. In our series, the venous drainage flow was towards the SOV, but the vessel was not significantly enlarged and very posterior in the orbital apex. Therefore, we considered that trans-orbital venous access may be impossible. Additional drainage into the Sylvian vein and the superior petrosal sinus was observed in two patients and drainage into the IPS in one patient. Although cannulation of the thrombosed IPS has been advocated [3, 30] and trans-venous embolisation of DCCF through the SPS and the Sylvian vein has been reported [17, 23], we considered that these manouevres may be risky and difficult. Therefore, trans-arterial embolisation was considered an alternative for the treatment of the fistulae.
In our study, the microcatheter was first navigated and wedged into the feeder proximal to the fistula. Then, low concentration NBCA was slowly injected through the microcatheter, across the fistula and into the abnormal CS, subsequently permeating into the peri-fistulous collateral network and polymerising in the CS, thus casting the affected CS and eliminating the pathological arterio-venous connection. Using this technique, the arterial pedicle beyond the tip of the wedged microcatheter becomes a functional extension of the microcatheter, similar to the microcatheter being across the fistula to embolise the immediately receptive venous apparatus and resembling embolisation via the trans-venous route. However, compared with trans-venous embolisation, this technique has the following advantages: (1) the fistula site and the immediately receptive venous apparatus is the targeted occlusion, preserving the functional venous pathways that drain normal brain parenchyma, reducing the likelihood that shunt flow may be diverted into alternative venous pathways and predisposing to intraparenchymal haemorrhage [12, 14, 16, 25]; (2) treatment is not limited by venous access problems, including thrombosed or stenotic dural sinuses and high-grade lesions draining directly into cortical veins; (3) specific complications of trans-venous embolisation may be avoided in certain circumstances, e.g., cannulation of a thrombosed IPS could result in the laceration of the clival dura and catheterisation of a thrombosed or extremely tortuous SOV could cause dissection, perforation and thrombosis of the vessel [9, 14, 26, 29].
In the present study, four patients showed complete obliteration of the fistula on post-embolisation angiography. One patient had a residual fistula after the procedure, but showed complete obliteration on the 5-month follow-up angiogram. No recurrence was noted in four patients with complete fistula obliteration on immediate post-embolisation angiography during clinical and angiographic follow-up. These results are comparable with those of the published series in which investigators used trans-venous embolisation for the treatment of DCCF [5, 6, 14–16, 29]. Furthermore, our obliteration rate on post-embolisation angiography and follow-up angiography was higher than those previously reported with earlier trans-arterial techniques which resulted in occlusion of arteries proximal to the fistula site, permitting later re-establishment of arterio-venous shunting through adjacent collateral networks [10].
The success of trans-arterial embolisation of a DCCF depends on complete obliteration of the shunting sites. PVA particles are liable to cause occlusion of the feeding arteries proximal to fistula site, resulting in the delayed recanalisation [7]. Microcoils may afford favourable control and occlude the shunting sites, but a prerequisite for complete occlusion of a DCCF is that the microcatheter must be navigated via an artery into the venous side of the fistula [18]. Otherwise, recanalisation and recurrence will still occur because of proximal occlusion of arterial feeders. Onyx is a cohesive and non-adherent liquid embolic agent, which has a favourable permeability and consistency. Theoretically, it is the optimal embolic agent for trans-arterial embolisation of a DCCF. However, catheter rupture or catheter entrapment potentially occurs during Onyx injection. Moreover, the maximum dose of dimethylsulfoxide that can be safely injected intra-arterially in cerebral vasculature is undetermined [1, 2]. Low concentration NBCA has a low viscosity and can be easily injected through a microcatheter. It has been used in trans-arterial embolisation of dural arterio-venous fistulae and is believed to be more controllable [19, 24]. However, there exists some risk that the glue spills into the draining veins or fragmentates after polymerisation due to excessive collateral inflow or delayed polymerisation, resulting in embolisation of the draining vein and incomplete closure of the shunt. In our series, it was difficult to catheterise into the venous side of the fistula, and therefore, trans-arterial embolisation with microcoils may not have completely obliterated the shunting points. Additionally, there is a need for further experience of the potential of Onyx for use as a permanent embolic agent in DCCF. Therefore, we embolised the fistulae via the trans-arterial route with low concentration NBCA and which proved to be quite suitable. However, we encountered the challenge of how to control the injection of low concentration NBCA. Our experience indicates the following suggestions to facilitate controlling the injection of low concentration NBCA: (1) the microcatheter must be advanced as far distally as possible and a true flow-arrest state must be established; (2) injection of glue must be performed under high resolution fluoroscopic guidance, very slowly and with minimal injection pressure; (3) in all patients with high-flow shunts, perform interruption of the collateral inflow before the definitive NBCA injection to minimise fragmentation as well as premature polymerisation of the definitive glue column and to prevent uncontrolled systemic venous embolisation.
Possible complications associated with trans-arterial NBCA embolisation of DCCF include cranial nerve palsies, systemic venous embolisation, trans-collateral embolisation into normal cerebral arteries and rupture of the catheterised vessel. Nerve palsies are related to glue moving into branches supplying cranial 3 to 7 and 9 to 11, progressive thrombosis and glue-induced inflammatory reactions inside the CS. Catheterisation as close to the fistula site as possible to prevent glue reflux and an in-depth understanding of the anatomy and potentially dangerous anastomoses of the feeders for the definitive NBCA injection will minimise the risk of nerve palsies. In our series, we did not experience palsies resulting from glue obstructing branches supplying cranial nerves. However, two patients experienced delayed sixth nerve palsy and aggravation of chemosis but gradually recovered from the symptoms with heparin and steroid therapy after 1 week. This complication has been documented by Wakhool et al. [29]. Complete thrombosis inside the CS with increased mass effect was considered the most likely cause. However, glue-induced inflammatory response inside the CS was also presumed a theoretical risk of cranial nerve palsy [29]. Systemic venous embolisation, such as cortical vein infarction, proximal SOV occlusion and pulmonary embolisation are other potential complications. In our series, no patient experienced the proximal SOV occlusion and pulmonary embolisation. These complications probably occur due to the use of low concentration NBCA. There have been reports of reflux of NBCA into the proximal SOV [28, 29]. Although patients tolerated proximal SOV embolisation without sequelae, it is also undesirable and can result in occlusion of episcleral venous drainage. One patient was observed to have reflux of NBCA into the Sylvian vein in this study. Although no clinical sequelae occurred in this patient, such occlusion of the draining vein may cause venous infarction and haemorrhage. Therefore, care must also be taken to prevent the reflux of glue into cortical veins and the SOV as well as propagation of glue into the pulmonary artery via the draining vein. Additionally, trans-collateral embolisation into normal cerebral arteries and rupture of the catheterised vessel may occur. Such complications did not occur in our patients, but the effort to prevent these complications should be made. The spillage of glue via the arterio-venous fistula into the ICA and subsequently into the distal middle cerebral artery territory due to forceful injection has been reported. [29]. Careful check for potentially dangerous anastomoses and injection of glue in a slow, controlled manner probably prevents these complications.
Conclusions
Trans-arterial embolisation with low concentration NBCA using the technique of a wedged microcathaeter is still a safe and effective treatment for DCCF when frequently used trans-venous approaches are not feasible. However, care must be taken to prevent inadvertent arterial and venous embolisation.
References
Arat A, Inci S (2006) Treatment of a superior sagittal sinus dural arterio-venous fistula with Onyx: technical case report. Neurosurgery 59(1 Suppl 1):169–170
Barrow DL, Spector RH, Braun IF, Landman JA, Tindall SC, Tindall GT (1985) Classification and treatment of spontaneous carotid-cavernous sinus fistulae. J Neurosurg 62:248–256
Benndorf G, Bender A, Lehmann R, Lanksch W (2000) Trans-venous occlusion of dural cavernous sinus fistulae through the thrombosed inferior petrosal sinus: report of four cases and review of the literature. Surg Neurol 54:42–54
Bellon RJ, Liu AY, Adler JR, Norbash AM (1999) Percutaneous trans-femoral embolisation of an indirect carotid-cavernous fistula with cortical venous access to the cavernous sinus. Case report. J Neurosurg; 90:959–963
Biondi A, Milea D, Cognard C, Ricciardi GK, Bonneville F, van Effenterre R (2003) Cavernous sinus dural fistulae treated by trans-venous approach through the facial vein: report of seven cases and review of the literature. AJNR Am J Neuroradiol 24:1240–1246
Cheng KM, Chan CM, Cheung YL (2003) Trans-venous embolisation of DCCFs by multiple venous routes: a series of 27 cases. Acta Neurochir (Wien) 145:17–29
Dawson RC, Joseph GJ, Owens DS, Owens DS, Barrow DL (1998) Trans-venous embolisation as the primary therapy for arterio-venous fistulae of the lateral and sigmoid sinuses. AJNR Am J Neuroradiol 19:571–576
Goldberg RA, Goldey SH, Duckwiler G, Vinuela F (1996) Management of cavernous sinus-dural fistulae. Indications and techniques for primary embolisation via the superior ophthalmic vein. Arch Ophthalmol 114:707–714
Halbach VV, Higashida RT, Hieshima GB, Hardin CW, Pribram H (1989) Trans-venous embolisation of dural fistulae involving the cavernous sinus. AJNR Am J Neuroradiol 10:377–383
Halbach VV, Higashida RT, Hieshima GB, Reicher M, Norman D, Newton TH (1987) Dural fistulae involving the cavernous sinus: results of treatment in 30 patients. Radiology 163:437–442
Hara T, Hamada J, Kai Y, Ushio Y (2002) Surgical trans-venous embolisation of a carotid-cavernous dural fistula with cortical drainage via a petrosal vein: two technical case reports. Neurosurgery 50:1380–1383
Jahan R, Gobin YP, Glenn B, Duckwiler GR, Viñuela F (1998) Trans-venous embolisation of a dural arterio-venous fistula of the cavernous sinus through the contra-lateral pterygoid plexus. Neuroradiology 40:189–193
Kai Y, Hamada J, Morioka M, Yano S, Kuratsu J (2007) Treatment of cavernous sinus dural arterio-venous fistulae by external manual carotid compression. Neurosurgery 60:253–257
Kim DJ, Kim DI, Suh SH, Kim J, Lee SK, Kim EY, Chung TS (2006) Results of trans-venous embolisation of cavernous dural arterio-venous fistula: a single-center experience with emphasis on complications and management. AJNR Am J Neuroradiol 27:2078–2082
Kirsch M, Henkes H, Liebig T, Weber W, Esser J, Golik S, Kühne D (2006) Endovascular management of dural carotid-cavernous sinus fistulae in 141patients. Neuroradiology 48:486–490
Klisch J, Huppertz HJ, Spetzger U, Hetzel A, Seeger W, Schumacher M (2003) Trans-venous treatment of carotid cavernous and dural arterio-venous fistulae: results for 31 patients and review of the literature. Neurosurgery 53:836–856
Kuwayama N, Endo S, Kitabayashi M, Nishijima M, Takaku A (1998) Surgical trans-venous embolisation of a cortically draining carotid cavernous fistula via a vein of the Sylvian fissure. AJNR Am J Neuroradiol 19:1329–1332
Layton KF, Nelson MD, Kallmes DF (2006) Trans-arterial coil embolisation of the venous component of aggressive type 4 dural arterio-venous fistulae. AJNR Am J Neuroradiol 27:750–752
Liu HM, Huang YC, Wang YH, Tu YK (2000) Trans-arterial embolisation of complex cavernous sinus dural arterio-venous fistulae with low-concentration cyanoacrylate. Neuroradiology 42:766–770
Liu HM, Wang YH, Chen YF, Cheng JS, Yip PK, Tu YK (2001) Long-term clinical outcome of spontaneous carotid cavernous sinus fistulae supplied by dural branches of the internal carotid artery. Neuroradiology 43:1007–1014
Meyers PM, Halbach VV, Dowd CF, Lempert TE, Malek AM, Phatouros CC, Lefler JE, Higashida RT (2002) Dural carotid cavernous fistula: definitive endovascular management and long-term follow-up. Am J Ophthalmol 134:85–92
Miller NR, Monsein LH, Debrun GM, Tamargo RJ, Nauta HJ (1995) Treatment of carotid-cavernous sinus fistulae using a superior ophthalmic vein approach. J Neurosurg 83:838–842
Mounayer C, Piotin M, Spelle L, Moret J (2002) Superior petrosal sinus catheterisation for trans-venous embolisation of a dural carotid cavernous sinus fistula. AJNR Am J Neuroradiol 23:1153–1155
Nelson PK, Russell SM, Woo HH, Alastra AJ, Vidovich DV (2003) Use of a wedged microcatheter for curative trans-arterial embolisation of complex intracranial dural arterio-venous fistulae: indications, endovascular technique, and outcome in 21 patients. J Neurosurg 98:498–506
Oishi H, Arai H, Sato K, Iizuka Y (1999) Complications associated with trans-venous embolisation of cavernous dural arterio-venous fistula. Acta Neurochir (Wien) 141:1265–1271
Quiñones D, Duckwiler G, Gobin PY, Goldberg RA, Viñuela F (1997) Embolisation of dural cavernous fistulae via superior ophthalmic vein approach. AJNR Am J Neuroradiol 18:921–928
Toulgoat F, Mounayer C, Túlio Salles Rezende M, Piotin M, Spelle L, Lazzarotti G, Desal H, Moret J (2006) Trans-arterial embolisation of intracranial dural arterio-venous malformations with ethylene vinyl alcohol copolymer (Onyx18). J Neuroradiol 33:105–114
Troffkin NA, Given CA (2007) Combined trans-arterial N-butyl cyanoacrylate and coil embolisation of direct carotid-cavernous fistulae. Report of two cases. J Neurosurg; 106:903–906
Wakhloo AK, Perlow A, Linfante I, Sandhu JS, Cameron J, Troffkin N, Schenck A, Schatz NJ, Tse DT, Lam BL (2005) Trans-venous n-butyl-cyanoacrylate infusion for complex dural carotid cavernous fistulae: technical considerations and clinical outcome. AJNR Am J Neuroradiol 26:1888–1897
Yamashita K, Taki W, Nishi S, Sadato A, Nakahara I, Kikuchi H, Yonekawa Y (1993) Trans-venous embolisation of dural carotid cavernous fistulae: technical considerations. Neuroradiology 35:475–479
Yu SC, Cheng HK, Wong GK, Chan CM, Cheung JY, Poon WS (2007) Trans-venous embolisation of dural carotid-cavernous fistulae with trans-facial catheterisation through the superior ophthalmic vein. Neurosurgery 60:1032–1037
Author information
Authors and Affiliations
Corresponding authors
Additional information
Comment
An extensive literature has been written on dural arterio-venous fistulas (DAVFs) of the cavernous sinus, and various managements have been proposed to treat these lesions. Endovascular approach of the cavernous sinus through venous route (via the inferior petrosal sinus, pterygoid plexus, ophthalmic vein via direct puncture or facial navigation) with coiling of the venous compartment draining the shunt is considered to be the best and safest treatment for these complex lesions, and transarterial embolization has been rarely proposed. The authors report in this paper their experience in five patients with dural shunts of the cavernous sinus that have been treated by transarterial embolization with glue. This paper is interesting as it opens a debate concerning the interest and risks of this technique, and the use of liquid emboli in these lesions. The problems raised by transarterial embolization in DAVFs of the cavernous sinus are indeed triple: one must navigate through tiny tortuous arterial feeders arising from internal or external carotid arteries that are nearly always neuro-dural arteries also vascularizing cranial nerves (CN), one must occlude selectively the shunting zone without being proximal in order to respect the CN vascularization, and one has to avoid the dangerous arterial anastomoses at the skull base. The main challenge in transarterial embolization of DAVFs of the cavernous sinus is thus much more anatomical than technical. All these procedures have to be performed under General Anaesthesia in order to allow precise depiction of all feeders taking in charge the shunt. The cavernous sinus region is indeed an important anatomic crossroads between external and internal carotid vascularizations, and transarterial embolization has thus to be performed in perfect anatomic conditions with precise recognitions of the dangerous points of the artery that is catheterized and occluded. Major complications can occur (CN palsies, erratic emboli in the ophthalmic artery or in the intracranial internal carotid artery) if the procedure is poorly achieved. Proper permanent occlusion of the shunt is only obtained with liquid emboli. Particles create transitory occlusions and rarely cure the lesion: their use should thus be nowadays limited to flow redistributions in order to allow secondarily better endovascular occlusions through a main feeder.
Transarterial deposition of coils is inadequate because of the proximal occlusion that will be created. Only fluid agents are useful emboli here: the risk they carry are not related to their physical characteristics but rely on anatomic traps that are scattered all along the traject of the neuro-dural arteries before reaching the shunt itself. Glue is an ?old? embolic agent that has proven since more than 30 years its efficiency in the endovascular management of arterio-venous malformations. Injected diluted and in wedge position in the arterial feeder, it can penetrate deeply into the DAVF; furthermore, because of its three effects(inflammatory, thrombotic, thermic), glue may allow secondary thrombosis of the shunt. It is a cheap product in comparison to Onyx that is considered by certain teams nowadays as a reference embolus . The long term follow up of Onyx is however not known at this stage. The debate that could be created between the supporters of Onyx and those of glue is thus a wrong debate that should not mask the need of precise anatomic knowledge in cavernous DAVFs. The therapeutic decisions have to be taken on a case-by-case basis. Venous approach should be privileged whenever possible. Glue can be injected also directly into the venous compartment draining the shunt : the cavernous sinus being in fact a plexus, occlusion of one channel may not compromise the venous drainage of other compartment. Compression of the superior ophthalmic vein at the internal canthus remains an appropriate technique in cases of DAVFs draining exclusively into that system. Transarterial embolization of these lesions should only be offered in specific indications after careful evaluation. It should be balanced against other therapeutic managements known to also offer good results in these lesions, as radiosurgery.
Georges Rodesch
Hôpital Foch, Suresnes, France
An erratum to this article can be found at http://dx.doi.org/10.1007/s00701-009-0458-3
Rights and permissions
About this article
Cite this article
Li, MH., Tan, HQ., Fang, C. et al. Trans-arterial embolisation therapy of dural carotid–cavernous fistulae using low concentration n-butyl-cyanoacrylate. Acta Neurochir (Wien) 150, 1149–1156 (2008). https://doi.org/10.1007/s00701-008-0133-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-008-0133-0