Abstract
During mould surveys a number of Aspergillus strains were isolated from environmental air which did not fit any known species of the genus. They showed phenotypic as well as molecular genetic similarity with A. arenarius, A. arenarioides and A. peyronelii, three species without clear phylogenetic position. Multi-gene phylogenetic analysis comprising taxa across the subgenus Circumdati showed that these species cluster into a well-supported clade sister to sect. Candidi. We propose the status of a new section for this clade, sect. Petersonii sect. nov. The phenotypic descriptions after 14 days on 8 various agar media were provided for members of sect. Petersonii which, together with maximum growth temperature and molecular genetic data from four loci (ITS rDNA, β-tubulin, calmodulin and RPB2) supported the recognition of four species. Two species are newly described here as A. asclerogenus sp. nov. and A. petersonii sp. nov. Aspergillus arenarius is reduced to synonymy with A. peyronelii, a species revived and typified in this study. A dichotomous key based on the combination of morphology and physiology is provided for all recognized species of sect. Petersonii. In addition, other species from subg. Circumdati with ambiguous phylogenetic position based on previous studies were also included in our analysis resulting in the proposal of sections Robusti and Tanneri. All newly proposed sections also have strong phenotypic support.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aspergillus is a diverse genus encompassing approximately 350 species with high economic impact for humans (Samson et al. 2014). Such high numbers of species necessarily need a phylogeny- and phenotype-based subgeneric classification which is also user-friendly. The current classification into four subgenera and 20 sections (Houbraken et al. 2014; Hubka et al. 2015b) resolves the placement of the vast majority of species with only a few exceptions.
The subgenus Circumdati comprises important producers of mycotoxins, bioactive exometabolites, biotechnologically important enzymes and organic acids, some species are used in food fermentations, and some may cause food spoilage or human infections (Hubka et al. 2014, 2015b; Samson et al. 2011; Varga et al. 2011a, b; Visagie et al. 2014b). This subgenus is currently classified into seven sections; however, the phylogenetic position of several species belonging to subg. Circumdati remained unresolved in previous taxonomic studies. This was the case of A. janus and A. brevijanus previously classified as members of sect. Versicolores, Terrei or Flavipedes, and recently transferred to a new sect. Jani (Hubka et al. 2015b). The list of taxonomically problematic species includes also A. arenarius, A. peyronelii, A. arenarioides, A. robustus, A. tanneri and A. neoniveus (Houbraken et al. 2014; Houbraken and Samson 2011; Peterson 2008; Peterson et al. 2008; Visagie et al. 2014b). All these species either created peripheral clades of respective sections with low or moderate support or were classified as members of different sections by different studies.
The primary objective of this study was to integrate A. arenarius and its relatives into current subgeneric classification of Aspergillus. Isolates of these species were abundantly isolated during recent mould surveys from the indoor environment. The positions of other problematic species in the subg. Circumdati were also re-examined by using DNA sequence data from several protein-coding loci. Our phylogenetic data supported the proposal of three new sections in the subg. Circumdati, sect. Petersonii, Robusti and Tanneri. All sections were also strongly supported by unique phenotype. Other taxonomic novelties included description of two new species from the indoor environment in sect. Petersonii and synonymization of A. arenarius with A. peyronelii, revived in this study.
Materials and methods
Fungal isolates
Ten samples were collected as swab samples, two were air samples collected with a single stage bio-aerosol impaction sampler (EMSL VP-400 Microbial Sampler) (Peterson and Jurjević 2013). The media used for fungal isolation from air was malt extract agar (MEA), and dilution plates were used to isolate fungi that were taken by swabs. Swabs were placed in 10 mL of sterile water with 0.1 % Tween 20 and vortexed. Three dilutions were performed (102 ×, 103 ×, 104 ×) and plated out on MEA with chloramphenicol and dichloran-glycerol (DG18) agar. All isolates examined in this study were deposited into the Culture Collection of Fungi (CCF), Department of Botany, Charles University, Prague, Czech Republic; selected isolates were deposited into the Agricultural Research Service Culture Collection, Peoria, Illinois, USA (NRRL). Herbarium specimens of newly described species were deposited into the herbarium of the Mycological Department, National Museum in Prague (PRM). Provenance and GenBank accession numbers for DNA sequences of the isolates are detailed in Table 1.
Culture methods
Fungal isolates (Table 1) were grown at 25 °C for 14 days in darkness on Czapek yeast extract agar (CYA), MEA, CYA with 20 % sucrose (CY20S), Czapek yeast autolysate agar supplemented with 5 % NaCl (CYAS), DG18, oatmeal agar (OA), potato dextrose agar (PDA), and creatine agar (CREA) (Health Link®, Jacksonville, FL, USA) (Pitt 1980; Samson et al. 2010). Additional CYA and MEA cultures were incubated at different temperatures to determine the cardinal growth temperatures of the new species (20, 30, 35 and 37 °C) for 14 days (Table 2). The cultures were grown in duplicate as a three-point inoculation on each medium in 90 mm diam Petri dishes. Macromorphology of all species was described after 14 days of incubation when the colony color was fully expressed and all typical features were present.
Microscopy
Microscopic examination was detailed previously (Jurjevic et al. 2012). Scanning electron microscopy (SEM) was performed using a JEOL–6380 LV scanning electron microscope (JEOL Ltd. Tokyo, Japan) as described by Hubka et al. (2015a). A Nikon digital SLR camera with D70 lens was used for colony photography. Photographs were resized and fitted into plates with CorelDraw X6.
Molecular studies and phylogenetic analysis
ArchivePure DNA yeast and Gram2+ kit (5 PRIME Inc., Gaithersburg, Maryland) was used for DNA isolation from 7-day-old cultures according to manufacturer instructions as updated by Hubka et al. (2013). Forward primers ITS1F (5′-CTTGGTCATTTAGAGGAAGTAA), ITS1 (5′-TCCGTAGGTGAACCTGCGG) or ITS5 (5′-GGAAGTAAAAGTCGTAACAAGG) in combination with reverse primers ITS4S (5′-CCTCCGCTTATTGATATGCTTAAG) or NL4 (5′-GGTCCGTGTTTCAAGACGG) were used for amplification of ITS rDNA region and in some isolates also partial LSU rDNA region. Terminal primers were used for sequencing, internal primers ITS2 (5′-GCTGCGTTCTTCATCGATGC) and ITS3 (5′-GCATCGATGAAGAACGCAGC) were used in cases where sequencing with terminal primers did not produce sequences of sufficient quality. Partial β-tubulin (benA) was amplified with primer pair Bt2a (5′-GGTAACCAAATCGGTGCTGCTTTC) or Ben2f (5′-TCCAGACTGGTCAGTGTGTAA) and Bt2b (5′-ACCCTCAGTGTAGTGACCCTTGGC). All terminal primers were used for DNA sequencing. Calmodulin gene (caM) was amplified using the primers CF1M (5′-AGGCCGAYTCTYTGACYGA) or CF1L (5′-GCCGACTCTTTGACYGARGAR) and CF4 (5′-TTTYTGCATCATRAGYTGGAC). RPB2 gene encoding RNA polymerase II was amplified with primers fRPB2-5F (5′-GAYGAYMGWGATCAYTTYGG) or RPB2-F50-CanAre (5′-TTGAACATTGGTGTCAAGGC; designed in this study) and fRPB2-7cR (5′-CCCATRGCTTGYTTRCCCAT). Both terminal primers were used for sequencing. The reaction mixture and PCR protocol was described by Hubka and Kolařík (2012), RPB2 gene fragments were amplified by both standard and touchdown cycling conditions (Hubka and Kolařík 2012). PCR product purification and sequencing were performed at Macrogen Europe (Amsterdam, the Netherlands). Sequences were deposited into the EMBL (European Molecular Biology Laboratory) database under the accession numbers LN849386–LN849445 and LN873996–LN873998 listed in Table 1 (bold print).
Sequences were inspected and assembled using the Bioedit sequence alignment editor v7.0.0 (Hall 2004). Alignments of the regions were done using the FFT-NSi strategy as implemented in MAFFT v6.861b (Katoh et al. 2005). The best model for analysis was determined in MEGA6.
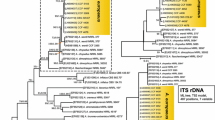
For the phylogenetic analysis across species in the subg. Circumdati, the benA, caM and RPB2 loci were combined and introns were extracted. The analysis involved 111 taxa, A. cervinus NRRL 5025T was used as an outgroup. There were a total of 1631 positions in the final dataset, 670 variable and 619 parsimony informative. The phylogenetic tree was calculated with maximum likelihood (ML) analysis based on the General Time Reversible model (GTR). A discrete Gamma distribution was used to model evolutionary rate differences among with possibility for some sites to be evolutionarily invariable (GTR+G+I). Codon positions included were 1st + 2nd + 3rd + noncoding and all positions with less than 70 % site coverage were eliminated (fewer than 30 % alignment gaps, missing data, and ambiguous bases were allowed at any position). Initial tree for the heuristic search was obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood approach, and then selecting the topology with superior log likelihood value. The tree with the highest log likelihood (−23606.7) is shown (Fig. 1). Statistical support for tree nodes was calculated with 1000 bootstrap iterations. Bayesian inference analysis (BI) was performed using MrBayes v3.1 (Ronquist and Huelsenbeck 2003), the same substitution model was used and metropolis-coupled Markov chain Monte Carlo search algorithm was run with 5 × 106 generations and four parallel chains. One tree was saved per 1000 generations, the burn-in and convergence of the chains were determined with TRACER v1.5 (available from http://tree.bio.ed.ac.uk/software/tracer).
Maximum likelihood tree showing the relationships of Aspergillus species belonging to subgenus Circumdati and their classification into sections. The analysis was based on the combined data from benA, caM and RPB2 loci, coding regions only, GTR+G+I model. Numbers on internodes are bootstrap proportions and Bayesian posterior probabilities. When bootstrap proportions were greater than 99 % and Bayesian probabilities greater than 0.99, the internode line is thick. Only supports higher than 50 % and 0.50, respectively, are shown. The ex-type strains are designated with superscript T
Phylogenetic trees based on single genes datasets were constructed for members of sect. Petersonii (Fig. 2). ML method was used with complete deletion option (all positions containing gaps and missing data were eliminated). Aspergillus pragensis CCF 3962T was used as an outgroup. Trees with the highest log likelihood score are shown (Fig. 2). The alignment of benA locus contained 21 taxa and 481 characters (50 variable, 27 parsimony informative, K2+G model), caM alignment contained 23 taxa and 793 characters (191 variable, 76 parsimony informative, K2+G model), RPB2 alignment contained 23 taxa and 1038 characters (211 variable, 66 parsimony informative, T92+G model), ITS rDNA alignment contained 22 taxa and 572 characters (57 variable, 7 parsimony informative, T92+G model). Alignments were deposited in TreeBASE (submission ID 18279).
Results
Phylogenic analysis
Four DNA regions were amplified and sequenced for the ex-type strains of A. peyronelii, A. arenarius, A. arenarioides and related species isolated in this study (Table 1). ML and BI phylogenetic analyses based on coding regions of benA, caM and RPB2 genes were performed to resolve the position of these taxa and other species with not fully resolved positions belonging to subg. Circumdati. Both ML and BI analyses across species diversity of subg. Circumdati supported arrangement of sections Circumdati, Candidi, Nigri, Jani, Terrei, Flavipedes and Flavi as recognized in the majority of recently published multi-gene phylogenetic studies (Fig. 1). The isolates of A. peyronelii and its relatives grouped into a separate and strongly supported clade outside currently recognized sections, supporting the status of a new section for these taxa. This section is described below as sect. Petersonii and is the most closely related to sect. Candidi whose members are, however, phenotypically dissimilar (see below). The isolates of A. robustus and A. tanneri also created well-supported clades related to sect. Circumdati (Fig. 1). The phylogenetic distances of these clades from sect. Circumdati and each from other correspond to distances between other well-supported sections in the subg. Circumdati. We believe that classification of these taxa into separate sections Robusti and Tanneri (see below) with strong phylogenetic and phenotypic support is the best option to resolve the position of these controversial species. These new sections will expand the number of single-species sections in the genus Aspergillus, i.e., sects. Silvati and Bispori (both from the subg. Nidulantes).
The section Nigri as a whole has only moderate support by ML analysis. The section is divided into several well-supported clades which correspond well with those described by Varga et al. (2011a). The phenotypic similarity of species across section Nigri supports their maintaining as one large section. In addition, the splitting of sect. Nigri into several separate sections would be devoid of practical significance for users and thus opposed to the general concept of sections. The same applies to A. neoniveus and the clade containing A. ambiguus and A. microcysticus which created moderately supported marginal clades of sect. Terrei. The position A. neoniveus was especially controversial in previous studies (see “Discussion”), but the phylogenetic and morphological similarity support its classification as a member of sect. Terrei.
Four major clades were supported in sect. Petersonii based on combined analysis as well as in single-gene trees calculated by ML analyses (Fig. 2). These four groups of isolates were also separated by morphological and physiological data and are recognized here as separate species, A. peyronelii, A. arenarioides and two new species described below as A. petersonii and A. asclerogenus. The interspecies relationships in sect. Petersonii remain unresolved because of weakly supported deeper branching. The ex-type isolate of A. peyronelii and A. arenarius grouped together in all trees (Figs. 1, 2) and thus A. arenarius is placed in synonymy with A. peyronelii based on priority rules. The genetic distances of sequences of both isolates did not exceed 1.2 %, a common intraspecies genetic diversity observed in aspergilli, including isolates of A. petersonii, a closely related species described below. The DNA sequences of all genetic loci used in this study are sufficient for differentiation of all four species recognized here. The discrimination power of ITS rDNA region is, however, very low in comparison to the remaining three loci, the situation is common across sections of the subg. Circumdati (Hubka et al. 2014, 2015b; Varga et al. 2011a, b; Visagie et al. 2014b). Whereas A. peyronelii and A. asclerogenus are clearly separated by ITS rDNA, the isolates of A. arenarioides and A. petersonii are distinguished by only single position in the alignment. This single substitution is, however, usable for barcoding purposes.
All strains from sect. Petersonii newly isolated in this study were identified as A. arenarioides and two new species described below (Table 1). The isolate ATCC MYA-4943 from which ITS sequence is deposited in GenBank under the designation A. callestemii (the description was not published) represents probably A. peyronelii (Fig. 2).
Discussion
Taxonomic position of A. peyronelii and its relatives
Taxonomic placement of A. peyronelii and A. arenarius has always been problematic. Sappa (1955) discussed the probable relationship of A. peyronelii to species from sect. Usti and Terrei. Aspergillus arenarius and A. peyronelii were placed into sect. Versicolores by Raper and Fennell (1965) and Kozakiewicz (1989) based on morphology. Kozakiewicz (1989) also places A. floriformis in synonymy with A. peyronelii based on identical ornamentation of conidia, osmotolerance and some other similarities found in the original descriptions. Relatedness of these species is very unlikely because A. floriformis in contrast to A. peyronelii produces globose Hülle cells and brown-pigmented conidiophores, and has high growth optimum (35 °C) and maximum (45 °C). All these characters indicate that A. floriformis belongs with high probability to subg. Nidulantes as also recognized by Samson and Mouchacca (1975) and Samson (1979).
Combined data from 5.8S and 28S rDNA and RPB2 placed A. arenarius in the neighborhood of sect. Candidi (Peterson 2008). Similarly, combined dataset of Cct8, Tsr1, RPB1 and RPB2 placed A. arenarius close to A. candidus, the species with very different phenotype (Houbraken and Samson 2011). The position of A. arenarius and closely related A. arenaroides was recently examined by Visagie et al. (2014a) by ITS data which placed them closely to a clade with uniseriate black aspergilli (sect. Nigri). The number of available cultures for A. peyronelii and A. arenarius has always been very limited and restricted almost exclusively to the ex-type isolates and NRRL 4899 which was mentioned by Raper and Fennell (1965) as possible new collection of A. peyronelii. New isolates closely related to these taxa were collected recently from indoor environment in Micronesia and described by Visagie et al. (2014a) as A. arenarioides. Some isolates collected in this study from indoor environments in Trinidad & Tobago and the USA again represented A. arenarioides, but also two new species described here as A. asclerogenus and A. petersonii. The position of all five above-mentioned species was analyzed in combined phylogenetic analysis based on β-tubulin, calmodulin and RPB2 data together with taxa across the entire subg. Circumdati. This analysis similarly to morphological data supported the status of a new section for these taxa—sect. Petersonii sect. nov. Sequence data and analysis of authentic material (see section Taxonomy) also indicated that A. arenarius is identical with A. peyronelii described earlier.
The distribution of species from sect. Petersonii seems to be restricted to tropical regions with several exceptions. Aspergillus peyronelii has been isolated only from soil (Somalia, India). The ITS sequence of strain ATCC MYA-4943 deposited in GenBank under designation A. callestemii probably belongs to A. peyronelii (Fig. 2, ITS tree), which was re-isolated in India 50 years after its first isolation there by E. Yuill (isolate NRRL 5012). Other species in sect. Petersonii were isolated mostly from the indoor environment in Micronesia or the Caribbean region (Trinidad and Tobago, Florida). The exception is the strain of A. petersonii isolated from baseball gloves in Illinois (USA).
The spectrum of exometabolites produced by sect. Petersonii members is in general unknown, but sclerotia analysis of Aspergillus peyronelii NRRL 5012 revealed three terphenyl-type metabolites and arenarins A–C (Oh et al. 1998). Arenarins A–C have demonstrated pharmacological activity against human tumor cells (Oh et al. 1998). Whereas arenarins are only known from A. peyronelii, terphenyl-type metabolites were detected in members of sect. Candidi, A. ellipticus (sect. Nigri) and Penicillium raistrickii (Belofsky et al. 1998; Hubka et al. 2014; Rahbæk et al. 2000).
Position of other taxonomically ambiguous species in the subgenus Circumdati
The placement of other taxonomically unresolved or ambiguous species belonging to subg. Circumdati was investigated by multi-gene phylogeny together with A. peyronelii clade (Fig. 1). The position of A. robustus was controversial since the original description (Christensen and Raper 1978). Christensen and Raper (1978), and Raper and Fennell (1965) recognized its affinity to sect. Circumdati (A. ochraceus group) but also discussed possible relationship to A. alliaceus and A. lanosus (sect. Flavi). The placement of A. robustus was not fully resolved or contradictory in sequence-based phylogenetic studies. Based on D1 and D2 regions of LSU rDNA (Peterson 2000) and ITS rDNA data (Varga et al. 2000), the species created a separate clade unrelated to other Aspergillus sections without clear affinity to subg. Circumdati. More recent multi-gene phylogenetic studies placed A. robustus in the distant neighborhood of sect. Circumdati (Houbraken and Samson 2011; Peterson 2008; Visagie et al. 2014b). Similarly, A. tanneri described recently from two cases of invasive aspergillosis in chronic granulomatous disease patients was found to be phylogenetically most closely related but morphologically dissimilar to A. robustus and sect. Circumdati (Sugui et al. 2012). Position of these controversial taxa cannot be resolved by using the current system of sections and we believe that the creation of separate sections is the best option. Classification of these two species as members of sect. Circumdati would violate the otherwise very compact concept of the section in terms of morphology, phylogeny and production of exometabolites.
Aspergillus neoniveus (former Fennellia nivea) was the last species of our interest due to its controversial position in recent phylogenetic studies which placed it with low support into basal position toward sect. Terrei (Peterson 2008) or sects. Flavipedes and Jani (Peterson et al. 2008). Previous studies based on morphology usually classified this species into sect. Flavipedes (Samson 1979). Our analysis assigned this species with moderate support to a basal clade of sect. Terrei supporting current classification as did also Samson et al. (2011).
Taxonomic treatment
Aspergillus sect. Petersonii Ž.Jurjević & Hubka, sect. nov. [MycoBank MB#814442] —TYPE: Aspergillus petersonii Ž.Jurjević & Hubka
Description: Section Petersonii contains species with whitish, yellowish, light brown or green colonies. Conidiophores biseriate, stipes hyaline, brownish with age, smooth, finely roughened to crustaceous, vesicles do not exceed 20 µm in diam with variable shape, pyriform, subglobose, elongate near angular or Penicillium-like (Fig. 3); conidia globose to ellipsoidal, green in mass, smooth to roughened by light microscopy, microverrucose, tuberculate to lobate-reticulate in SEM (Figs. 4, 5). Sclerotia globose to ellipsoidal (Figs. 4, 5), pale yellow to brown, observed in all species with exception of A. asclerogenus. Sexual state is unknown. None of the species assigned to this section are able to grow on CYA at 40 °C. The section now encompasses four species in a strongly supported clade in the subg. Circumdati. Most closely related section Candidi differs significantly by color of sporulation (white or yellow), predominantly globose vesicles commonly reaching or exceeding the diameter of 20 µm and production of black or purple-black sclerotia. The species of sect. Petersonii are predominantly indoor and soil-borne fungi known almost exclusively from the tropical region (see Table 1).
Micromorphology of members of Aspergillus section Petersonii by optical microscopy. Aspergillus peyronelii NRRL 5012 (a–d): conidia (a), conidiophores (b–d); Aspergillus arenarioides CCF 4938 (e, h) and CCF 4939 (f, g): conidia (e), conidiophores (f–h). Aspergillus petersonii CCF 4999T (i–l): conidia (i), conidiophores (j–l); Aspergillus asclerogenus CCF 4947T (m–p): conidia (m), conidiophores (n–p). Scale bars 5 μm
Scanning electron microscopy pictures of Aspergillus petersonii and A. asclerogenus. Aspergillus petersonii CCF 4999T (a–d) and CCF 4945 (e): sclerotium (a), detail of vesicle with phialides (b), stipe (c), conidia (d, e); Aspergillus asclerogenus CCF 4947T (f–i): conidiophore (f, g), conidia (h, i). Scale bars a = 100 μm; b–i = 5 μm
Scanning electron microscopy pictures of Aspergillus peyronelii and A. arenarioides. Aspergillus peyronelii NRRL 5012 (a–e): sclerotium (a), detail of vesicle with phialides (b), phialides (c), conidia (d, e); Aspergillus arenarioides CCF 4928T (f–h): sclerotium (f), conidia (g, h). Scale bars a, f = 100 μm; b–e, g, h = 5 μm
New species
Aspergillus asclerogenus Ž.Jurjević & Hubka, sp. nov. —TYPE: Trinidad and Tobago, Tunapuna, isol. ex indoor air sample, home, Aug 2008, Ž. Jurjević, (holotype—PRM 933843; dried colony of CCF 4947T). Ex-holotype culture CCF 4947T = NRRL 58502T [MycoBank MB#814441] (Figs. 3m–p, 4f–i, 6).
Etymology: Named after the absence of sclerotia in culture in contrast to related species.
Description: Colonies on CYA at 25 °C (Fig. 6) attained 17–21 mm diam in 14 days, velutinous, rising ca. 4–6 mm, centrally lightly concave, radially moderate deep to deep sulcate, reverse brown, conidial heads grey-greenish-blue, abundant at the center of colony, mycelium white to pale buff; on 20 °C same as at 25 °C, reverse pale brown to brown; at 30 °C colonies similar to CYA at 25 °C, on 35 °C conidia inconspicuous, very poor sporulation, no growth at 37 °C (Table 2). Colonies on MEA at 25 °C (Fig. 6) attained 17–18 mm diam in 14 days, velutinous, low, plain, radially moderate deep sulcate, reverse brown; sporulation abundant, conidial heads radiate to loosely columnar, greyish-blue-green; mycelium white with yellowish shades at margins; at 20 and 30 °C colonies similar to MEA at 25 °C, good sporulation, conidial heads buff, inconspicuous, abruptly rising ca. 3–4 mm; no growth at 37 °C (Table 2). Colonies on CY20S at 25 °C (Fig. 6) attained 13–18 mm diam in 14 days, reverse uncolored; good sporulation, conidial heads columnar, grey-green, mycelium white, submerged. Colonies on OA at 25 °C (Fig. 6) attained 18–20 mm diam in 14 days, velutinous, low, radially very lightly sulcate, reverse grey; sporulation very good, conidial heads buff to greyish-green, mycelium white to buff. Colonies on PDA at 25 °C (Fig. 6) attained 20–22 mm diam in 14 days, velutinous, rising ca. 3 mm diam, radially moderate deep to deep sulcate, reverse brown; very good sporulation at the center of colony (area with 8–12 mm diam), conidial heads greyish-blue-green, mycelium white with yellowish shades at margins 5–8 mm diam. Colonies on DG18 at 25 °C (Fig. 6) attained 15–17 mm diam in 14 days, velutinous, rising ca. 3–4 mm, radially moderate deep sulcate, reverse pale yellow; good to very good sporulation, conidial heads buff, inconspicuous, mycelium white covering the entire colony. Colonies on CYAS at 25 °C (Fig. 6) attained 14–23 mm diam in 14 days, velutinous to lightly floccose, rising ca. 3 mm, occasionally moderate deep sulcate, reverse buff to yellowish-brown; abundant sporulation over the entire colony, conidial heads greyish-blue-green, mycelium white near inconspicuous. Colonies on CREA at 25 °C (Fig. 6) attained 7–9 mm diam in 14 days, no acid production. No exudate, soluble pigment or sclerotia observed on all tested media.
Stipes (Figs. 3n–p, 4f, g) on MEA hyaline with age becoming brown, smooth to finely roughened, occasionally rough near crustaceous, villose in SEM (Fig. 4f, g), short if borne from aerial hyphae, long if borne from substrate; (15–)30–150(–250) × 2–3(–4) µm diam; vesicle (Fig. 3n–p) pyriform, occasionally subglobose to elongate near angular, occasionally borne at a small angle to the conidiophore, (4–)5–7(–9) µm diam, occasionally fertile on one side of vesicle, “medusa heads” frequently present (multiple conidiophores growing from vesicles); biseriate; metulae cylindrical (3–)4–7 × 2–3.5(–4.5) µm diam, occasionally finely rough to rough, villose in SEM (Fig. 4f), covering 1/3 to 2/3 of vesicle, occasionally entire vesicle; phialides ampulliform, (4–)5–7(–8) × 2–3 µm diam, sometimes villose in SEM (Fig. 4f, g); conidia globose to subglobose occasionally near ellipsoidal, (2–)2.5–3(–4) × 2–4 µm diam, smooth to finely rough, occasionally rough-walled, tuberculate in SEM (Fig. 3m, 4h, i).
Diagnosis: No sclerotia production, growth on MEA 4–6 mm and CYA 7–9 mm diam at 35 °C after 14 days, but no growth at 37 °C, “medusa heads” present (multiple conidiophores growing from vesicles).
Aspergillus petersonii Ž.Jurjević & Hubka, sp. nov. —TYPE: Trinidad and Tobago, Macoya, isol. ex indoor swab sample, office, Apr 2014, Ž. Jurjević, (holotype—PRM 933841; dried colony of CCF 4999T). Ex-holotype culture CCF 4999T = NRRL 66216T [MycoBank MB#814440] (Figs. 3i–l, 4a–e, 7).
Etymology: Named in honor of our colleague and good friend, Stephen W. Peterson for his significant contribution to the taxonomy of the genus Aspergillus.
Description: Colonies on CYA at 25 °C (Fig. 7) attained 17–21 mm diam in 14 days, velutinous, rising ca. 5–7 mm, radially moderate deep to deep sulcate, sclerotia pale buff to brown, commonly abundant as a crust, occasionally inconspicuous, overgrown with mycelium and spores, exudate clear to pale yellow, abundant, soluble pigment present in some isolates making the entire Petri dish faint brown (CCF 4948), reverse pale brown to orange-brown (CCF 4948); very good sporulation commonly at margins ca. 3–5 mm broad area, conidial heads radiate to loosely columnar, buff to greyish-blue, mycelium white with buff shades; sclerotia absent to abundant at 20 °C, poor to very good sporulation, at 30 °C poor to very good sporulation, conidial heads pale to buff, mycelium white to pale yellow, exudate clear to pale yellow, sclerotia pale to yellowish-brown, reverse buff; at 35 °C only isolate CCF 4944 grew up to 2 mm on CYA, isolate CCF 4999T did not grow at 35 °C after 2 weeks of cultivation, but left at room temperature for 6 additional days it grew to 6 mm diam. Colonies on MEA at 25 °C (Fig. 7) attained 15–18 mm diam in 14 days, velutinous to occasionally lightly floccose, plain and low at margins, occasionally moderate deep sulcate, rising ca. 3–4 mm, sclerotia pale yellow to brown, commonly abundant at the center of colony, ca. 10 mm diam, or in some isolates inconspicuous, overgrown with mycelium and spores, exudate clear to pale brown, sparse to abundant, soluble pigment absent, reverse light brown; sporulation mostly abundant, conidial heads radiate to loosely columnar, greyish-blue to greyish green-blue, fragmentary heads resembling penicillate fructifications occasionally present, poor, white to pale yellowish mycelium occasionally present, sometimes inconspicuous; on 20 °C abundant sporulation, exudate clear, sclerotia absent; on 30 °C very good sporulation, sclerotia present, sparse to abundant, pale to yellowish-brown, exudate clear, faint brown soluble pigment present only in CCF 4948, reverse brown; no growth at 35 °C (Table 2). Colonies on CY20S at 25 °C (Fig. 7) attained 16–20 mm diam in 14 days, velutinous, occasionally very lightly radially sulcate, low, plain, exudate absent, no sclerotia, no soluble pigments, reverse uncolored to greenish-grey centrally; sporulation abundant, conidial heads radiate to loosely columnar, greyish-blue to grey-bluish-green, mycelium white occasionally only visible at margins (ca. 2–3 mm wide zone). Colonies on OA at 25 °C (Fig. 7) attained 18–20 mm diam in 14 days, velutinous to somewhat floccose, radially lightly to moderate deep sulcate, rising ca. 3 mm; exudate clear to yellow, abundant, soluble pigment absent, reverse pale brown; sporulation good to abundant, conidial heads pale to pale yellow, mycelium white to buff, commonly beneath hyphae is a mat of brownish-yellow sclerotia, occasionally sparse. Colonies on PDA at 25 °C (Fig. 7) attained 13–20 mm diam in 14 days, velutinous to lightly floccose, rising ca. 3–5 mm diam, radially moderate deep to deep sulcate, exudate clear to yellow, abundant, soluble pigment when present faint brownish (CCF 4948), reverse pale buff to brown or orange-brown; poor to abundant sporulation, conidial heads radiate to loosely columnar, pale to greyish-blue, mycelium white, covering abundance of white to brown or brownish-yellow sclerotia. Colonies on DG18 at 25 °C (Fig. 7) attained 23–30 mm diam in 14 days, velutinous, plain, rising ca. 3–6 mm, moderate deep sulcate, exudate absent, no soluble pigment, no sclerotia, reverse white to yellow; abundant sporulation, conidial heads radiate to loosely columnar, greenish-grey to blue, mycelium white occasionally with buff shades, covering the entire colony. Colonies on CYAS at 25 °C (Fig. 7) attained 20–28 mm diam in 14 days, velutinous, occasionally moderate deep to deep sulcate, plain, rising ca. 3–6 mm, exudate when present clear to brownish, soluble pigment faint brown, present only in CCF 4948, occasionally yellowish-brown sclerotia, sparse, reverse pale brown to brown; very abundant sporulation, conidial heads radiate, greenish-grey to greyish-blue, mycelium white with pale buff shades. Colonies on CREA at 25 °C (Fig. 7) attained 9–15 mm diam in 14 days, no acid production.
Stipes (Figs. 3j–l, 4c) on MEA hyaline with age in brownish shades, smooth to finely roughened, occasionally with age becoming rough near crustaceous, villose in SEM (Fig. 4c), short if borne from aerial hyphae, long if borne from substrate; (30–)75–350(–550) × (–2.5)4–6(–7) µm diam; vesicle pyriform to spatulate, occasionally subglobose, occasionally borne at a small angle to the conidiophore, (6–)7–14(–17) µm diam; biseriate; metulae cylindrical (3–)4–10(–14) × 2.5–3.5(–4.5) µm diam, occasionally finely rough to rough, villose in SEM (Fig. 4b), covering 1/3 to 2/3 of vesicle, occasionally entire vesicle; phialides ampulliform, (5–)6–9(–15) × 2–3(–4) µm diam, occasionally finely roughened, villose in SEM (Fig. 4b), occasionally fertile on one side of vesicle; conidia globose to subglobose occasionally ellipsoidal, (2.5–)3–4(–5–6 occasionally, 7–9 rare) × 2.5–5 µm diam, smooth to finely roughened or rough-walled (Fig. 3i), tuberculate in SEM (Fig. 4d, e). Hülle cells absent. Sclerotia globose to ellipsoidal, buff to pale brown, or brownish-yellow, present on CYA, CYAS, MEA, OA and PDA, 250–600 µm diam (Fig. 4a).
Diagnosis: Mostly abundant sporulation on MEA, conidial heads greyish-blue to greyish green-blue, sclerotia mostly abundant at the center of colony. No growth at 35 °C after 14 days on MEA, or occasionally very restricted growth on CYA up to 2 mm.
Emended descriptions
Aspergillus arenarioides Visagie, Hirooka & Samson, Stud. Mycol. 78: 110. 2014. [MycoBank MB#809195] (Figs. 3e–h, 5f–h, 8).
Emended description: Colonies on CYA at 25 °C (Fig. 5) attained 14–23 mm diam in 14 days, velutinous, rising ca. 4–6 mm, radially moderate deep to deep sulcate, covered with crust of pale yellow to brown sclerotia, exudate clear to pale yellow or pale yellowish-brown, abundant, soluble pigment absent, isolate NRRL 4899 produces somewhat dump earthy odor, reverse brown; sporulation inconspicuous, conidial heads grey blue-green, mycelium white occasionally with greyish shades; on 20 °C sporulation sparse or absent, conidial heads greyish-green, sclerotia brown, abundant, exudate clear, absent to abundant; at 30 °C colonies similar to CYA at 25 °C. Colonies on MEA at 25 °C (Fig. 8) attained 15–23 mm diam in 14 days, velutinous, rising ca. 5 mm, sclerotia pale yellow to brown, abundant, covering almost entire colony as a crust or occasionally in concentric rings, or in some isolates overgrown with mycelium, sclerotia absent at NRRL 4899, exudate clear to pale yellow or yellowish-brown, abundant, soluble pigment absent; reverse brown; sporulation poor to good, conidial heads pale to greyish-blue-green, mycelium white occasionally with buff shades, fragmentary heads resembling penicillate fructifications occasionally present; at 20 °C and 30 °C colonies similar to MEA at 25 °C. Colonies on CY20S at 25 °C (Fig. 8) attained 15–25 mm diam in 14 days, velutinous, soluble pigments absent; reverse uncolored, sclerotia buff to yellow occasionally in concentric rings, in some isolates absent; sporulation poor to good, conidial heads grey green, mycelium white. Colonies on OA at 25 °C (Fig. 8) attained 17–29 mm diam in 14 days, velutinous, occasionally radially moderate deep sulcate, centrally rising ca. 3 mm, exudate clear, abundant, soluble pigment absent, reverse brown; sporulation abundant occasionally inconspicuous, covered with crust of yellow to brown sclerotia, mycelium white with orange-yellow shades, conidial heads grey green. Colonies on PDA at 25 °C (Fig. 8) attained 17–24 mm diam in 14 days, velutinous, rising ca. 5–6 mm diam, radially moderate deep to deep sulcate, exudate clear to yellow occasionally brown, abundant, soluble pigment absent, reverse brown; sporulation abundant to sparse (NRRL 4899), conidial heads grey-green to pale blue, occasionally inconspicuous, covered with crust of yellow to brown sclerotia, occasionally absent (NRRL 4899), mycelium white with yellow shades. Colonies on DG18 at 25 °C (Fig. 8) attained 19–31 mm diam in 14 days, velutinous, rising ca. 4–5 mm, radially moderate deep sulcate, exudate clear, sparse, when present sclerotia yellow, often sparse covered with hyphae, soluble pigment absent, reverse pale yellow; good sporulation, conidial heads grey to blue-green, mycelium white. Colonies on CYAS at 25 °C (Fig. 8) attained 20–28 mm diam in 14 days, conidial heads pale blue-green to grey-green, abundant, occasionally inconspicuous, covered with crust of pale yellowish to brown sclerotia, in some isolates sclerotia sparse or absent, mycelium white occasionally with yellowish shades, velutinous, rising ca. 5–6 mm, moderate deep sulcate, exudate clear to brown, abundant, soluble pigment absent, sclerotia absent, reverse pale yellow to brown or dark brown. Colonies on CREA at 25 °C (Fig. 8) attained 13–24 mm diam in 14 days, no acid production.
Stipes (Fig. 3f–h) on MEA hyaline with age in brownish shades, smooth to finely roughened, occasionally rough nearly crustaceous, short if borne from aerial hyphae, long if borne from substrate, (20–)60–250(–375) × (2.3–)3–5 µm diam, on CYAS up to 550 µm long; biseriate; vesicle pyriform to subglobose, occasionally somewhat elongate to near angular, occasionally borne at a small angle to the conidiophore, (5–)6–9(–12) µm diam; metulae cylindrical (4–)5–8(–10) × 2.5–4(–5) µm diam, covering 1/3 to 3/3 of vesicle; phialides ampulliform, (6–)7–9(–12) × (2.3–)2.5–3(–3.5) µm diam, occasionally fertile on one side of vesicle; conidia globose to ellipsoidal, (2.3–)2.5–3.5(–5) × 2.5–3.5(–5) µm diam (Figs. 3e, 5g, h), finely roughened to rough. Hülle cells absent. Sclerotia globose to ellipsoidal, pale yellow to brown, 150–500 µm diam (Fig. 5f).
Aspergillus peyronelii Sappa, Allionia 2: 248. 1955. [MycoBank MB#292855] (Figs. 3a–d, 5a–e, 9) —Described from: Somalia, near Goluin, isol ex tropical soil in the thorn savannah, before 1955, F. Sappa. —LECTOTYPE (designated here): Plate 1, subfigures 1-4, in Sappa 1955, Allionia 2: 249 [MycoBank MBT#201633]. —EPITYPE (designated here): a dried herbarium specimen derived from the culture IMI 139271 (PRM 933831) [MycoBank MBT#201634]. Ex-epitype culture is IMI 139271T = CCF 4942T (both sterile but verified by this study). The culture CBS 122.58 (contaminant) and NRRL 4754 (probably mixed culture) will be replaced by new material from this study (personal communication with J. Houbraken and S.W. Peterson).
Emended description (based on strain NRRL 5012): Colonies on CYA at 25 °C (Fig. 9) attained 17–18 mm diam in 14 days, velutinous, rising ca. 4–6 mm, radially moderate deep to deep sulcate, sclerotia covering entire colony as a brown crust, overgrown with light layer of hyphae, commonly agar is cracking around the colony, exudate clear to brown, sparse, soluble pigment brown, strong, reverse orange-brown to brown; very poor sporulation, conidial heads brownish-green, inconspicuous, mycelium white; on 30, 35, and 37 °C colonies similar to CYA at 25 °C, except soluble brown pigment is more intense, mycelium more dirty white to dirty yellowish-brown. Colonies on MEA at 25 °C (Fig. 9) attained 18–20 mm diam in 14 days, velutinous, rising centrally ca. 3 mm, radially moderate deep to deep sulcate, exudate clear to pale yellow, soluble pigment absent, reverse brown; conidial heads absent, entire colony covered with heavy layer of yellowish-brown to brown sclerotia, mycelium white, visible only at margins; on 20 °C colonies are velutinous, abruptly rising ca. 3 mm, lightly to moderate deep sulcate, exudate clear, sparse, reverse brown; mycelium white, sclerotia pale yellow; on 30, 35 and 37 °C colonies similar to MEA at 25 °C. Colonies on CY20S at 25 °C (Fig. 9) attained 19–22 mm diam in 14 days, velutinous, no exudate or soluble pigments, sclerotia absent, reverse buff to brown centrally; conidial heads absent, mycelium white. Colonies on OA at 25 °C (Fig. 9) attained 17–20 mm diam in 14 days, velutinous, rising ca. 4 mm, radially moderate deep sulcate, exudate clear, abundant, soluble pigment absent, reverse brown; conidial heads absent, entire colony covered with heavy layer of brown sclerotia, white mycelium only visible at margins. Colonies on PDA at 25 °C (Fig. 9) attained 18–20 mm diam in 14 days, velutinous, rising ca. 5 mm, radially moderate deep to deep sulcate, exudate clear, abundant, soluble pigment absent, reverse brown; very poor sporulation, conidial heads inconspicuous, entire colony covered with heavy layer of brown sclerotia, white mycelium only visible at margins. Colonies on DG18 at 25 °C (Fig. 9) attained 19–24 mm diam in 14 days, velutinous, radially moderate deep to deep sulcate, exudate and soluble pigment absent, sclerotia absent, reverse buff; poor sporulation, conidial heads white to cream, inconspicuous, mycelium white. Colonies on CYAS at 25 °C (Fig. 9) attained 16–24 mm diam in 14 days, velutinous, rising ca. 4 mm, radially moderate deep to deep sulcate, exudate brown, sparse, sclerotia absent, soluble pigment in brownish shades, reverse orange-brown to brown; sporulation good, conidial heads radiate to loosely columnar, white to pale buff, inconspicuous, mycelium white with orange-yellow shades. Colonies on CREA at 25 °C (Fig. 9) attained 9–14 mm diam in 14 days, no acid production.
Stipes (Fig. 3b–d) on CYAS hyaline, become brownish with age, smooth, occasionally finely roughened to rough, slightly villose in SEM, short if borne from aerial hyphae, long if borne from substrate, (25–)50–200(–300) × 2–4 µm diam; biseriate; vesicle pyriform to subglobose, occasionally somewhat elongate near angular, (4–)5–9(–12) µm diam; metulae cylindrical (4–)5–8(–9) × 3–4.5(–5) µm diam, covering 1/3 to 2/3 of vesicle occasionally entire vesicle, slightly villose in SEM; phialides ampulliform, (5–)6–9(–10) × 2-3(–3.5) µm diam, occasionally fertile on one side of vesicle, occasionally finely roughened, villose in SEM (Fig. 5b, c); conidia globose to subglobose occasionally ellipsoidal, 2–3(–4) × 2–3 µm diam, smooth to finely roughened (Fig. 3a), microverrucose in SEM (Fig. 5d, e). Hülle cells absent. Sclerotia globose to ellipsoidal, brown, 150–350(–500) µm diam (Fig. 5a).
Notes 1 — examination of the type specimen of A. peyronelii (Fig. 10): Herbarium specimen Herb. IMI 139271 derived from the living culture CBS 122.58 was designated as lectotype of A. peyronelii (Samson and Gams 1985). Small bits of material were cut from the type specimen using insect minuten pins. The fungal material was either wetted in 70 % ethanol for 5–30 min before mounting or placed directly in lacto-phenol cotton blue mounting medium and covered with a glass cover slip. Slides were prepared from the colony center area, the middle colony area and the periphery, in order to see mycelium of different ages. When viewed at 400× magnification, the bits of material were composed of branched hyphae 1–1.5 µm diam and often fragmented. No structures identifiable as spores were encountered. The material was searched for phialides and vesicles, and these structures were also not found. Sterile structures suggestive of sclerotia or primordia of ascomata were white instead of brown as expected in mature colonies which were not zonate as described by Sappa (1955).
Herbarium specimen Herb. IMI 139271 designated as lectotype of Aspergillus peyronelii by Samson and Gams (1985). The specimen itself was a three-point inoculated on PDA agar and derived from culture CBS 122.58
A piece of clear tape ca. 4 × 20 mm was touched to the colony surface and mounted in lactic acid fuchsin dye. The entire area of the tape was scanned. There were no conidia, phialides and vesicles. In conclusion, the lectotype selected by Samson and Gams (1985) bears no resemblance to the species described by Sappa (1955).
Notes 2 — examination of authentic material of A. peyronelii (living cultures): We examined the cultures derived from the same strain which is considered to be an authentic Sappa’s strain (no designation in protologue) and received independently from several culture collections: CBS 122.58, IMI 139271 and NRRL 4754.
The culture CBS 122.58 received as lyophilisate was grown on MEA. The culture grew rapidly at 25 as well as at 37 °C and produced first only abundant, white aerial mycelium with white masses of sterile mycelium suggestive of primordia of sclerotia or ascomata when examined by stereo microscope. The structures suggestive of A. nidulans by morphology were observed after several weeks of incubation. These structures included masses of Hülle cells, in some subcultures also fertile ascomata after nearly 2 months, covered by Hülle cells and with red-brown ascospores with two equatorial ridges and also few biseriate conidiophores with brownish stipes producing globose, slightly roughened conidia, 2.5–3 µm in diam. Our observation was also supported by the results of DNA sequencing. Calmodulin sequence showed 100 % identity in BLAST searches with the ex-type strain of A. delacroxii (former Emericella echinulata). The living culture of CBS 122.58 was sent on our request from CBS collection to exclude contamination during manipulation with the lyophilisate. The results of our examination including sequence data were identical.
The culture IMI 139271 was received as living culture after problems with its reviving (personal communication with Esther Madden, IMI collection). The culture was white, sterile and slow-growing. Restricted growth was also observed at 37 °C. Sterile, white masses of mycelium observed after 2–3 weeks of cultivation on MEA and PCA were observed and resembled sclerotia or primordia of ascomata but they remained sterile. No structures identifiable as spores or Hülle cells were found even after prolonged incubation. The DNA sequences of this isolate (Table 1) were closely related to the ex-type of A. arenarius. The close relatedness of A. peyronelii to A. arenarius is apparent from the original description and was also mentioned by Raper and Fennell (1965) without specification of clear distinguishing features. The isolate NRRL 4899 identified here as A. arenarioides was suspected by Raper and Fennell (1965) that it might represent an additional isolate of A. peyronelii. All this information and data convinced us that isolate IMI 139271 represents a real Sappa′s culture and isolate CBS 122.58 was probably subsequently contaminated and no longer represents the authentic material examined by Sappa.
The culture NRRL 4754 was received as lyophilisate and was again characterized by prevalent white aerial mycelium and sterile microscopic nature. This isolate was sequenced in the past by S.W. Peterson, but the sequences were not used in the final phylogenetic study (Peterson 2008). In BLAST searches of these sequences (EF669715, EF669678, EF669699, EF669673), there are no hits with any Aspergillus species, the closest matches in GenBank are to pleosporalean species. Consequently, other ITS rDNA sequences deposited in GenBank under name A. peyronelii represent misidentifications (AB704782, KF031024, JX868795, HE805122) probably due to similarity of their sequences with that of NRRL 4754.
Our trials for re-sequencing of isolate NRRL 4754 in this study were unsuccessful at the beginning due to repeated low quality of resulting sequences (noisy signal or double sequence data). Previous and our sequencing results led us to the idea that NRRL 4754 could be a mixed culture and we tried to clean the culture. As the monosporic isolation techniques could not be used due to sterility of the culture, we transferred under the control of the microscope the pieces of mycelium and individual hyphae by sterilized glass needle onto a new agar plate. Several subcultures were subjected to DNA sequencing with resulting sequences identical in several cases to those of IMI 139271. These subcultures derived from NRRL 4754 were also morphologically identical to IMI 139271. The sequences of hypothetical pleosporalean species were not obtained again. The cleaned culture was deposited into the CCF collection of fungi as CCF 4942.
Notes 3 — nomenclatural and taxonomic notes: Original material represented by illustration is extant for A. peyronelii, however, the species name was lectotypified (Samson and Gams 1985) by a specimen Herb. IMI 139271 (Fig. 10) dried from the living culture CBS 122.58 (the culture was not mentioned in the protologue and consequently is not a part of original material, but is considered to be authentic). The same herbarium specimen was later cited as neotype by Pitt and Samson (1993, 2000). We designated above a lectotype (iconotype) to supersede types designated by Samson and Gams (1985) and Pitt and Samson (1993, 2000); Art. 9.19 (McNeill et al. 2012).
Based on our findings, we have doubts about the nature of specimen Herb. IMI 139271. It is sterile and derived from the culture CBS 122.58 which is currently represented by a contaminant. It was not possible to verify if the contamination occurred before 1969 (IMI specimen creation) or more recently because we did not try to isolate DNA from it. We deposited new herbarium specimens derived from culture IMI 139271 (PRM 933831) and cleaned culture NRRL 4754 (PRM 933830). The sequence data from four loci deposited in this study (Table 1) can serve for unambiguous identification of A. peyronelii.
Dichotomous key to species from Aspergillus sect. Petersonii
-
1a.
Growth on CYA and MEA at 37 °C after 14 days……………A. peyronelii
-
1b.
No growth on CYA and MEA at 37 °C after 14 days……………2
-
2a.
Sclerotia abundant on CYA and MEA after 14 days……………3
-
2b.
Sclerotia absent on CYA and MEA after 14 days……………A. asclerogenus
-
3a.
Vesicles on MEA (6-)7-14(-17) µm diam, no growth on MEA at 35 °C after 14 days……………A. petersonii
-
3b.
Vesicles on MEA (5-)6-9(-12) µm diam, restricted growth on MEA at 35 °C after 14 days (4–9 mm)……………A. arenarioides
Aspergillus sect. Robusti Ž.Jurjević & Hubka, sect. nov. [MycoBank MB#814443] —TYPE: Aspergillus robustus M.Chr. & Raper, Mycologia 70: 200. 1978.
Description: Section Robusti is currently a single-species section created for A. robustus. This section is most closely related to sect. Circumdati and shares with it yellow sporulation, production of biseriate conidiophores with large and predominantly globose vesicles, and production of sclerotia. Aspergillus robustus differs from all members of sect. Circumdati by phototropic conidiophores, lower temperature optimum and maximum (is unable to grow on CYA at 30 °C according to Visagie et al. (2014b)). The sclerotia of A. robustus are finally black in contrast to white, yellow or brown sclerotia in sect. Circumdati. Completely different exometabolite spectrum also supports our proposal of a separate section for A. robustus (Frisvad et al. 2004).
The description of A. robustus with illustrations was published by Christensen and Raper (1978) and recently by Visagie et al. (2014b).
Aspergillus sect. Tanneri Ž.Jurjević & Hubka, sect. nov. [MycoBank MB#814444] —TYPE: Aspergillus tanneri Kwon-Chung, Sugui & S.W.Peterson, J. Clin. Microbiol. 50: 3312. 2012.
Description: Section Tanneri is currently a single-species section created for A. tanneri. This section is most closely related to sects. Robusti and Circumdati. Aspergillus tanneri is readily distinguished from members of both mentioned sections by its small pyriform vesicles, lack of sclerotia, very poor sporulation, uncolored reverse of colonies without production of soluble pigments and better growth at 37 °C than at 25 °C. The species is pathogenic to humans, a condition very uncommon in sect. Circumdati and unknown in A. robustus.
References
Belofsky GN, Gloer KB, Gloer JB, Wicklow DT, Dowd PF (1998) New p-terphenyl and polyketide metabolites from the sclerotia of Penicillium raistrickii. J Nat Prod 61:1115–1119
Christensen M, Raper KB (1978) Aspergillus robustus, a new species in the A. ochraceus group. Mycologia 70:200–205
Frisvad JC, Frank JM, Houbraken J, Kuijpers AFA, Samson RA (2004) New ochratoxin A producing species of Aspergillus section Circumdati. Stud Mycol 50:23–43
Hall T (2004) Bioedit 7.0.0. North Carolina State University, Raleigh
Houbraken J, Samson RA (2011) Phylogeny of Penicillium and the segregation of Trichocomaceae into three families. Stud Mycol 70:1–51
Houbraken J, de Vries RP, Samson RA (2014) Modern taxonomy of biotechnologically important Aspergillus and Penicillium species. Adv Appl Microbiol 86:199–249
Hubka V, Kolařík M (2012) β-tubulin paralogue tubC is frequently misidentified as the benA gene in Aspergillus section Nigri taxonomy: primer specificity testing and taxonomic consequences. Persoonia 29:1–10
Hubka V, Kolařík M, Kubátová A, Peterson SW (2013) Taxonomical revision of Eurotium and transfer of species to Aspergillus. Mycologia 105:912–937
Hubka V, Lyskova P, Frisvad JC, Peterson SW, Skorepova M, Kolarik M (2014) Aspergillus pragensis sp. nov. discovered during molecular re-identification of clinical isolates belonging to Aspergillus section Candidi. Med Mycol 52:565–576
Hubka V, Nissen C, Jensen R, Arendrup M, Cmokova A, Kubatova A, Skorepova M (2015a) Discovery of a sexual stage in Trichophyton onychocola, a presumed geophilic dermatophyte isolated from toenails of patients with a history of T. rubrum onychomycosis. Med Mycol. doi:10.1093/mmy/myv1044
Hubka V, Novakova A, Kolarik M, Jurjevic Z, Peterson SW (2015b) Revision of Aspergillus section Flavipedes: seven new species and proposal of section Jani sect. nov. Mycologia 107:169–208
Jurjevic Z, Peterson SW, Horn BW (2012) Aspergillus section Versicolores: nine new species and multilocus DNA sequence based phylogeny. IMA Fungus 3:59–79
Katoh K, Kuma K, Toh H, Miyata T (2005) MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucleic Acids Res 33:511–518
Kozakiewicz Z (1989) Aspergillus species on stored products. Mycol Pap 161:1–188
McNeill J, Barrie FR, Buck WR, Demoulin V, Greuter W, Hawksworth DL, Herendeen PS, Knapp S, Marhold K, Prado J (2012) International Code of Nomenclature for algae, fungi, and plants (Melbourne Code) adopted by the Eighteenth International Botanical Congress, Melbourne, Australia, July 2011. Koeltz Scientific Books, Königstein
Oh H, Gloer J, Wicklow D, Dowd P (1998) Arenarins A-C: new cytotoxic fungal metabolites from the sclerotia of Aspergillus arenarius. J Nat Prod 61:702–705
Peterson SW (2000) Phylogenetic relationships in Aspergillus based on rDNA sequence analysis. In: Samson RA, Pitt JI (eds) Integration of modern taxonomic methods for Penicillium and Aspergillus classification. Harwood Academic Publishers, Amsterdam, pp 163–178
Peterson SW (2008) Phylogenetic analysis of Aspergillus species using DNA sequences from four loci. Mycologia 100:205–226
Peterson SW, Jurjević Ž (2013) Talaromyces columbinus sp. nov., and genealogical concordance analysis in Talaromyces clade 2a. PLoS ONE 8:e78084
Peterson SW, Varga J, Frisvad JC, Samson RA (2008) Phylogeny and subgeneric taxonomy of Aspergillus. In: Varga J, Samson RA (eds) Aspergillus in the genomic era. Wageningen Academic Publishers, Wageningen, pp 33–56
Pitt JI (1980) The genus Penicillium and its teleomorphic states Eupenicillium and Talaromyces. Academic Press, London
Pitt JI, Samson RA (1993) Species names in current use in the Trichocomaceae (Fungi, Eurotiales). In: Greuter W (ed) NCU-2: Names in Current Use in the Families Trichocomaceae, Cladoniaceae, Pinaceae, and Lemnaceae. Koeltz Scientific Books, Königstein, pp 13–57
Pitt JI, Samson RA (2000) Types of Aspergillus and Penicillium and their teleomorphs in current use. In: Samson RA, Pitt JI (eds) Integration of modern taxonomic methods for Penicillium and Aspergillus classification. Harwood Academic Publishers, Amsterdam, pp 51–79
Rahbæk L, Frisvad JC, Christophersen C (2000) An amendment of Aspergillus section Candidi based on chemotaxonomical evidence. Phytochemistry 53:581–586
Raper KB, Fennell DI (1965) The genus Aspergillus. Williams & Wilkins Co., Baltimore
Ronquist F, Huelsenbeck JP (2003) MRBAYES 3: bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Samson RA (1979) A compilation of the Aspergilli described since 1965. Stud Mycol 18:1–40
Samson RA, Gams W (1985) Typification of the species of Aspergillus and associated teleomorphs. In: Samson RA, Gams W (eds) Advances in Penicillium and Aspergillus Systematics. Plenum Press, New York, pp 31–54
Samson RA, Mouchacca J (1975) Additional notes on species of Aspergillus, Eurotium and Emericella from Egyptian desert soil. Antonie Leeuwenhoek 41:343–351
Samson RA, Houbraken J, Thrane U, Frisvad JC, Andersen B (2010) Food and indoor fungi. CBS KNAW Biodiversity Center, Utrecht
Samson RA, Peterson SW, Frisvad JC, Varga J (2011) New species in Aspergillus section Terrei. Stud Mycol 69:39–55
Samson RA, Visagie CM, Houbraken J, Hong SB, Hubka V, Klaassen CHV, Perrone G, Seifert KA, Tanney JB, Varga J, Yaguchi T, Frisvad JC (2014) Phylogeny, identification and nomenclature of the genus Aspergillus. Stud Mycol 78:141–173
Sappa F (1955) Nuove species di Aspergillus dei terreni della savanna spinosa Somala. Allionia 2:247–257
Sugui JA, Peterson SW, Clark LP, Nardone G, Folio L, Riedlinger G, Zerbe CS, Shea Y, Henderson CM, Zelazny AM (2012) Aspergillus tanneri sp. nov., a new pathogen that causes invasive disease refractory to antifungal therapy. J Clin Microbiol 50:3309–3317
Varga J, Tóth B, Rigó K, Téren J, Hoekstra R, Kozakiewicz Z (2000) Phylogenetic analysis of Aspergillus section Circumdati based on sequences of the internal transcribed spacer regions and the 5.8 S rRNA gene. Fungal Genet Biol 30:71–80
Varga J, Frisvad JC, Kocsubé S, Brankovics B, Tóth B, Szigeti G, Samson RA (2011a) New and revisited species in Aspergillus section Nigri. Stud Mycol 69:1–17
Varga J, Frisvad JC, Samson RA (2011b) Two new aflatoxin producing species, and an overview of Aspergillus section Flavi. Stud Mycol 69:57–80
Visagie CM, Hirooka Y, Tanney JB, Whitfield E, Mwange K, Meijer M, Amend AS, Seifert KA, Samson RA (2014a) Aspergillus, Penicillium and Talaromyces isolated from house dust samples collected around the world. Stud Mycol 78:63–139
Visagie CM, Varga J, Houbraken J, Meijer M, Kocsube S, Yilmaz N, Fotedar R, Seifert KA, Frisvad JC, Samson RA (2014b) Ochratoxin production and taxonomy of the yellow aspergilli (Aspergillus section Circumdati). Stud Mycol 78:1–61
Acknowledgments
This research was supported by the Ministry of Education, Youth and Sports of the Czech Republic (244-260194) and by the project “BIOCEV—Biotechnology and Biomedicine Centre of the Academy of Sciences and Charles University” (CZ.1.05/1.1.00/02.0109) from the European Regional Development Fund, and also by EMSL Analytical, Inc., USA. Molecular genetic analyses were supported by the projects of Charles University Grant Agency (GAUK 1130214 and 607812). We would like to thank to Prof Karol Marhold for consultation on nomenclatural issues, Dr Miroslav Hyliš for assistance with scanning electron microscopy, Dr. Milada Chudíčková for excellent technical assistance and Veronica Jurjević for assistance with editing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Pavel Lizoň.
Rights and permissions
About this article
Cite this article
Jurjević, Ž., Kubátová, A., Kolařík, M. et al. Taxonomy of Aspergillus section Petersonii sect. nov. encompassing indoor and soil-borne species with predominant tropical distribution. Plant Syst Evol 301, 2441–2462 (2015). https://doi.org/10.1007/s00606-015-1248-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-015-1248-4