Abstract
Taxus mairei is an endangered plant with high medicinal value in China. In the present study, inter-simple sequence repeat markers and high-performance liquid chromatography (HPLC) were used to investigate genetic diversity, taxol content, and the inter-relationship of these two variables in 11 populations. Genetic diversity was high at the species level [percentage of polymorphic bands (PPB) = 91.73 %; Nei’s gene diversity (h) = 0.2428; Shannon’s information index (I) = 0.3771], but relatively lower at the population level (PPB = 54.14–67.67 %; h = 0.1809–0.2121; I = 0.2721–0.3211). Hierarchical analysis of molecular variance (AMOVA) revealed moderate genetic differentiation among populations (Ø ST = 16.13 %), in line with the low gene differentiation coefficient (G ST = 0.1697) and relatively strong gene flow (N m = 2.4480). Both UPGMA and principal coordinates analysis supported the clustering of all 11 populations into three groups. A Mantel test indicated a significant correlation between geographic and genetic distances (r = 0.405; P < 0.005). Taxol content varied significantly among populations, ranging from 0.0069 to 0.0127 % based on the HPLC analysis. The taxol content was not significantly associated with genetic diversity, but was significantly, negatively associated with population latitude (r = −0.620; P < 0.005). This implies that local temperature may significantly affect the taxol content, although the role of heredity cannot be neglected. Our findings provided important references for resource protection and sustainable management of this valuable plant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Taxus mairei is a Tertiary relict tree within the family Taxaceae, and mainly occurs in southern China, Nepal, India and Vietnam (Poudel et al. 2012; Zhang et al. 2012). It is an evergreen plant with ornamental, medicinal and timber uses (Editorial Committee of the Flora of China 1978). This plant (especially its bark) has long been exploited as a source of taxol, a compound with anti-cancer activity. As a result, it has been seriously over-harvested. Its consequent scarcity, combined with a naturally low fertility and slow growth rate, has caused it to become very vulnerable and has led to its being listed as a rare and endangered plant in China (Yu 1999).
Following the official approval of taxol as a novel drug for curing ovarian cancer in 1992, wild Taxus resources have become insufficient to meet the market and clinical demands. Despite the availability of a chemical synthesis method, human-planted trees are still the primary source of taxol due to the complexity of the procedure and the resultant low yields (Shen and Wu 1997; Yang et al. 2008). Screening for strains with good genetic stability and high taxol content would contribute to the sustainable utilization of Taxus plants.
Previous studies demonstrated that T. mairei has high taxol content and is the Taxus species with the highest growth rate and broadest distribution range. These characteristics offer the advantage of excellent provenance selection and make it well-suited to large-scale cultivation (Su et al. 2000; Zheng 2003). A strategy for improved development and utilization of this plant, based on the premise of reasonable protection of germplasm resources, is urgently needed. Key components in developing such a strategy include elucidating genetic diversity and taxol content differences among germplasm resources, analyzing the relationship between genetic variation and taxol content, and evaluating environmental influences on genetic variation and taxol content (Milligan et al. 1994).
Previous population genetic studies of T. mairei have involved only a small sample size or a limited number of molecular markers (Ru et al. 2008; Jiang et al. 2009; Zhang et al. 2009a; b; Li et al. 2011a, b), and studies of its taxol content have mainly focused on the influence of different habitats, stalk position and age (Ke et al. 2009; Li et al. 2011a, b; Yu et al. 2012; Zhang and Du 2012). Chen et al. (1999) investigated relationships among molecular characteristics, morphological features and habitats of high taxol-content plants by using random amplified polymorphic DNA (RAPD) markers and high-performance liquid chromatography (HPLC); however, no detailed effort was made to analyze the correlation between genetic variation and taxol content.
In this study, 11 T. mairei populations were sampled across its geographic range in China. Our major objectives were to: (1) evaluate the level and partitioning of genetic variability within/among populations using inter-simple sequence repeat (ISSR) markers; (2) determine the taxol content in each population and analyze the distribution pattern of content variability among populations by high-performance liquid chromatography (HPLC); (3) analyze the relationship between taxol content and genetic diversity, and elucidate the influence of environment on taxol content; and (4) eventually provide references for the effective conservation and sustainable utilization of this valuable plant.
Materials and methods
Plant materials
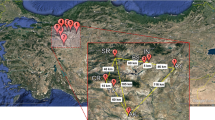
A total of 219 individuals from 11 natural populations were sampled across the major production areas of T. mairei in China, including Zhejiang, Jiangxi, Yunnan, Sichuan and Hunan provinces. Fresh leaves were collected, dried in ziplock bags with silica gel, transported back to the laboratory and kept in a −80 °C freezer until the ISSR analysis. For taxol content determination, three individuals were randomly sampled from each population, and branches of approximately the same age and growth conditions were collected. Voucher specimens were deposited in the herbarium of the Laboratory of Biological Resources and Application Technology, College of Life Science and Technology, Tongji University, Shanghai, China (Table 1; Fig. 1).
Geographic distribution of the 11 sampled populations of Taxus mairei in China. For population abbreviations, see Table 1
DNA extraction and ISSR-PCR amplification
Total genomic DNA was extracted from dried leaves using a modified CTAB method (Doyle and Doyle 1987). The DNA was dissolved in 0.1× TE buffer (10 mM Tris–HCl and 1 mM EDTA, pH 8.0), and maintained at −20 °C for long-term storage or at 4 °C for immediate use.
ISSR primers used in this study were synthesized by Shanghai Sangon Biological Engineering Technology and Service Co. (Shanghai, China) according to the primer set published by the University of British Columbia (Vancouver, BC, Canada). One hundred ISSR primers were initially screened, and 13 primers which yielded strong, discernible bands were used for assaying all 219 samples (Table 2). PCRs were performed in 20-μL reaction volumes containing 2.0 mM MgCl2, 0.25 mM of each dNTP, 1.0 U Taq DNA polymerase (Takara, Dalian, China), 0.2 μM primer and 50 ng template DNA. Amplifications were performed in a Mastercycler Gradient PCR Machine (Eppendorf, Germany) using the following program: initial denaturation at 94 °C for 4 min; followed by 40 cycles of 94 °C for 45 s, an appropriate annealing temperature (see Table 2) for 45 s, and 72 °C for 1.5 min; and a final extension at 72 °C for 5 min. The amplified products were separated in 1.5 % agarose gels in 1× TAE buffer at 100 V for 1 h, stained with ethidium bromide and photographed under UV light using a UVP-GDS8000 Gel Documentation System (UVP, USA).
HPLC assays
Taxol was extracted by the methanol ultrasonic method (Liu 2008). Branches were placed in an oven at 45 °C for 24 h and then ground into powder. After sifting through five screens, 10 g of powder was mixed with 100 ml of 95 % methanol and subjected to ultrasonic extraction for 2 h. The extract was filtered, and 80 ml of 95 % methanol was added to the sediment. After ultrasonic extraction for 1 h, the extract was again filtered; 60 ml of 95 % methanol was added to the sediment, followed by ultrasonic extraction for 1 h and re-filtering. The three filtrates were combined and allowed to dry at 50 °C. The dried residue was dissolved in methanol ultrasonically and diluted to constant volume in a 10-ml volumetric flask. The diluted samples were filtered through a 0.45-μm filter membrane and used for the HPLC analysis.
HPLC was performed using an Agilent 1100 HPLC System equipped with an ultraviolet detector. Taxol extracts (20 μl) were directly injected onto a Hypersil ODS column (Agilent, Germany) of 5 μm particle size, 250 mm length and 4.6 mm diameter. A mobile phase of acetonitrile/ultra-pure water (48:52 v/v) at a flow rate of 1.2 ml min−1 was used. The absorption wavelength was 228 nm. Three repeats were made in measuring the taxol content of each sample.
Statistical analyses
Because ISSR markers are dominantly inherited, each band was assumed to represent the phenotype at a single biallelic locus (Williams et al. 1990). Only reproducible and discernible fragments ranging in size from 150 to 2,000 bp were included in the statistical analysis. To construct the binary data matrix, ISSR bands were scored as presence (1) or absence (0) characters.
POPGENE v1.32 (Yeh et al. 1997) was used to calculate various genetic diversity parameters, including the percentage of polymorphic bands (PPB), Shannon’s information index (I), Nei’s gene diversity (h), the gene differentiation coefficient (G ST) and gene flow (N m). Nei’s genetic distance (D) among populations were also computed using this program, and the resultant genetic distance matrix was then used to construct an UPGMA (Unweighted Pair Group Method with Arithmetic Mean) dendrogram. Grouping of populations was carried out by principal coordinates analysis (PCoA) using the software package GenAlEx v6.3 (Peakall and Smouse 2006). A Mantel test (Mantel 1967) between genetic and geographic distances was conducted using GenAlEx v6.3 to test for isolation-by-distance (IBD). This package was also employed to perform a hierarchical analysis of molecular variance (AMOVA; Excoffier et al. 1992), which statistically assessed the partitioning of genetic variability within/among populations.
HPLC data were analyzed using SPSS v17.0 (SPSS Inc., USA). A significance test of taxol content differences among populations was conducted by the single-factor analysis of variance (one-way ANOVA; P < 0.05). A UPGMA dendrogram was constructed and principal components analysis (PCA) was performed from the taxol content data. SPSS v17.0 was also employed to analyze possible associations of taxol content with latitude, longitude and genetic diversity. Taxol content and genetic distances were calculated and analyzed using the Mantel test.
Results
Genetic diversity
The 13 selected ISSR primers yielded 133 reproducible bands, of which 122 were polymorphic across the 219 T. mairei samples from 11 populations. At the population level, the percentage of polymorphic bands (PPB) varied between 54.14 and 67.67 %, with an average of 60.42 %; this estimate was substantially higher at the species level (PPB = 91.73 %) (Table 1). Nei’s gene diversity (h) ranged from 0.1809 to 0.2125 and Shannon’s information index (I) from 0.2721 to 0.3211 at the population level. Both parameters displayed a similar trend to PPB. As indicated by these three parameters, the highest levels of genetic diversity occurred in populations JIFX (PPB = 67.67 %; h = 0.2102; I = 0.3211) and JXJG (PPB = 64.66 %; h = 0.2121; I = 0.3211), while the lowest level in population SCMY (PPB = 54.14 %; h = 0.1809; I = 0.2721).
Genetic differentiation
The AMOVA analysis revealed a moderate level of genetic differentiation, with 16.13 % of total genetic variability residing among populations (Table 3). This pattern was further confirmed by the gene differentiation coefficient (G ST = 0.1697) and the substantial gene flow (N m = 2.4480) among populations.
Nei’s genetic distances ranged from 0.0254 (YNSM vs. YNLJ) to 0.0866 (JXFX vs. SCMY), with an average of 0.0584. The UPGMA cluster analysis clustered the 11 populations into three groups. Group I comprised the single population from Sichuan Province (SCMY). Group II included eight populations from Zhejiang, Jiangxi and Hunan provinces (JXFX, JXJG, ZJSA, ZJSC, ZJZJ, ZJSZ, HNZZ and HNYY), while Group III comprised the remaining two populations from Yunnan Province (YNLJ and YNSM) (Fig. 2a). The relationship implied by the UPGMA analysis was confirmed by the PCoA plot (Fig. 2b), in which the first two factors accounted for 29.88 % (axis 1) and 22.89 % (axis 2) of the total genetic variance, respectively.
A Mantel test revealed a significant correlation between geographic and genetic distances among populations (r = 0.405; P = 0.003; 999 permutations), indicating the role of geographic isolation in shaping the present population genetic structure of T. mairei.
Taxol content
The taxol content of T. mairei populations varied between 0.0069 and 0.0127 %. Population JXJG possessed the highest taxol content (0.0127 %), followed by populations YNSM (0.0122 %) and YNSM (0.0122 %). The lowest taxol contents were uncovered in populations SCMY (0.0076 %), YNLJ (0.0071 %) and ZJZJ (0.0069 %). A test of significance revealed significant differences in taxol content among populations (Table 1).
A UPGMA cluster analysis based on taxol content clustered the 11 populations into two groups. Group I consisted of populations sampled from Hunan (HNYY and HNZZ), Jiangxi (JXFX), Sichuan (SCMY), Zhejiang (ZJZJ, ZJSZ and ZJSA) and Yunnan (YNLJ) provinces, while Group II comprised the other three populations sampled from Jiangxi (JXJG), Yunnan (YNSM) and Zhejiang (ZJSC) provinces (Fig. 3a). This was further confirmed by the PCA plot (Fig. 3b).
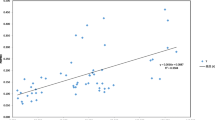
ISSR and taxol content analyses revealed that populations JXJG and YNSM were highly genetically diverse and possessed high taxol contents, while populations YNLJ and SCMY were characterized by low genetic diversity and low taxol contents. Although these results suggest a possible correlation between genetic diversity and taxol content, the SPSS analysis failed to reveal significant correlations between taxol contents and PPB (r = 0.36; P = 0.277), h (r = 0.329; P = 0.323) or I (r = 0.354; P = 0.286). Analysis of the taxol content versus latitude or longitude indicated that the taxol content was significantly negatively correlated to population latitude (r = −0.620; P = 0.042). Mantel test suggested no significant correlation between taxol content differences and genetic distance (r = 0.102; P = 0.275).
Discussion
A meta-analysis of genetic diversity revealed that gymnosperms have high genetic variability, with an average PPB estimate of 70.9 % (Hamrick and Godt 1990). In our study, we uncovered an even higher species-level genetic diversity (PPB = 91.73 %), in line with previous findings of Ru et al. (2008) (PPB = 91.79 %), Zhang et al. (2009a) (PPB = 98.4 %) and Zhang et al. (2009b) (PPB = 98.89 %).
The high genetic diversity of T. mairei can be attributed to four major factors. Firstly, paleogeological and paleobiological studies revealed that T. mairei is a relict species whose origin can date back to the Tertiary glacial period. Due to its long lifespan and overlapping generations, considerable genetic variability may have been accumulated and conserved under various selection pressures during the evolutionary process. Hence, high intraspecific genetic diversity is exhibited in this plant (Tan and Chen 2006). Secondly, T. mairei is the most widely distributed taxon within the genus Taxus. The diversity of germplasm resources coupled with environmental heterogeneity could contribute to the accumulation of genetic variability (Yang et al. 2009). Thirdly, T. mairei is an anemophilous plant and reproduces by seed under natural conditions, both characteristics being beneficial for maintaining genetic diversity. Besides, it is a late successional species. As suggested by Hamrick (1996), competitive pressure of such species is mainly intraspecific, which may promote the formation of intraspecific genetic variability (Xi 1986).
POPGENE analysis revealed moderate genetic differentiation among T. mairei populations (G ST = 0.1697); this estimate of G ST was close to the average values estimated for gymnosperm species (G ST = 0.18; Nybom and Bartish 2000), long-lived perennial herbaceous species (G ST = 0.19; Nybom 2004) and widely distributed outcrossing species (G ST = 0.170; Hamrick and Godt 1996). Meanwhile, the G ST estimate obtained in our study approximated the value previously reported for T. mairei using RAPD-based data (G ST = 0.181; Ru 2008), was slightly higher than the value for T. mairei using ISSR-based data (G ST = 0.1211; Zhang 2009a), but substantially lower than the value for its congener T. wallichiana using cpDNA PCR–RFLP-based data (G ST = 0.694; Gao et al. 2007).
Among-population genetic differentiation can be attributed to a variety of factors, e.g., genetic drift, breeding system, gene flow, habitat fragmentation and population isolation (Hogbin and Peakall 1999; Schaal et al. 1998; Slatkin 1987). And in our study, the moderate genetic differentiation among populations can be largely explained by gene flow, breeding system and habitat change. Typically, a homogenizing effect emerges at N m > 1, in which case population differentiation is prevented (Wright 1931). In our study, strong gene flows occurred among the 11 populations (N m = 2.4480), being sufficient to counteract genetic differentiation caused by genetic drift.
In general, long-distance gene flow, which occurs in plants with wind-dispersed pollen and animal-dispersed seeds, can counteract population differentiation across a wide geographical range (Loveless and Hamrick 1984). T. mairei is an anemophilous plant with extraordinarily high pollen dispersal ability. A steady pollen exchange occurs among populations. In addition, T. mairei seeds are enclosed in a clayey fleshy aril. At the seed maturity stage, the aril is red and has a sweet taste, attracting birds to eat the aril and spread the seeds. This process facilitates seed/gene flow among T. mairei populations (Deng et al. 2008).
As proposed by Mohapatra et al. (2009), one possible contributor to high within-population genetic differentiation coupled with low among-population genetic differentiation is the divergence from a common ancestral population. As a glacial relict species, it can be inferred that the extant populations of T. mairei are derived from formerly restricted ranges and may thus originate from the same source population(s). In addition, T. mairei has undergone habitat fragmentation in modern times; the effect of habitat fragmentation is still relatively limited, and has not resulted in substantially genetic differentiation among populations, as supported by Zhang et al. (2012).
Genetic distances between T. mairei populations were relatively small, but a significant correlation was found between geographic and genetic distances. UPGMA clustered the 11 populations into three groups, in line with their geographical distribution. Populations sampled from Zhejiang, Jiangxi and Hunan provinces (JXFX, JXJG, ZJSA, ZJSC, ZJZJ, ZJSZ, HNZZ and HNYY) formed one group; populations sampled from Yunnan Province (YNLJ and YNSM) formed a second group, and the third group comprised a single population from Sichuan Province (SCMY). Relatively large genetic distances were found between the third group and the former two, indicating the obvious occurrence of geographical distribution patterns among the populations. The observed patterns were similar to those of its congener T. wallichiana Zucc. We speculate that geographical barrier is among the major factors that contribute to the formation of the geographical distribution patterns of T. mairei. In contrast to the eastern regions, Yunnan Province is a relatively closed area surrounded by the Hengduan Mountains and the Himalayas. Meanwhile, the unique geographical features of the Sichuan Basin and the geographical barriers of the Daba Mountains separate Sichuan Province from the eastern region.
Taxol, a diterpenoid compound present in the bark, branches and leaves of Taxus plants, is a complex secondary metabolite that has been found to improve microtubule polymerization and stabilize polymerized microtubules (Horwitz et al. 1993). The accumulation of secondary metabolites is influenced by a combination of genetic and environmental factors. Compared with primary metabolites, secondary metabolites have a stronger correlation with and correspondence to the environment. Previous studies have suggested the significant impacts of temperature, humidity and soil fertility on taxol biosynthesis (Yang et al. 2010; Su et al. 2012). Consistent with these reports, our study uncovered obvious taxol content differences among populations from different locations.
Although Mantel test revealed no significant association between taxol content and genetic variation, a significant, negative association was observed between taxol content and latitudes of populations. This result appeared to be supported by the UPGMA cluster analysis. Of the three basic geographic elements (i.e., latitude, altitude and longitude), latitude is supposed to have the greatest influence on temperature, with an average contribution of 44.4 % (Fang 1992). We thus deduce that the observed differences in taxol content among T. mairei populations are mainly due to temperature variations arising from latitude differences.
Considering the high intraspecific genetic diversity, loss of genetic diversity should not be a major contributor to this plant’s endangered status. Other factors, such as anthropogenic destruction of natural habitats, poor seed production, low seed survival rates, poor natural population regeneration capacity and over-harvesting, may be responsible for its decline.
A combination of in situ and ex situ conservation approaches is commonly employed to protect endangered plants. Based on our findings, populations with high genetic diversity and taxol content, such as populations JXJG and ZJSC should be given a priority for in situ conservation. Meanwhile, samples from different populations should be collected for ex situ conservation. In particular, populations that are relatively genetically distant, such as populations from Sichuan and Yunnan provinces, can be used as cross parents so as to strengthen gene flow and maintain a maximum of genetic variability. By comparatively analyzing genetic diversity and taxol content, our results provide a theoretical foundation for the resource protection, utilization, cultivation and breeding of this valuable plant.
References
Chen YH, Bai SM, Cheng KD, Zhang S, Li JX (1999) RAPD analysis on high-taxane-contained accessions of Taxus chinensis var. mairei. Acta Bot Sin 41:829–832
Deng QS, Zhu QQ, Lu CH (2008) Natural regeneration of Taxus chinensis var. mairei and its seed dispersal by frugivorous birds. Chin J Ecol 27:712–717
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Photochem Bull 19:11–15
Editorial Committee of the Flora of China (1978) Flora of China, vol 7. Science Press, Beijing
Excoffier L, Smouse PE, Quattro JM (1992) Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics 131:479–491
Fang JY (1992) Study on the geographic elements affecting temperature distribution in China. Acta Ecol Sin 12:97–104
Gao LM, Möller M, Zhang XM, Hollingsworth ML, Liu J, Mill RR, Gibby M, Li DZ (2007) High variation and strong phylogeographic pattern among cpDNA haplotypes in Taxus wallichiana (Taxaceae) in China and North Vietnam. Mol Ecol 16:4684–4698
Hamrick JL, Godt MJW (1990) Allozyme diversity in plant species. In: Brown AHD, Clegg MT, Kahlar AL, Weir BS (eds) Plant population genetics, breeding, and genetic resources. Sinauer Associates, Inc., Sunderland, pp 43–63
Hamrick JL, Godt MJW (1996) Effect of life history traits on genetic diversity in plant species. Philos Trans R Soc London B Biol Sci 351:1291–1298
Hogbin PM, Peakall R (1999) Evaluation of the contribution of genetic research to the management of the endangered plant Zieria prostrata. Conserv Biol 13:514–522
Horwitz SB, Cohen D, Rao S, Ringel I, Shen HJ, Yang CP (1993) Taxol: mechanism of action and resistance. J Natl Cancer Inst Monogr 15:55–61
Jiang JM, Shen YF, Sun YM (2009) AFLP analysis of genetic diversity of Taxus chinensis var. mairei. Chin Wild Plant Resour 28:37–40
Ke CT, Tong C, Wang YZ, Huang JF, Ni JZ, Yang HY (2009) Taxol and 10-DAB contents of different provenance Taxus chinensis var. marei and related affecting factors. Chin J Ecol 28:231–236
Li J, Liu Y, Shi MH, Tang Q, Wu ZN, Dou GF, Meng ZY (2011a) Determination of paclitaxel content in different parts of Taxus wallichiana var. mairei by HPLC internal standard method. Mil Med Sci 35:624–626
Li NW, He SA, Shu XC, Wang Q, Xia B, Peng F (2011b) Genetic diversity and structure analyses of wild and ex-situ conservation populations of Taxus chinensis var. mairei based on ISSR marker. J Plant Resour Environ 20:25–30
Liu WY (2008) Pharmaceutical analysis. People’s Medical Publishing House, Beijing
Loveless MD, Hamrick JL (1984) Ecological determinants of genetic structure of plant populations. Annu Rev Ecol Syst 15:65–95
Mantel N (1967) The detection of disease clustering and a generalized regression approach. Cancer Res 27:209–220
Milligan BG, Leebens MJ, Strand AE (1994) Conservation genetics: beyond the maintenance of marker diversity. Mol Ecol 3:423–435
Mohapatra KP, Sharma RN, Mohapatra T (2009) Genetic analysis and conservation of endangered medicinal tree species Taxus wallichiana in the Himalayan region. New For 37:109–121
Nybom H (2004) Comparison of different nuclear DNA markers for estimating intraspecific genetic diversity in plants. Mol Ecol 13:1143–1155
Nybom H, Bartish IV (2000) Effect of life history traits and sampling strategies on genetic diversity estimates obtained with RAPD markers in plants. Perspect Plant Ecol Evol Syst 3:93–114
Peakall R, Smouse PE (2006) GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes 6:288–295
Poudel RC, Möller M, Gao LM, Ahrends A, Baral SR, Liu J, Thomas P, Li DZ (2012) Using morphological, molecular and climatic data to delimitate yews along the Hindu Kush-Himalaya and adjacent regions. PLoS ONE 7:e46873
Ru WM, Qin YY, Zhang GP, Zhang JT (2008) Genetic diversity of rare and endangered plant Taxus chinensis var. mairei. Bull Bot Res 28:698–704
Schaal BA, Hayworth DA, Olsen KM, Rauscher JT, Smith WA (1998) Phylogeographic studies in plants: problems and prospects. Mol Ecol 7:465–474
Shen ZW, Wu LF (1997) Advances in taxol research. Prog Chem 9:1–13
Slatkin M (1987) Gene flow and the geographic structure of natural populations. Sci 236:787–792
Su YJ, Wang T, Li XY, Fan GK, Ke YY, Zhu JM, Liao WB (2000) Analysis on the amounts of Taxol in different location of Taxus chinensis Var. mairei. Nat Prod Res Dev 13:19–20
Su JR, Zang CF, Liu WD, Li SF, Zhang ZJ (2012) Effect of light quality on growth and taxanes contents of Taxus yunnanensis. For Res 25:419–424
Tan LP, Chen ZF (2006) Taxus resources in China. J NW For Univ 21:113–117
Williams JGK, Kubelik AR, Livak KJ, Rafalski JA, Tingey SV (1990) DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res 18:6531
Wright S (1931) Evolution in Mendelian population. Genetics 16:91–159
Xi YZ (1986) Studies on pollen morphology of Taxaceae of China. Acta Phytotaxon Sin 24:249
Yang FJ, Pang HH, Zhang XK, Sun JY, Zu YG (2008) Quantitative changes of anti-cancer active components in Taxus chinensis var. mairei branches and leaves. Chin J Appl Ecol 4:911–914
Yang YL, Song XD, Dong JX, Liu GF, Li HY (2009) Resources and distribution of Taxus in the world. For Eng 25:5–10
Yang FJ, Pang HH, Zu YG, Zhang XK, Gao YX (2010) Relationships between the growth and Taxol content of Taxus chinensis var. mairei and environment factors. Bull Bot Res 30:742–746
Yeh FC, Yang R-C, Boyle TBJ, Ye Z-H, Mao JX (1997) POPGENE, the user-friendly shareware for population genetic analysis. Molecular Biology and Biotechnology Centre, University of Alberta, Canada
Yu YF (1999) The landmark of wild plant conservation in China. Plant 5:3
Yu SS, Sun QW, Zhang XP, Tian SN, Bo PL (2012) Content and distribution of active components in cultivated and wild Taxus chinensis var. mairei plants. Chin J Appl Ecol 23:2641–2647
Zhang XY, Du YT (2012) Paclitaxel distribution in entire plant of Taxus chinensis var. mairei by HPLC. Biomass Chem Eng 46:25–29
Zhang R, Zhou ZC, Jin GQ, Luo WJ (2009a) Genetic diversity and genetic differentiation of Taxus wallichiana var. mairei provenances. Sci Sil Sin 45:50–56
Zhang XM, Gao LM, Michael M, Li DZ (2009b) Molecular evidence for fragmentation among populations of Taxus wallichiana var. mairei, a highly endangered conifer in China. Can J For Res 39:755–764
Zhang XM, Li DZ, Gao LM (2012) Phylogeographical study on Taxus wallichiana var. mairei (Lemée & Léveillé) L.K.Fu & Nan Li. Acta Bot. Boreal.-Occident. Sin. 32:1983–1989
Zheng D-Y (2003) Study on the taxol content in different parts of Taxus growing in China. J Fujian Coll For 23:160–163
Acknowledgments
This study was supported by the Program of Lishui Administration of Science and Technology, Zhejiang Province, China (No. 2011JYZB03).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xi, XJ., Guo, J., Zhu, YG. et al. Genetic diversity and taxol content variation in the Chinese yew Taxus mairei . Plant Syst Evol 300, 2191–2198 (2014). https://doi.org/10.1007/s00606-014-1040-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-014-1040-x