Abstract
Available resources could influence the trade-offs among different reproductive components in plants. Here, we created three nutrient levels to test the nutrient effects on trade-offs among sexual reproduction, clonal propagation and vegetative growth in a monoecious clonal herb Sagittaria pygmaea. The results of this study showed that the plant exhibited different trade-off patterns among different nutrient levels. When the nutrient level was low, there were weak trade-offs between sexual reproduction and vegetative growth and between clonal propagation and vegetative growth; when the nutrient level was moderate, we found a strong trade-off between sexual reproduction and clonal propagation; but when the nutrient level was high, we found no trade-offs among these three different reproductive components. These results indicated that the plant could adjust its trade-off patterns to fit the nutrient variation and suggested that trade-offs are unlikely to constrain the evolution of reproductive strategy in this species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The trade-offs among different structures or functions are a central concept of life history evolution (Stearns 1989; Roff 1992; Cheplick 1995). In general in plants, the total resources are limited; increasing resources to one function may lead to a decrease to the others (Bell and Koufopanou 1986; Stearns 1992; Sullivan 1994). For example, increasing sexual reproduction may reduce asexual reproduction (Bazzaz et al. 1987; Westley 1993; Suzuki 2001); increasing current reproduction may also be manifest as a decrease in future survival and reproduction (Reekie and Bazzaz 1992; Cheplick 1995).
The trade-offs among components of reproduction in plants can be influenced by available resources and should reflect an alternative component of adaptation to the environment (Ronsheim and Bever 2000; van Kleunen et al. 2003). Trade-offs among different structures or functions will be more evident when available resources are limited and the trade-offs may not be critical when the available resource level is higher (Lambers and Poorter 1992). Because the trade-offs may vary with different resource levels (Venable 1992; Cheplick 1995), experimental investigation of life history trade-offs necessitates the use of multiple resource levels whenever possible. However, among the published literatures, most were conducted within the same resource level (Caswell 1985; Cain et al. 1995), only few studies investigated the trade-offs among different resource levels (Venable 1992; Cheplick 1995).
The trade-offs under the low nutrient level could become weaker when the sexual structures are photosynthetic and vegetative structures are resource sinks (Bazzaz 1997; Cain et al. 1995; Saikkonen et al. 1998; Mendez 1999). Temporal separation between vegetative growth, sexual reproduction and clonal propagation could also reduce the trade-offs (Bazzaz 1997; Gardner and Mangel 1999). These make the experimental manipulation more difficult and the results more mixed, but they can be alleviated by hand-pollination. Hand pollination increased investment in fruits whereas photosynthesis likely contributes little to construction and maintenance costs of fruits (Thompson and Eckert 2004).
In this study we manipulated sexual reproductive investment through two pollination treatments (0 and 100% flowers pollinated/individual) and three nutrient levels to investigate trade-offs among sexual reproduction, clonal propagation and vegetative growth in a monoecious clonal herb Sagittaria pygmaea Miq. Field observation indicated that the growth of this species in natural habitats appears strongly influenced by nutrients. It was expected that the trade-offs would be more evident when available resources were lowest but in a higher resource level the competing functions may no longer be critical.
Materials and methods
Study species
Sagittaria pygmaea (Alismataceae) is an emergent or submersed aquatic herb in East Asia. It occupies shallow water along marshes, ponds, stream banks and rice fields. The species is monoecious and is smaller than any other species in the genus Sagittaria. S. pygmaea is 7–15 cm high and has 3–5 sequential inflorescences; each inflorescence typically has only 0–2 female and 3–8 male flowers (Chen 1989). The species possesses the capability of sexual reproduction through seeds and clonal reproduction via corms.
Experimental design
In July 2006, we obtained 192 corms of S. pygmaea from a wild rice field in Zhijiang (30°20′N/111°35E), Hubei province, China and stored them in the dark at 5°C. We cultivated them in a common garden in Zhijiang in March 2007. Each corm was planted in a randomized array in plug trays and developing individuals were transplanted 1 month later in a 10-cm pot 0.5 m away from each other in a 4 × 8 pattern. All the plants were bagged using fine bridal veil at the onset of flowering.
Half of the individuals were assigned to the following pollination treatment: bridal veil netting was left undisturbed thus flowers were unpollinated and failed to set seed. Another half of the individuals were assigned to the following pollination treatment: all flowers on each inflorescence were hand-pollinated. For each pollination treatment, we assigned three nutrient levels: (1) each individual received water without any fertilizer (low); (2) each individual received 20-20-20 (N–P–K) fertilizers at 1.5 g per plant every 2 weeks (medium); (3) each individual received 20-20-20 (N–P–K) fertilizers at 1.5 g per plant every week (high).
At the initial signs of senescence of the above ground parts, plants were harvested. The sexual structures (inflorescence stalks, peduncles and fruits), the vegetative structures (leaves), and clonal structures (corms) were sorted and dried for 2 days to a dry mass in desiccators at 50°C and weighed to 0.001 g.
Statistical analysis
We used two-way ANOVA to test the effects of pollination treatment and nutrient treatment on sexual, vegetative, and clonal components of the plant. We also used the two-way ANOVA to test the effects of pollination treatment and nutrient treatment on subcomponents of sexual structure (inflorescence stalks, peduncles and fruits). In addition, one-way ANOVA was used to compare the effect of pollination on sexual, vegetative, and clonal components and their subcomponents of the plants. All the analyses were performed using SAS/STAT (SAS Institute 1998).
Results
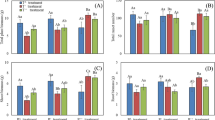
ANOVA revealed that the nutrient levels had significant effects on sexual, clonal and vegetative biomass and their subcomponents’ biomass (Table 1). Pollination treatments had different impacts on different reproductive structures among the three nutrient levels. Under the low nutrient level, pollinating all the flowers increased the sexual mass by 83% (Fig. 1; Table 2), including increased investment in the fruits and peduncles (fruits: F 1,63 = 16.34, P = 0.0002; peduncles: F 1, 63 = 24.37, P < 0.0001) but not in the inflorescence stalks (F 1, 63 = 1.73, P = 0.1928). Pollination also increased the clonal biomass by 53% but decreased the vegetative biomass by 19% (Fig. 1; Table 2). When the nutrient level was moderate, pollination increased the sexual mass by 58% (Fig. 1; Table 2), which involved increasing investment in fruits (fruits: F 1, 63 = 10.37, P = 0.0002) but not the peduncles and the inflorescence stalks (peduncles: F 1, 63 = 2.91, P = 0.0932; stalks: F 1, 63 = 0.52, P = 0.4742). But it led to a decrease by 37% of the clonal biomass and it had no impact on the vegetative growth (Fig. 1; Table 2). Under the high nutrient level, pollination increased the sexual mass by 64% which involved increasing investment in the fruits, peduncles and the inflorescence stalks (fruits: F 1, 63 = 23.23, P < 0.0001; peduncles: F 1, 63 = 3.81, P = 0.0556; stalks: F 1, 63 = 4.65, P = 0.0350). But it had no effect on the clonal biomass and the vegetative biomass (Fig. 1; Table 2).
On average, pollinating all flowers increased the sexual biomass by 0.0908 g and the clonal biomass by 0.2002 g but decreased the vegetative biomass by 0.1095 g in the low nutrient level. Thus, increasing every 1 g sexual biomass led to 1 g of investment to asexual biomass. However, in the middle nutrient level, pollinating all the flowers increased the sexual biomass by 0.1559 g but decreased the clonal biomass by 0.3496 g, which indicated that pollination from none to all caused 3 g of clonal biomass to be lost for every 2 g of investment in sexual reproduction.
Discussion
Our results showed that the trade-offs among different components of the plants exhibited different patterns at different nutrient levels.
At the low nutrient level, we found weak trade-offs between sexual reproduction and vegetative growth and between clonal propagation and vegetative growth. At this nutrient level, the total resources were limited, the plant could benefit from directing more resources to vegetative growth, which just needed few resources and could produce additional resources through photosynthesis of increased leaves. Thus, the plant exhibited trade-offs between vegetative growth and other two reproductive components. Under the same nutrient level, when we used pollination to increase sexual reproduction (sexual mass), we could not find any decrease in clonal propagation (corms mass). In general, sexual reproduction and clonal propagation often represent the future survival through the whole life history of this species while vegetative growth often represents the current survival. Under this nutrient level, the resources allocated to sexual reproduction and clonal propagation may both be limited since they are unable to afford those two reproductive components. The additional resources from the photosynthesis, which was caused by increased vegetative growth and peduncles, might have reinforced the two reproductive modes, leading to positive relations between sexual reproduction and clonal propagation. The plant could benefit more from increasing both sexual reproduction and clonal propagation to increase future reproduction because the current nutrition may not fit the survival of the species.
At the middle nutrient level, we found a strong trade-off between sexual and clonal propagation. Increased sexual biomass of 58% caused a 37% decrease in clonal propagation. The vegetative mass remained consistent when increasing the sexual reproduction. These indicated that the plant could allocate more resources to sexual and clonal reproduction but little to the vegetative growth. When the nutrient level was higher, the plant had more resources and could benefit more from allocating to reproduction than to vegetative growth. Because the resources were still within the scope of limitation, a trade-off between sexual reproduction and clonal propagation was apparent. The trade-off among components of reproduction in plants commonly has fitness significance for the individuals (Westley 1993). In general, sexual reproduction via seeds is better for founding new populations because of their small size and adaptation to dispersal (Eriksson 1997), while clonal propagation via bulbils, corms, or rhizomes is considered to be more successful in stable habitats (Philbrick and Les 1996). S. pygmaea could just produce one female flower to set seed on each inflorescence, indicating a limited seed production compared to some other species in Sagittaria. However, the species had strong clonal ability through corms and could spread quickly in the rice field when nutrient availability is better for growth (Chen 1989). S. pygmaea is one of the smallest species in Sagittaria and the total resources allocated to sexual reproduction may be limited and could not allow more flowers to set seed at the current nutrient level. However, the plant could adjust its resources to increase investment in clonal propagation when seed production is limited. These results suggest that the resource trade-off between sexual reproduction and clonal propagation might be an adaptive mechanism to ensure reproduction.
At the high nutrient level, increased sexual reproduction by pollination did not change the clonal and vegetative mass, which suggests that there were no trade-offs among sexual reproduction, clonal propagation and vegetative growth. This might due to high resource states which could enhance productivity of the plants, and this will cause more available resources and photosynthate to allocate to all functions and distinct allocation “decisions” among all competing functions, leading to no trade-offs among the three reproductive structures (Cheplick 1995).
The evolutionary significance of trade-offs in resource allocation depends on whether they affect the individual fitness to the environmental variation (Bazzaz 1997; Thompson and Eckert 2004). As stated above, the S. pygmaea could adjust trade-off patterns among different reproductive components to enhance the fitness to the nutrient availability, which suggests that trade-offs are unlikely to constrain the evolution of reproductive strategy in this species.
References
Bazzaz FA (1997) Allocation of resources in plants: state of the science and critical questions. In: Bazzaz FA, Grace J (eds) Plant resource allocation, Academic Press, San Diego, pp 1–37
Bazzaz FA, Chiariello NR, Coley PD, Pitelka LF (1987) Allocating resources to reproduction and defense. Bioscience 37:58–67
Bell G, Koufopanou V (1986) The cost of reproduction. Oxford Surv Evol Biol 3:83–131
Cain ML, Kahn B, Silander JA, Reynolds HL (1995) Genetic variability and tradeoffs among reproductive traits in white clover (Trifolium repens). Canad J Bot 73:505–511
Caswell H (1985) The evolutionary demography of clonal reproduction. In: Jackson JBC, Buss LW, Cook RE (eds) Population biology and evolution of clonal organisms, Yale University Press, New Haven, pp 187–224
Chen JK (1989) Systematic and evolutionary botanical studies on Chinese Sagittaria, Wuhan University Press, Wuhan
Cheplick GP (1995) Life history trade-offs in Aphibromus scabrivalvis (Poaceac): allocation to clonal growth, storage, and cleistogamous reproduction. Amer J Bot 82:621–629
Eriksson O (1997) Clonal life histories and the evolution of seed recruitment. In: de Kroon H, van Groenendael J (eds) The ecology and evolution of clonal plants, Backhuys, Leiden, pp 211–226
Gardner SN, Mangel M (1999) Modeling investments in seeds, clonal offspring, and translocation in a clonal plant. Ecology 80:1202–1220
Lambers H, Poorter H (1992) Inherent variation in growth rate between higher plants: a research for physiological causes and ecological consequences. Adv Ecol Res 23:187–261
Mendez M (1999) Effects of sexual reproduction on growth and vegetative propagation in the perennial geophyte Arum italicum (Araceae). Pl Biol 1:115–120
Philbrick CT, Les DH (1996) Evolution of aquatic angiosperm reproductive systems. Bioscience 46:813–826
Reekie EG, Bazzaz FA (1992) Cost of reproduction as reduced growth in genotypes of two congeneric species with contrasting life histories. Oecologia 90:21–26
Roff DA (1992) The evolution of life histories, Chapman and Hall, New York
Ronsheim ML, Bever JD (2000) Genetic variation and evolutionary trade-offs for sexual and asexual reproductive modes in Allium vineale (Liliaceae). Amer J Bot 87:1769–1777
Saikkonen K, Koivunen S, Vuorisalo T, Mutikainen P (1998) Interactive effects of pollination and heavy metals on resource allocation in Potentilla anserina L. Ecology 79:1620–1629
SAS institute (1998) SAS/STAT user’s guide, SAS Institute Inc Cary, North Carolina
Stearns SC (1989) Tradeoffs in life history evolution. Func Ecol 3:259–268
Stearns SC (1992) The evolution of life histories, Oxford University Press, Oxford
Sullivan G (1994) A trade-off between sexual and asexual reproduction in the dioecious clonal macrophyte Vallisneria americana: environmental and genetic influences. Ph.D. dissertation, Binghamton University, Binghamton
Suzuki A (2001) Resource allocation to vegetative growth and reproduction at shoot level in Eurya japonica (Theaceae): a hierarchical investment? New Phytol 152:307–312
Thompson FL, Eckert CG (2004) Trade-offs between sexual and clonal reproduction in an aquatic plant: experimental manipulations versus phenotypic correlations. J Evol Biol 17:581–592
van Kleunen M, Fischer M, Schmid B (2003) Experimental life-history evolution: selection on the allocation to sexual reproduction and its plasticity in a clonal plant. Evolution 56:2168–2177
Venable DL (1992) Size-number trade-off and the variation of seed size with plant resource status. Amer Naturalist 140:287–304
Westley LC (1993) The effect of inflorescence bud removal on tuber production in Helianthus tuberosus L. (Asteraceae). Ecology 74:2136–2144
Acknowledgments
We thank Dr. Stephen C. Maberly for the language checking. Dr. Li Wei and Yu Qian for helpful suggestions on the manuscript, Wang Yuanyuan and first author’s parents for their managements of the plants. This study was supported by grants from the National Natural Science Foundation of China (No: 30800061) and the Program for New Century Excellent Talents in University (from Ministry of Education, People’s Republic of China) granted to WQF (NCET-05-0619).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Liu, F., Chen, JM. & Wang, QF. Trade-offs between sexual and asexual reproduction in a monoecious species Sagittaria pygmaea (Alismataceae): the effect of different nutrient levels. Plant Syst Evol 277, 61–65 (2009). https://doi.org/10.1007/s00606-008-0103-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-008-0103-2