Abstract
Bacterial infectious diseases are severe threats to human health and increase substantial financial burdens. Nanomaterials have shown great potential in timely and accurate bacterial identification, detection, and monitoring to improve the cure rate and reduce mortality. Recently, carbon dots have been evidenced to be ideal candidates for bacterial identification and detection due to their superior physicochemical properties and biocompatibility. This review outlines the detailed recognition elements and recognition strategies with functionalized carbon dots (FCDs) for bacterial identification and detection. The advantages and limitations of different kinds of FCDs-based sensors will be critically discussed. Meanwhile, the ongoing challenges and perspectives of FCDs-based sensors for bacteria sensing are put forward.
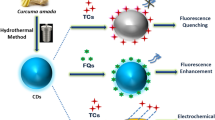
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bacterial infectious diseases have attracted extensive attention worldwide because they have become a threat to human health and increased substantial financial burdens. World Health Organization (WHO) reported that ca. 45% of all global diseases are related to infectious diseases, which lead to 26% of all deaths globally and around $2.9 − 6.7 billion in annual medical costs in the USA [1]. A recent review showed that the annually societal economic burden accounted for bacterial resistance was $77 billion for China, €1.5 billion for Europe, and 0.1–2.5% of yearly gross domestic product for Sub-Saharan Africa, respectively [2]. Although the use of antibiotics has cured most bacterial infectious diseases, bacterial resistance to current antibiotics has become increasingly serious. The WHO warned that the available antibiotics would lose their effectiveness in monitoring bacteria in the following 1–2 decades [3]. It has been predicted that antimicrobial resistance would lead to 10 million deaths every year by 2050 [4]. Additionally, the economic burden accounted for antibiotic resistance has been estimated to be $100 trillion USD for global and $20 trillion USD for China [5]. Prompt and accurate bacterial identification and detection are crucial to diagnosing and treating bacterial infectious diseases. Additionally, they play a key role in decreasing the emergence of drug-resistant bacteria.
Besides traditional detection methods including polymerase chain reaction (PCR), traditional plate counting, and enzyme-linked immunosorbent assay, optical and electrochemical assays based on nanomaterials have been developed as alternative strategies for bacterial identification and quantification [6,7,8]. Carbon dots (CDs) are a new kind of fluorescent carbon-based nanomaterials with a small size of less than 10 nm accidentally discovered in 2004 [9]. Typically, CDs are composed of an sp2 carbon core and oxygen/nitrogen-containing groups on their surface [10]. Up to now, the widely accepted classification of CDs is to categorize CDs into carbon nanodots (CNDs), graphene quantum dots (GQDs), and polymer dots (PDs) [11]. This classification was performed based on their different carbon core and surface chemical state [12,13,14]. CNDs are quasi-spherical nanoparticles with multilayers of graphene and a crystalline graphitic core. They can be subdivided into carbon nanoparticles (CNPs) and carbon quantum dots (CQDs) based on the difference that there are amorphous structures in CNPs and an obvious crystal lattice in CQDs. Different from CQDs, there are not only layered graphene crystalline structures in the carbon cores of GQDs but also chemical bonds on their edges. Since PDs are generally prepared by the polymerization of monomers/polymers, they possess cross-linked and polymerized structures on the surface of the carbon core. Though they are different from each other, they still have aroused much attention due to their properties of tunable photoluminescence, excellent electrochemical property, exceptional surface chemistry, and good biocompatibility. Therefore, CDs have been widely applied in the fields of sensing, bioimaging, biomedicine, catalysis, and optoelectronics [15, 16]. However, pristine CDs have some limitations including low fluorescence quantum yield and production yield, poor selectivity to targets, and few active sites. Fortunately, functionalizing CDs through surface modification and heteroatom-doping can effectively address the above drawbacks. The surface modification will provide more active sites for their applications, and heteroatom-doping aims to endow CDs with different intrinsic properties by changing their electronic structures [17,18,19]. Additionally, combining CDs with other nanomaterials will also improve their physicochemical properties. Therefore, the application scope of functionalized carbon dots (FCDs) has been greatly broadened, and some new applications in microorganisms [20,21,22] and plant systems [23] have been also launched gradually.
As far as we know, there have been a few reviews about FCDs applied in bacteria research such as bacterial detection, imaging, labeling, and eradication [20,21,22]. However, these available reviews specifically focus on the bacterial eradication and related antibacterial mechanisms of FCDs, rare of which have summarized the detection mechanisms of FCDs-based sensors for bacterial identification and detection. Accordingly, in this review, the detailed recognition elements and recognition strategies for bacteria sensing with FCDs-based sensors will be highlighted (Fig. 1). These sensors will be divided into biosensors and chemical sensors from the view of the types of recognition elements. Their advantages and limitations will be compared and discussed. Additionally, this review will also present the challenges associated with FCDs for bacteria sensing, and suggest future detection principles for the development of recognition element-mediated sensors with excellent selectivity and sensitivity for bacteria sensing.
Bacterial identification and detection
From the view of recognition elements, the available FCDs-based sensors for bacterial identification and detection can be divided into two categories: biosensors and chemical sensors. Biosensors can identify and detect concrete types of bacteria via molecular recognition since specific biomolecules (antibodies, aptamers, deoxyribonucleic acid (DNA), peptides, etc.) are used as recognition elements. In chemical sensors, not only molecular recognition but also non-covalent recognition in terms of electrostatic interaction, hydrogen bonding, and hydrophobic/hydrophilic interaction is employed, which are more suitable for bacterial identification from gram types. They identify and detect bacteria from more aspects including surface physicochemical properties, metabolites, and intracellular leakage. To realize bacterial identification and detection, various chemical materials are adopted as recognition elements like mannose, mannoside, quaternary ammonium salt, boric acid, and silane in chemical sensors.
Biosensors
The employment of biomolecules as recognition elements ensures the high selectivity and sensitivity of biosensors. The special selectivity of biosensors enables them to analyze targeted analytes without complicated pre-treatment of samples. Besides the real-time analysis, their potential for portable in situ analysis promises the application of biosensors to point-of-care diagnostics. Here, we classify the biosensors into label biosensors and label-free biosensors. In label biosensors, the recognition elements are biomolecules like antibodies, aptamers, DNA, and peptides, which are directly modified on the surface of CDs. The sensitivity of these label biosensors is closely related to the number of bound target molecules since the detection signal depends on the number of labels. In contrast, the biomolecules participate in bacterial detection but are not modified on the surface of CDs in label-free biosensors. And the detection signal changes rely on the physicochemical properties changes of CDs during the detection procedure in label-free biosensors. Though both label and label-free biosensors can provide comparatively high sensitivity and fast response, label biosensors endow less stability and more complex operations compared to label-free biosensors in practical applications. Additionally, the detection mechanism of label biosensors is basically based on the specific interaction between recognition elements of bacteria, while the detection signal change is the synergetic result of multiple kinds of response mechanisms in label-free biosensors. Tables 1 and 2 summarize the details of some label and label-free FCDs-based biosensors for bacteria detection, respectively.
FCDs-based label biosensors
Antibody-modified CDs-based biosensors
Antibodies are large Y-shaped proteins composed of four polypeptide chains (two heavy and two light chains) secreted by plasmocytes, which are commonly utilized as bioreceptors for bacterial identification and detection. The antigen-binding sites are located on the ends of their fragment antigen-binding regions, which confer a highly specific affinity to target antigens on the bacterial surface. A typical process of antibody-antigen binding involves multiple types of molecular interactions, including electrostatic forces, hydrogen bonding, van der Waals and hydrophobic interactions, and conformational changes. These interactions lead to the formation of a stable immunocomplex. These biosensors realized bacterial identification and detection based on fluorescence resonance energy transfer (FRET) [24], turn-on fluorescence [25, 26], and electrochemical signal [28, 30].
-
A)
FRET
In a FRET system, the fluorescence of antibody-modified CDs was quenched with the quencher via FRET and recovered with the addition of the target antigens. Therefore, FRET systems are suitable for various antigens via altering the antibodies. For example, a FRET system for the detection of Salmonella typhi has been constructed with graphene oxide (GO) as a fluorescence acceptor and antibody-conjugated iron porphyrin bio-mimicked graphene quantum dots (Fe–N-GQDs/Ab) as donors (Fig. 2) [24]. The π-π stacking interaction between Fe–N-GQDs/Ab and GO resulted in the fluorescence quenching of Fe–N-GQDs/Ab. With the addition of Salmonella typhi Vi antigen, the specific antibody-antigen interaction increased the distance between Fe–N-GQDs and GO, which recovered the fluorescence of Fe–N-GQDs.
-
B)
Turn-on fluorescence
The mechanism for FRET with GO as an acceptor and Fe–N-GQDs as a donor [24]
In turn-on fluorescence assays, immunomagnetic bead technique [25] or encapsulating CDs with silica nanospheres [26] have been employed to improve their sensitivity. Generally, a sandwich-structured immunocomplex generates after the immunomagnetic beads and FCDs simultaneously incubated with bacteria for about 1 h in an immunomagnetic sensor. The formed immunocomplex is enriched by magnetic separation and the fluorescence measurement is carried out. In a typical procedure, the fluorescence of FCDs is quenched upon binding to the targeted bacteria, providing a signal for the detection of the infection. For instance, a cell-based fluorescent CD-microsphere immunosensor has been prepared for E. coli O157:H7 detection based on the high affinity of Staphylococcal Protein A on the S. aureus cell surface to the fragment crystallizable region of antibodies with a limit of detection (LOD) of 2.4 × 102 CFU mL−1, which was comparable to that of real-time PCR methods [25].
-
III)
Electrochemical method
Electrochemical methods consist of various electrochemical transducers and analyte signal detection elements. Electrochemical transducers react with targeted analytes to generate electrochemical signals such as current, potential, resistance, and impedance, which could be used for qualitative and quantitative analysis of targeted analytes. The electrochemical methods have received much attention due to their superiorities over less dosage, rapid analysis, cost-effectiveness, and portability. Vedashree et al. have constructed an electrochemical sandwich immunoassay for the detection of S. aureus [28]. In this immunoassay, gold nanoparticles binding S. aureus antibodies (AuNPs-Ab) were modified on the surface of graphite electrodes. Then S. aureus and GQDs bound S. aureus antibodies (Ab-GQDs) were added in order onto the modified graphite electrodes. Once the sandwich complex AuNPs-Ab/S. aureus/Ab-GQDs formed, the current varied with the current change proportional to the concentration of S. aureus. The detection of S. aureus was realized with a LOD of less than 1 CFU mL−1.
-
IV)
Electrochemiluminescence
Electrochemiluminescence (ECL) is a technique fusing electrochemical measurement and chemiluminescence strategy. A typical electrochemiluminescence assay consists of two important components: coreactants and ECL initiation element. The coreactants induce the formation of an intermediate with ionic radicals, which will further interact together and release the ECL signal for detection. The ECL assays have been widely applied in various fields due to the advantages of affordable price, low background signal, high sensitivity and selectivity, and rapid analysis. For example, Sakda Jampasa et al. obtained immunocomplexes on the surface of carboxyl graphene (GOOH)–modified screen-printed carbon electrode, which were composed of capture antibody (Ab1), antigen, and nitrogen-doped CD (NCD) labeled with secondary antibody (Ab2-NCD) (Fig. 3a) [29]. The formation of these immunocomplexes initiated an obvious ECL signal increment with the addition of K2S2O8 in the following mechanism (Fig. 3b). Firstly, NCD and K2S2O8 were simultaneously electrochemically reduced to generate NCD·− and SO4·−, respectively. Secondly, NCD·− reacted with SO4·− to form the excited state NCDs*. Finally, the NCD* transferred its energy to NCD and released an optical signal. When this constructed ECL sensor was applied to the detection of L. monocytogenes, this signal was in a linear relationship with the L. monocytogenes concentration, which has been successfully applied to detect L. monocytogenes in real food products with satisfactory results. Additionally, inspired by the report that nitrogen-doped GQDs (N-GQDs) could emit strong ECL signal with K2S2O8 as a coreactant on the glassy carbon electrode, Chen’s group utilized polydopamine (PDA) surface imprinted polymer (SIP) and N-GQDs to develop an ECL biosensor for the detection of E. coli O157:H7 [30]. The PDA SIP for E. coli O157:H7 was first formed on the electrode surface. Then N-GQDs labeled with E. coli O157:H7 polyclonal antibody (pAb) and E. coli O157:H7 were added on the electrode surface, and the bioconjugation of SIP-E. coli O157:H7/pAb-N-GQDs generated an intensive ECL signal with the addition of K2S2O8. Therefore, the ECL sensing system could realize the detection of E. coli O157:H7.
Schematic illustrations of a NCD-conjugated secondary antibodies (upper low) and the developed ECL sensor for L. monocytogenes detection (down low); b the mechanism for ECL signal increment of NCD with the addition of K2S2O8 [29]
Aptamer-modified CDs-based biosensors
Although the specificity of antibodies is high, the high expense and poor reproducibility have limited their wide application. Consequently, as a class of single-stranded deoxyribonucleic acid (ssDNA) or ribonucleic acid (RNA) that can specifically bind to targeted analytes, aptamers have aroused attention due to their advantages of lower cost, higher thermal stability, and higher specificity. The existing aptamer-modified CDs-based biosensors consist of fluorescent aptasensors and electrochemical aptasensors.
-
A)
Fluorescence method
Fluorescence methods possess the properties of rapid response speed, excellent sensitivity, and high selectivity. Most fluorescent aptasensors used aptamer-modified CDs (CDs-aptamers) as fluorescence donors and nanomaterials like gold nanoparticles (AuNPs) [33, 34], GO [32, 38], and Fe3O4 [42] as fluorescence acceptors. The fluorescence of the system recovered with the addition of target bacteria. As shown in Fig. 4 a, both CDs and AuNPs were functionalized with ssDNA and tethered together with a linker DNA containing the complementary sequences of S. aureus–specific aptamer [33]. The nanometal surface energy transfer between CDs and AuNPs quenched the fluorescence of CDs. When aptamer was added, the strong competitiveness of aptamer to linker DNA released CDs to recover their fluorescence. The stable binding complex between the aptamer and S. aureus resulted in the preferential binding of the aptamer to S. aureus compared to the aptamer/DNA duplex. Consequently, the fluorescence of CDs was quenched again due to the reassembly of CDs with AuNPs. The variable fluorescence response was directly linear to S. aureus concentration with a LOD of 10 CFU mL−1.
In addition, ratiometric fluorescent aptasensors have also been developed for the quantitative detection of trace amounts of bacteria due to their low background signal and high accuracy and sensitivity for bioanalysis. In these aptasensors, the fluorescence of CDs-aptamers recovered due to the specific interaction of CDs-aptamers to target bacteria releasing them from the quenchers. As shown in Fig. 4 b, the energy transferred from ssDNA-modified ortho-phenylenediamines carbon dot (o-CD-ssDNA) to nitrogen-doped CNDs (NCND) modified GO via π-stacking interaction, resulting in fluorescence quenching [38]. Based on the preferential interaction of o-CD-ssDNA to A. baumannii, o-CD-ssDNA was released from GO with the fluorescence recovery, while the fluorescence of NCND only slightly changed. The fluorescence intensity ratio of o-CD-ssDNA/NCND was dependent on the concentration of A. baumannii with an LOD of 3.0 × 102 CFU mL−1.
-
B)
Electrochemical method
Electrochemical aptasensors are the other widely adopted detection technique for bacteria detection due to their advantages over high sensitivity, fast response, low cost, and simple operation. Ranjbar et al. [39] modified cellulose nanofibers (CNFs) with AuNPs/CDs nanocomposite for immobilization of the thiolated aptamer. The AuNPs/CDs/CNFs have been applied for the impedimetric detection of S. aureus with an LOD of 1 CFU mL−1. In Jiang et al.’s work, they constructed a photoelectrochemical biosensing platform with excellent performances by coupling tungsten oxide hydrate nanosheets (WO3·H2O) with N-GQDs for the detection of E. coli O157:H7 [40]. WO3·H2O possessed broad photoabsorption from visible light to near-infrared light, and N-GQDs displayed excellent photoelectric performance. The photoelectric response of as-fabricated heterojunction to E. coli O157:H7 has been greatly enhanced via the combination of these two properties. This method displayed the advantages of rapid response, high selectivity and sensitivity, wide linear range, and ultralow LOD of around 0.05 CFU mL−1.
DNA-modified CDs also exhibit superior selectivity and sensitivity. Recently, a CDs-Fe3O4-based electrochemical aptasensor has been developed for E. coli O157:H7 detection [41]. The introduction of Fe3O4 enhanced the electrical conductivity of CDs on the surface of the glassy carbon electrode, while the intensity decreased with the addition of probe DNA due to the hindrance of [Fe(CN)6]3−/4− diffusion from DNA molecules. When E. coli O157:H7 was added, the oxidation peak potential of the proposed sensor was in a linear relationship with the logarithm of the E. coli O157:H7 concentration and with a LOD of 6.88 CFU mL−1. Pangajama et al. also found the excellent conductivity of nanocomposite, carbon dots/ZnO nanorod/polymerizing aniline (CDs/ZnO/PANI), enhanced the sensitivity of the proposed electrochemical sensor [52]. The composite was functionalized with oligonucleotide to capture the genomic DNA of E. coli O157:H7 on the sensor surface. The sensor could realize the detection of E. coli O157:H7 in water samples.
Peptide-modified CDs-based biosensors
Cecropin P1 is a naturally produced cationic antibacterial peptide consisting of 31 amino acids with 10 free amines, which could selectively bind to E. coli cell walls. Therefore, cecropin P1 has also been used as a biorecognition element and conjugated to carboxylated GQDs (CGQDs) via the reaction between its amine groups and the carboxyl groups of CGQDs [43]. The lipopolysaccharide in Gram-negative bacteria is negatively charged due to its phosphate groups, which can interact with the positively charged amino groups on cecropin P1 [53]. This electrostatic interaction allows the cecropin P1-conjugated CGQDs to selectively bind to the cell walls of gram-negative bacteria. The obtained cecropin P1-conjugated CGQDs could reserve the binding ability to Gram-negative bacteria cell walls. The fluorescence of the cecropin P1-conjugated CGQDs was quenched upon binding to the E. coli, providing a signal for the detection of E. coli in real samples.
FCDs-based label-free biosensors
-
A)
Fluorescence method
Excellent fluorescence is one of the most important properties of CDs, based on which many label-free biosensors for bacteria detection have been developed. For example, a controllable Guanine-quadruplexes (G4) release-based dual-recognition ratiometric fluorescence sensing of MRSA was constructed (Fig. 5a) [44]. In this biosensor, polyethyleneimine-functionalized CDs were used as a platform to assemble with DNA molecular beacon (MB) and Van to form dual-recognition CDs (Van-MB-CDs) via a cross-linking amide reaction. The designed MB contained an MRSA aptamer with a partially complementary sequence and a complementary G-rich sequence on each end. With the addition of MRSA, Van-MB-CDs bound with MRSA, which destructed MB to release the G-rich DNA sequence and form a G4 structure. Then, N-methyl mesoporphyrin IX (NMM) bound to the G4 structure to emit an obvious red fluorescence signal at 605 nm. Using the fluorescence intensity of Van-MB-CDs at 440 nm as a reference, the F605 nm/F440 nm was linear with the concentration of MRSA with a LOD of 8.2 CFU mL−1. The dual-recognition moieties designed in this biosensor effectively shortened the detection process and enhanced the detection accuracy. In a triple-module biosensor, the authors successfully detect H. pylori based on combining three modules together: H. pylori–specific aptamer modified with Ca2+-doped superparamagnetic nanoparticles for capturing H. pylori, a bifunctional co-polymer of chloroprotoporphyrin IX iron (III)-polyethylene glycol-desferrioxamine with high affinity to H. pylori and Fe3+, and Fe3+-quenched CDs (Fig. 5b) [45]. This novel biosensor possessed a low LOD of 1 CFU mL−1 in a wide range of 10–107 CFU mL−1.
a Diagrams for the preparation of the dual-recognition Van-MB-CDs and their application as a ratiometric fluorescence sensor for MRSA detection by coupling with NMM based on MRSA-triggered controllable release of G4 [44]; b schematic illustration of a triple-module biosensor for detecting H. pylori [45]; c schematic illustration of the detection of S. aureus with CDs@BONs [49]
In the other work, CDs were initially encapsulated into breakable organosilica nanocapsules (BONs) to form core–shell CDs@BONs via the cohydrolyzation of tetraethyl orthosilicate and bis[3-(triethoxysilyl)propyl]disulfide (Fig. 5c) [49]. Then both CDs@BONs and immunomagnetic nanoparticles were modified with anti-S. aureus antibodies to specifically capture S. aureus simultaneously. Once the organosilica shells were broken by NaBH4 reduction, hundreds of CDs released from BONs would significantly amplify the fluorescence signals. The sensitivity reached 30 CFU mL−1, which was more than 100 times that of previously reported immunoassays with directly antibody-conjugated CDs as labels.
-
B)
Electrochemical method
Since electrochemical methods are sensitive and rapid for bacteria detection, the FCDs used as an electrochemical indicator for bacteria detection have been reported [54]. Recently, an electrochemical immunosensor based on the intrinsic peroxidase-like activity of GQDs has been used for the detection of Y. enterecolitica (Fig. 6a) [47]. GQDs were firstly laminated on a gold electrode to catalyze the reduction of H2O2 on the surface of the gold electrode and generated a significant signal at the mA range via the improvement of electron transfer between Au and GQDs. Once the antibody was immobilized and Y. enterecolitica was continuously added, the generation of antigen–antibody complex on the electrode restrained the electron transfer from GQDs to the Au electrode, which resulted in the gradual signal decrease of the immunosensor. As a label-free immunosensor, the sensor could be used for any pathogenic bacteria detection with a rapid, specific, and sensitive response.
It has been reported that the high content of sp2 carbon in GQDs contributes to the electron transport on the electrode surface, and the oxygen groups on the surface of GQDs will enlarge the luminescence signals [56]. Enlightened by this property, Li et al. prepared GQD composites (GQDs/AgNPs) by in situ thermal reduction Ag(NH3)2OH to silver nanoparticles (AgNPs) on the surface of GQDs (Fig. 6b) [55]. The obtained GQDs/AgNPs were applied to amplify the luminescence signals in a tris(2,2′-bipyridyl)ruthenium(II)/tripropylamine (Ru(bpy)32+/TPA) assay. Both Ru(bpy)32+ and TPA would lose an electron to form Ru(bpy)33+ and TPA+· with the electric excitation. The formed TPA· from the deprotonation of TPA+· further reacted with Ru(bpy)33+ to generate excited Ru(bpy)32+*. The optical signal was obtained as excited Ru(bpy)32+* returned to the steady state of Ru(bpy)32+. As the E. coli antibody was modified onto the electrode, the proposed ECL sensor could selectively detect E. coli in a linear range of 10–107 CFU mL−1. Moreover, integrating this ECL sensor with a portable smartphone could be applied to point-of-care testing.
-
III)
Colorimetric immunoassay
Colorimetric strategies endow some advantages of simple operation, low-cost, visual detection with naked eyes, without sophisticated instruments. When targeted bacteria appeared in colorimetric biosensors, an obvious color change is observed. Therefore, they are the potential for on-site analysis and point-of-care diagnosis. Accordingly, a colorimetric immunoassay has been constructed for the detection of S. aureus with magnetic nanoparticles decorated with CDs (Mag-CDs) and immunoglobulin Y antibody–coated silver nanoclusters (IgY-AgNCs) (Fig. 7a) [50]. In this typical sandwich-type immunoassay, Mag-CDs captured S. aureus via Van der Waals force and electrostatic interaction between Mag-CDs and S. aureus. IgY-AgNCs efficiently bond to S. aureus via the special interaction between antibodies and S. aureus. As a result, sandwich-type immunocomplexes formed. Meanwhile, IgY-AgNCs played as an enzyme mimic to catalyze the oxidation of o-phenylenediamine to form a yellow product. The color of the solution deepened from colorless to deep yellow with the increasing concentration of S. aureus, and the absorbance was proportional to the S. aureus concentration. This visual sensor was highly selective and sensitive with the LOD of 4.9 CFU mL−1 in the linear range of 10–106 CFU mL−1.
Different from the conventional Ab1-bacteria-Ab2 sandwich pattern, Wang’s group employed a nanoparticle-bacteria-antibody sandwich strategy and proposed a label-free lateral flow immunoassay (LFIA) for the detection of S. enteritidis (Fig. 7b) [51]. In this work, nitrogen-rich carbon nanoparticles (pNC) with positive charge were absorbed on the surface of S. enteritidis cells via electrostatic interaction and hydrogen bonding, and further specifically captured the anti-bacteria monoclonal antibody (McAb) coated on the test line (T-line). And the color on T-line varied with the accumulation of pNC-S. enteritidis complex. The LOD of this immunoassay was low to 102 CFU mL−1 in the linear range of 102–108 CFU mL−1.
Chemical sensors
Though the biosensors exhibited the advantages over the high selectivity and sensitivity to bacteria with low detection limit, there are also some limitations for biosensors, such as expensive cost, professional techniques, low stability, and false signal. As a complementary strategy to biosensors, chemoselective ligand-based chemical sensors are appealing to researchers owing to their cheaper cost, higher resistance to volatile environments, and faster response time. Up to now, various antibiotics (amikacin [57, 58], colistin [59], vancomycin [60], and ampicillin [61]) and chemical materials (mannose [62,63,64], boric acid [65, 66], and ionic liquid [67]) have been adopted for the preparation of CDs to ensure the detection selectivity. Furthermore, since the surface physicochemical properties, metabolites, and intracellular leakages of bacteria differ from one another, these differences can be used as recognition elements for bacterial identification and detection. Accordingly, the CDs-based chemical sensors for bacterial identification and detection would be discussed from the following three bacterial characteristics: (1) specific structures on the bacterial cell surface, (2) metabolites, and (3) intracellular leakages. Some of the FCDs-based chemical sensors for bacteria detection are summarized in Table 3.
Molecular recognition with bacterial surface structures
Besides the functional groups on the surface of CDs, the selectivity of CDs to different bacteria species mainly depends on the structures and compositions of bacteria cell walls. As is known, the cell walls of Gram-positive bacteria are simple and consist of only one interconnected peptidoglycan layer with negatively charged teichoic acids. As for Gram-negative bacteria, there is a thin peptidoglycan layer between two layers of phospholipids [75, 84]. Accordingly, the methods for bacteria detection with the surface physicochemical properties as recognition elements mainly employ the following mechanism: covalent interaction, electrostatic interaction, and hydrophobic interaction.
-
A)
Covalent interaction
Covalent interaction involves the formation of a chemical bond between the recognition element and the bacterial surface structure. Special molecules on the surface of the cell membrane could be chosen as recognition elements for bacterial identification and detection. For example, mannose [62,63,64] and mannoside [85] can strongly and selectively bind to the FimH lectin units on the tips of the wild-type 1 pili on the surface of E. coli, and boronic acid shows preferential affinity to the diol groups of lipopolysaccharide on the bacterial cell surface to form cyclic boronate ester bond [65]. Therefore, mannose, mannoside, and boronic acid have been used as sources to synthesize FCDs for the successful detection of E. coli. For example, electroconductive boronic acid–modified polymer dot (B-PD)–coated electrode has been adopted in an electrochemical sensor [69]. The presence of boronic acid provided distinguishing sites for selective bacterial capture via diol-diol interactions between boronic acid and bacterial membranes. The designed sensor was sensitive to bacteria with an LOD of 100.8 CFU mL−1 for E. coli and 10 CFU mL−1 for S. aureus. Moreover, bacterial detection could be carried out in-line via transmitting the electronic signal from the B-PD-coated electrode to a smartphone. In another example, vancomycin (known as a glycopeptide antibiotic)-modified CDs can effectively identify Gram-positive bacteria via the formation of hydrogen bonds between vancomycin and the terminal peptide of d-alanyl-d-alanine on the cell walls of the Gram-positive bacteria [60].
-
B)
Electrostatic interaction
There are lipoteichoic acids and teichoic acids on the surface of Gram-positive bacteria and lipopolysaccharide on the surface of Gram-negative bacteria. These constituents make Gram-positive and Gram-negative bacteria negatively charged, which provide anionic sites for their electrostatic interaction with positively charged CDs. Up to now, positively charged CDs have been prepared with amikacin (an amino glycoside antibiotic) [57, 58], and colistin (a polycationic peptide antibiotic) [59]. These positively charged CDs could distinguish negatively charged E. coli via electrostatic interaction with the phosphate groups in lipid A of E. coli. Wang et al. prepared positively charged and hydrophobic NCD with energetic ionic liquid (1,3-dibutylimidazolium dicyandiamide) as a carbon source, which could selectively label yeast cell S. cerevisiae [67]. Besides, functionalized CDs incorporated with MgFe2O4 [86], CsWO3 [87], NiFe2O4 [88], Fe3O4 [89], and AgNPs [90] are also applied to bacteria detection via electrostatic interaction between bacteria and nanocomposites in corresponding work.
-
III)
Hydrophobic interaction
Hydrophobic interaction involves the interaction between the anchoring groups (e.g., alkyl chains and lipids) of amphiphilic FCDs and the plasma membranes of targeted bacteria, without affecting cell viability [91]. Previous work reported that the attachment capacities and modes of the amphiphilic CDs to the bacterial membranes were dependent on the cell surface physicochemical properties [73, 92], and the high hydrophobicity of the materials is beneficial to their affinity to negative bacteria [93]. Zhao and co-workers obtained hydrophobic CDs with near-infrared emission derived from perilla and found these CDs could selectively stain Gram-positive bacteria [74]. To investigate the identification ability of the obtained CDs, they were incubated with six different kinds of bacteria for 30 min, respectively. Results showed that CDs only selectively stained Gram-positive bacteria but Gram-negative bacteria. The detailed interaction mechanism study revealed that all the CDs, Gram-positive bacteria, and Gram-negative bacteria were negatively charged. Therefore, the electronic interaction was excluded from explaining the phenomena. On the other hand, the water-octanol partition coefficient (log P) of CDs was measured to be 1.1. This value was larger than 0, indicating the relative hydrophobicity of CDs. Previous work has found that the materials with smaller log P showed higher affinity to Gram-positive bacteria and fungi than Gram-negative bacteria, while this affinity gradually weakened with the log P gradually increasing, and the materials displayed higher affinity toward Gram-negative bacteria than Gram-positive bacteria and fungi [93]. Therefore, its authors concluded that the selective identification of Gram-positive bacteria was due to hydrophobic interactions between CDs with suitable hydrophobicity and the cell membrane of Gram-positive bacteria. To enhance the selectivity and sensitivity to bacterial identification and detection, the combination of two or three kinds of the above mechanism has been considered. And these assays usually were applied to the gram-type identification via fluorescence imaging or staining. For example, Wu’s group has prepared two types of quaternized CDs. One type of CDs was prepared by conjugating lauryl betaine onto the surface of amine-functionalized CDs via carboxyl-amine reaction [75], while the other one was prepared with glycerol and dimethyloctadecyl[3-(trimethoxysilyl) propy]ammonium chloride (Si-QAC) [76] (Fig. 8a). Since both lauryl betaine and Si-QAC are compounds with a long alkyl chain–containing quaternary ammonium, the obtained quaternized CDs endowed both positive charge and hydrophobicity, and could selectively interact with Gram-positive bacteria via both electrostatic and hydrophobic interactions. In Yan and co-workers’ work, three excitation peaks and single-color emission CQDs (T-SCQDs) have been prepared with glucose, glycine, and l-tryptophan as sources via the hydrothermal method (Fig. 8b) [94]. The obtained T-SCQDs could lastingly and selectively track Gram-positive bacteria via specifically targeting peptidoglycan. The detail interaction mechanism study showed that the selectivity of T-SCQDs to Gram-positive bacteria was attributed to three reasons: (1) the electrostatic interaction between negatively charged peptidoglycan and the cationic amino groups of T-SCQDs; (2) the hydrophobic interaction between the short peptides cross-linked with peptidoglycan and the benzopyrrole structure of T-SCQDs; (3) the generation of hydrogen bonds between the hydrophilic groups on the surface of peptidoglycan and the hydroxyl groups on the surface of T-SCQDs. These reasons were also reported for both S. aureus and E. coli strongly anchored on CDs which were decorated with magnetic nanoparticles and chitosan [95].
Recognition with bacterial metabolites
-
A)
Enzymes
It has been reported that the redox enzymes generated during the metabolic process of Gram-negative bacteria can reduce Cu2+ to Cu+ [96]. Inspired by this phenomenon, a ratiometric fluorescent sensor has been constructed for the selective detection of Gram-negative bacteria based on a core-satellite nanostructure (BCD@SiO2@BSA-AuNC). In this sensor, blue CDs (BCDs) encapsulated in the core of silica and target-sensitive bovine serum albumin stabilized gold nanoclusters (BSA-AuNC) covalently linked on silica surface were used as reference signal and response signal, respectively [77] (Fig. 9a). The introduced Cu2+ coordinated with the amino acids in BSA resulting in the obvious fluorescence quenching of BSA-AuNC and the unchanged fluorescence of BCDs. When the Gram-negative bacteria were added, the reduction of Cu2+ to Cu+ with Gram-negative bacteria alleviated the fluorescence quenching of BSA-AuNC. Consequently, the detection and quantification of Gram-negative bacteria were realized.
In the other assay for E. coli detection, enzyme-mediated redox relay reaction with CD-manganese dioxide (MnO2) nanosheets was used as a fluorescent sensing platform (Fig. 9b). Initially, the fluorescence of CDs quenched via the FRET process between CDs and MnO2 nanosheets [78]. As E. coli was added, the reductases like NADH-quinone reductase produced in the respiratory pathway reduced p-benzoquinone (BQ) to hydroquinone (HQ). The obtained HQ further reduced MnO2 nanosheets to free Mn2+ ions, which disintegrated MnO2 nanosheets and enhanced the fluorescence of CDs. Since the reduction of BQ to HQ is a two-electron transfer pathway prevalent only occurred in the respiratory pathway of E. coli, the proposed assay excellently distinguished E. coli from other bacterial species.
There are still some other kinds of enzymes such as lipase, gelatinase, hyaluronidase, phosphatase, and nitroreductase produced in the metabolic process [97], which also can be used for bacterial identification. For instance, a new CDs-hydrogel hybrid has been employed for bacterial detection via modulating the fluorescence of CDs embedded in hydrogel by cleaving the scaffolds’ ester bonds by bacterially secreted esterases and lipases [79].
-
B)
pH variations
The acidic products (such as lactic acid, acetic acid, CO2) generated in the metabolic process of bacteria will decrease the pH of the medium. These pH variations promise the design of pH-sensitive assays for bacteria detection [80,81,82]. Zhao and co-colleagues established a sugar-metabolism-triggered bacterial identification strategy with pH-sensitive CDs (p-CDs) [80]. When glucose, bacteria, and p-CDs were mixed, E. coli and S. aureus could be distinguished from the other two kinds of investigated bacteria (S. typhimurium and B. subtilis) since the fluorescence of the solutions containing E. coli and S. aureus effectively reduced. However, when lactose was used instead of glucose, only the fluorescence of the solution containing E. coli decreased (Fig. 10). These phenomena were attributed to the expression of β-galactosidase in the sugar metabolism of E. coli, which can further hydrolyze lactose into glucose and galactose and give rise to the pH decrease. This strategy was also suitable for multiple-bacterial identification.
Normalized fluorescence intensity of p-CDs with various types of bacteria with or without a glucose and b lactose [80]
Meanwhile, the pH-sensitive redox property of CDs has been utilized to develop non-invasive monitoring of bacterial growth platforms by encapsulating bacterial cells and CDs in alginate microspheres [82]. Since CDs were close to the growing bacterial cell, the tiny changes in the microenvironment could be monitored. The developed platform was sensitive and with a fast response to bacteria with a low bacterial count of < 103 CFU mL−1 within 20 min.
Recognition with intracellular leakage
Different from most reported work, Gong’s group developed an intracellular leakage-trigged signal-on solid-state ECL assay for the detection of E. coli [83]. In this assay, N,S co-doped CDs-poly dimethyl diallylammonium chloride grafted carbon nanospheres (labeled as (dCDs/PDDA)n) were self-assembled to multilayer as ECL luminophores and coreacted with peroxydisulfate (PS) ions. Meanwhile, the molecularly imprinted electrospun nanofibers (ESNFs) were incorporated with (dCDs/PDDA)n layer as recognition elements (ESNFs-(dCDs/PDDA)n@CNs). Upon the addition of E. coli, PS ions destroyed the integrity of the E. coli cell membrane, and the intracellular leakage K+ (the most dominant intracellular cations)-triggered ECL enhancement was achieved via prompting the involved 1O2-mediated ECL process (Fig. 11). Since the ECL enhancement linearly depended on the concentration of E. coli, the proposed assay was applied to the E. coli detection. To the best of our knowledge, this assay was the only one to detect bacteria with inner content leakage triggered signal. The assay effectively avoided the possible risks of bacterial inactivation, bacterial membrane crack, and even intracellular leakage.
a Schematic illustration of the intracellular leakage K+-trigged ECL enhancement for E. coli detection and b the possible mechanism of the proposed ECL system [83]
Array-based sensors
Given the fact that the real samples are complex and contain multiple pathogenic microorganisms, traditional single-response sensors cannot meet the detection requirement. To address this problem, the array-based sensing strategy has aroused much attention due to its ability to distinguish various kinds of microorganisms spontaneously. In general, a representative sensor array is composed of multiple recognition elements or receptors that specifically interact with the targets and produce detectable optical or electrochemical signal changes. These signal changes can be analyzed using statistical methods and displayed in patterns with clear boundaries between different regions.
To date, there are several sensor arrays that have been developed for the identification of bacteria. Zheng et al. constructed a sensor array with CDs modified with three different receptors (boronic acid, polymixin, and vancomycin). This sensor array could effectively identify six kinds of bacteria (E. coli, P. aeruginosa, Desulfovibrio desulfuricans, Staphylococcus Sciuri, L. monocytogenes, and S. aureus) with linear discrimination analysis (Fig. 12a), which is a mathematical statistical method widely used to separate classes of objects in sensing arrays [98]. In addition, Wang et al. [99] have synthesized single concentration-dependent CDs and utilized the emission spectra differences of CDs with different excitations to realize the identification of eight microorganisms. The latter work possessed the advantages of simple operation without surface modification. Unlike these two reports, Shaulof’s group explored a sensor array based on the capacitance of interdigitated electrodes (IDEs) coated with CDs responding to vapor molecules with different polarities [100]. The CDs-IDE sensor array featured distinct capacitance changes to different gas produced by various bacteria during the metabolic process. The excellent sensitivity and selectivity of the sensor array endowed its excellent predictability for real-time monitoring of bacterial proliferation and discriminating bacteria. Accordingly, Alafeef et al. [1] synthesized pH-responsive yttrium-doped carbon nanoparticles (Y-doped CNP) and incorporated them into a 3D gel matrix to provide a dual approach for bacteria detection (Fig. 12b). The fluorescence of Y-doped CNP changed with pH which varied with the culture time of different kinds of bacteria. This simple, easy-to-use, and cost-effective detection strategy was successful to track the growth of bacteria over time.
Conclusions and perspectives
In this review, we provide a comprehensive analysis of the latest advances in the mechanisms and applications of FCDs in bacterial identification and detection. The excellent physicochemical properties (good water solubility, biocompatibility, and easy functionalization) promise wide applications of FCDs in the bacterial field. However, there are still some challenges that need to be overcome:
-
A)
Challenges from FCDs
Firstly, most of the FCDs used in the existing bacterial sensors were prepared with chemical materials, and only a small minority of them are derived from biomasses since biomasses are more environment-friendly and greener, abundant in raw materials, and low in cost for large-scale production. Moreover, the quantum yields of these CDs are not high, which is not conducive to their applications in bacterial imaging and detection. Secondly, only non-metal atom–doped CDs are utilized in bacterial applications; the bacterial applications of metal ion–doped CDs remain blank. However, metal ion–doped CDs possess more excellent physicochemical properties like electrochemical properties, intrinsic peroxidase-like activity, and catalytic activity. Thirdly, although the application of CDs in microorganism detection mainly focuses on bacteria detection, it is also significant to detect viruses and fungi.
-
B)
Challenges from the design strategies of FCDs-based bacterial sensors
Firstly, most sensors for bacterial identification and detection were based on the fluorescence and electrochemical properties of FCDs; the other physicochemical properties like intrinsic peroxidase-like activity and electrochemiluminescence of FCDs can also be taken into consideration for the design of new sensors for bacterial research. Secondly, most of the bacterial identification and detection were carried out from the view of the variation of surface physiochemical properties of different bacteria species; the assays could be developed based on the differences from the generated products and environment changes during the metabolic process and the intracellular leakage of different bacteria. Thirdly, though chemical sensors possess the advantages of a lower price, good stability, and easy operation, their sensitivity and selectivity need to be further improved; that is, more suitable recognition elements are awaiting discovery. Finally, FCDs-based commercial products like fluorescent ink, eye drops, test strips, and light-emitting diodes have been previously reported. However, the available FCDs sensors have not been commercialized for bacterial detection in practical applications. Once the stability and reproducibility of these sensors have been assured, they can be used reliably for practical applications. Furthermore, combined with the excellent properties of FCDs and mobile devices, new commercialization advances will be made in bacterial detection in the form of mobile applications, test strips, or diagnostic kits. These commercialized products will realize rapid, real-time, and convenient bacterial detection in the environment and food.
References
Alafeef M, Dighe K, Pan D (2019) Label-free pathogen detection based on yttrium-doped carbon nanoparticles up to single-cell resolution. ACS Appl Mater Inter 11(46):42943–42955. https://doi.org/10.1021/acsami.9b14110
Li T, Wang ZL, Guo JH, de la Fuente-Nunez Cesar, Wang JQ, Han B, Tao H, Liu J, Wang XM (2023) Bacterial resistance to antibacterial agents: mechanisms, control strategies, and implications for global health. Sci Total Environ 860:160461. https://doi.org/10.1016/j.scitotenv.2022.160461
Ray PC, Khan SA, Singh AK, Senapati D, Fan Z (2012) Nanomaterials for targeted detection and photothermal killing of bacteria. Chem Soc Rev 41(8):3193–3209. https://doi.org/10.1039/C2CS15340H
O’Neill J (2014) Antimicrobial resistance: tackling a crisis for the health and wealth of nations. Rev Antimicrob Resist. https://amrreview.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf. Accessed 2 Mar 2020
Zhen XM, Li YY, Chen YX, Dong P, Liu S, Dong HJ (2018) Effect of multiple drug resistance on total medical costs among patients with intraabdominal infections in China. PLoS One 13(3):e0193977. https://doi.org/10.1371/journal.pone.0193977
Chen J, Andler SM, Goddard JM, Nugen SR, Rotello VM (2017) Integrating recognition elements with nanomaterials for bacteria sensing. Chem Soc Rev 46(5):1272–1283. https://doi.org/10.1039/C6CS00313C
Li D, Kumari B, Makabenta JM, Gupta A, Rotello V (2019) Effective detection of bacteria using metal nanoclusters. Nanoscale 11(46):22172–22181. https://doi.org/10.1039/C9NR08510F
Yu T, Xianyu YL (2021) Array-based biosensors for bacteria detection: from the perspective of recognition. Small 17(21):2006230. https://doi.org/10.1002/smlP202006230
Xu XY, Ray R, Gu YH, Ploehn HJ, Gearheart L, Raker K, Scrivens WA (2004) Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J Am Chem Soc 126:12736–12737. https://doi.org/10.1021/ja040082h
Mintz KJ, Bartoli M, Rovere M, Zhou Y, Hettiarachchi SD, Paudyal S, Chen J, Domena JB, Liyanage PY, Sampson R, Khadka D, Pandey RR, Huang S, Chusuei CC, Tagliaferro A, Leblanc RM (2021) A deep investigation into the structure of carbon dots. Carbon 173:433–447. https://doi.org/10.1016/j.carbon.2020.11.017
Zhu SJ, Song YB, Zhao XH, Shao JR, Zhang JH, Yang B (2015) The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): current state and future perspective. Nano Res 8(2):355–381. https://doi.org/10.1007/s12274-014-0644-3
He C, Xu P, Zhang XH, Long WJ (2022) The synthetic strategies, photoluminescence mechanisms and promising applications of carbon dots: current state and future perspective. Carbon 186:91–127. https://doi.org/10.1016/j.carbon.2021.10.002
Li S, Li L, Tu HY, Zhang H, Silvester DS, Banks CE, Zou GQ, Hou HS, Ji XB (2021) The development of carbon dots: from the perspective of materials chemistry. Mater Today 51:188–207. https://doi.org/10.1016/j.mattod.2021.07.028
Arcudi F, Đorđević L, Prato M (2019) Design, synthesis, and functionalization strategies of tailored carbon nanodots. Acc Chem Res 52(8):2070–2079. https://doi.org/10.1021/acs.accounts.9b00249
Sun XC, Lei Y (2017) Fluorescent carbon dots and their sensing applications. TrAC Trend Anal Chem 89:163–180. https://doi.org/10.1016/j.trac.2017.02.001
KhairolAnuar NK, Tan HL, Lim YP, So’aib MS, Abu Bakar NF (2021) A review on multifunctional carbon-dots synthesized from biomass waste: design/fabrication, characterization and applications. Front Energy Res 9:626549. https://doi.org/10.3389/fenrg.2021.626549
Park Y, Yoo J, Lim B, Kwon W, Rhee SW (2016) Improving the functionality of carbon nanodots: doping and surface functionalization. J Mater Chem A 4(30):11582–11603. https://doi.org/10.1039/C6TA04813G
Li F, Yang DY, Xu HP (2019) Non-metal-heteroatom-doped carbon dots: synthesis and property. Chem Eur J 25(5):1165–1176. https://doi.org/10.1002/chem.201802793
Lin LP, Luo YX, Tsai P, Wang JJ, Chen X (2018) Metal ions doped carbon quantum dots: synthesis, physicochemical properties, and their applications. TrAC Trend Anal Chem 103:87–101. https://doi.org/10.1016/j.trac.2018.03.015
Anand A, Manavalan G, Mandal RP, Chang HT, Chiou YR, Huang CC (2020) Carbon dots for bacterial detection and antibacterial applications-a minireview. Curr Pharm Design 25(46):4848–4860. https://doi.org/10.2174/1381612825666191216150948
Cui FC, Ye YL, Ping JF, Sun XL (2020) Carbon dots: current advances in pathogenic bacteria monitoring and prospect applications. Biosens Bioelectron 156:112085. https://doi.org/10.1016/j.bios.2020.112085
Lin FM, Bao YW, Wu FG (2019) Carbon dots for sensing and killing microorganisms. C-J Carbon Res 5(2):33. https://doi.org/10.3390/c5020033
Li YD, Xu XK, Wu Y, Zhuang JL, Zhang XJ, Zhang HR, Lei BF, Hu CF, Liu YL (2020) A review on the effects of carbon dots in plant systems. Mater Chem Front 4(2):437–448. https://doi.org/10.1039/C9QM00614A
Kamal Z, ZareiGhobadi M, Mohseni SM, Ghourchian H (2021) High-performance porphyrin-like graphene quantum dots for immuno-sensing of Salmonella typhi. Biosens Bioelectron 188:113334. https://doi.org/10.1016/j.bios.2021.113334
Zhao Y, Li YZ, Zhang PP, Yan ZH, Zhou YG, Du YP, Qu CY, Song YJ, Zhou D, Qu SN, Yang RF (2021) Cell-based fluorescent microsphere incorporated with carbon dots as a sensitive immunosensor for the rapid detection of Escherichia coli O157 in milk. Biosens Bioelectron 179:113057. https://doi.org/10.1016/j.bios.2021.113057
Song Y, Ostermeyer GP, Du D, Lin YH (2021) Carbon nanodot-hybridized silica nanospheres assisted immunoassay for sensitive detection of Escherichia coli. Sensor Actuator B: Chem 349:130730. https://doi.org/10.1016/j.snb.2021.130730
Yang XZ, Feng LQ, Qin X (2018) Preparation of the Cf-GQDs-Escherichia coli O157: H7 bioprobe and its application in optical imaging and sensing of Escherichia coli O157: H7. Food Anal Method 11(8):2280–2286. https://doi.org/10.1007/s12161-018-1207-0
Sirdeshmukh VV, Apte HR, Kale AA (2019) Graphene quantum dots as promising probes in electrochemical immunoassay for rapid and sensitive detection of pathogenic Staphylococcus aureus. IEEE 13th International Conference on Nano/Molecular Medicine & Engineering (NANOMED). IEEE 108–114. https://doi.org/10.1109/NANOMED49242.2019.9130608
Jampasa S, Ngamrojanavanich N, Rengpipat S, Chailapakul O, Kalcher K, Chaiyo S (2021) Ultrasensitive electrochemiluminescence sensor based on nitrogen-decorated carbon dots for Listeria monocytogenes determination using a screen-printed carbon electrode. Biosens Bioelectron 188:113323. https://doi.org/10.1016/j.bios.2021.113323
Chen SF, Chen XQ, Zhang LJ, Gao JJ, Ma Q (2017) Electrochemiluminescence detection of Escherichia coli O157:H7 based on a novel polydopamine surface Imprinted polymer biosensor. ACS Appl Mater Inter 9(6):5430–5436. https://doi.org/10.1021/acsami.6b12455
Wang HY, Chi Z, Cong Y, Wang ZZ, Jiang F, Geng JY, Zhang P, Ju P, Dong QJ, Liu CG (2018) Development of a fluorescence assay for highly sensitive detection of Pseudomonas aeruginosa based on an aptamer-carbon dots/graphene oxide system. RSC Adv 8(57):32454–32460. https://doi.org/10.1039/C8RA04819C
Pan T, Shan XL, Jiang D, Qi L, Wang WC, Chen ZD (2021) Fluorometric aptasensor for determination of Escherichia coli O157:H7 by FRET effect between aminated carbon quantum dots and graphene oxide. Anal Sci 37(6):833–838. https://doi.org/10.2116/analsci.20P306
Yao S, Zhao C, Shang MY, Li J, Wang J (2021) Enzyme-free and label-free detection of Staphylococcus aureus based on target-inhibited fluorescence signal recovery. Food Chem Toxicol 150:112071. https://doi.org/10.1016/j.fct.2021.112071
Pebdeni AB, Hosseini M, Ganjali MR (2020) Fluorescent turn-on aptasensor of Staphylococcus aureus based on the FRET between green carbon quantum dot and gold nanoparticle. Food Anal Method 13(11):2070–2079. https://doi.org/10.1007/s12161-020-01821-4
Hu XT, Li YX, Xu YW, Gan ZY, Zou XB, Shi JY, Huang XW, Li ZH, Li YH (2021) Green one-step synthesis of carbon quantum dots from orange peel for fluorescent detection of Escherichia coli in milk. Food Chem 339:127775. https://doi.org/10.1016/j.foodchem.2020.127775
Wang RJ, Xu Y, Zhang T, Jiang Y (2015) Rapid and sensitive detection of Salmonella typhimurium using aptamer-conjugated carbon dots as fluorescence probe. Anal Method 7(5):1701–1706. https://doi.org/10.1039/C4AY02880E
Guo Z, Huang XW, Li ZH, Shi JY, Zhai XD, Hu XT, Liang NN, Zou XB (2020) Rapid and highly sensitive detection of Salmonella typhimurium in lettuce by using magnetic fluorescent nanoparticles. Anal Method 12(48):5861–5868. https://doi.org/10.1039/D0AY01744B
Bahari D, Babamiri B, Salimi A, Salimizand H (2021) Ratiometric fluorescence resonance energy transfer aptasensor for highly sensitive and selective detection of Acinetobacter baumannii bacteria in urine sample using carbon dots as optical nanoprobes. Talanta 221:121619. https://doi.org/10.1016/j.talanta.2020.121619
Ranjbar S, Shahrokhian S (2018) Design and fabrication of an electrochemical aptasensor using Au nanoparticles/carbon nanoparticles/cellulose nanofibers nanocomposite for rapid and sensitive detection of Staphylococcus aureus. Bioelectrochemistry 123:70–76. https://doi.org/10.1016/j.bioelechem.2018.04.018
Jiang D, Yang CQ, Fan YD, Polly Leung HM, Inthavong K, Zhang Y, Li ZY, Yang M (2021) Ultra-sensitive photoelectrochemical aptamer biosensor for detecting E. coli O157:H7 based on nonmetallic plasmonic two-dimensional hydrated defective tungsten oxide nanosheets coupling with nitrogen-doped graphene quantum dots (dWO3•H2O@N-GQDs). Biosens Bioelectron 183:113214. https://doi.org/10.1016/j.bios.2021.113214
Lin XF, Mei YQ, He C, Luo Y, Yang M, Kuang Y, Ma XM, Zhang HF, Huang QT (2021) Electrochemical biosensing interface based on carbon dots-Fe3O4 nanomaterial for the determination of Escherichia coli O157: H7. Front Chem 9:769648. https://doi.org/10.3389/fchem.2021.769648
Cui FC, Sun JD, de Dieu Habimana J, Yang XX, Ji J, Zhang YZ, Lei HT, Li ZJ, Zheng JY, Fan MH, Sun XL (2019) Ultrasensitive fluorometric angling determination of Staphylococcus aureus in vitro and fluorescence imaging in vivo using carbon dots with full-color emission. Anal Chem 91(22):14681–14690. https://doi.org/10.1021/acs.analchem.9b03916
Bruce JA, Clapper JC (2020) Conjugation of carboxylated graphene quantum dots with Cecropin P1 for bacterial biosensing applications. ACS Omega 5(41):26583–26591. https://doi.org/10.1021/acsomega.0c03342
Shen YZ, Wu TT, Chen HH, Ye YW, Xu JJ (2022) Ratiometric fluorescence detection of pathogenic bacteria based on dual-recognition nanoprobes with controllable G-quadruplex release. Chem Commun 58(3):447–450. https://doi.org/10.1039/D1CC05966A
Wang ZZ, Wang HY, Cheng XH, Geng JY, Wang L, Dong QJ, Liu CG, Chi ZM, Chi Z (2021) Aptamer-superparamagnetic nanoparticles capture coupling siderophore-Fe3+ scavenging actuated with carbon dots to confer an “off-on” mechanism for the ultrasensitive detection of Helicobacter pylori. Biosens Bioelectron 193:113551. https://doi.org/10.1016/j.bios.2021.113551
Gao R, Zhong ZT, Gao XM, Jia L (2018) Graphene oxide quantum dots assisted construction of fluorescent aptasensor for rapid detection of Pseudomonas aeruginosa in food samples. J Agr Food Chem 66(41):10898–10905. https://doi.org/10.1021/acs.jafc.8b02164
Savas S, Altintas Z (2019) Graphene quantum dots as nanozymes for electrochemical sensing of Yersinia enterocolitica in milk and human serum. Materials 12(13):2189. https://doi.org/10.3390/ma12132189
Shi YH, Sun YH, Qu XW, Zhou L, Yue TL, Yuan YH (2021) Preparation of species-specific monoclonal antibody and development of fluorescence immunoassay based on fluorescence resonance energy transfer of carbon dots for accurate and sensitive detection of Alicyclobacillus acidoterrestris in apple juice. Food Chem 347:129069. https://doi.org/10.1016/j.foodchem.2021.129069
Yang L, Deng WF, Cheng C, Tan YM, Xie QJ, Yao SZ (2018) Fluorescent immunoassay for the detection of pathogenic bacteria at the single-cell level using carbon dots-encapsulated breakable organosilica nanocapsule as labels. ACS Appl Mater Inter 10(4):3441–3448. https://doi.org/10.1021/acsami.7b18714
Yao S, Zhao C, Liu YS, Nie HR, Xi GL, Cao XL, Li ZL, Pang B, Li J, Wang J (2020) Colorimetric immunoassay for the detection of Staphylococcus aureus by using magnetic carbon dots and sliver nanoclusters as o-phenylenediamine-oxidase mimetics. Food Anal Method 13(4):833–838. https://doi.org/10.1007/s12161-019-01683-5
Wang ZH, Yao XL, Wang R, Ji YW, Yue TL, Sun J, Li T, Wang JL, Zhang DH (2019) Label-free strip sensor based on surface positively charged nitrogen-rich carbon nanoparticles for rapid detection of Salmonella enteritidis. Biosens Bioelectron 132:360–367. https://doi.org/10.1016/j.bios.2019.02.061
Pangajam A, Theyagarajan K, Dinakaran K (2020) Highly sensitive electrochemical detection of E. coli O157:H7 using conductive carbon dot/ZnO nanorod/PANI composite electrode. Sens Bio-Sens Res 29:100317. https://doi.org/10.1016/j.sbsr.2019.100317
Ebbensgaard A, Mordhorst H, Aarestrup FM, Hansen EB (2018) The role of outer membrane proteins and lipopolysaccharides for the sensitivity of Escherichia coli to antimicrobial peptides. Front Microbiol 9:2153. https://doi.org/10.3389/fmicb.2018.02153
MartínezPeriñán E, GarcíaMendiola T, Enebral Romero E, del Caño R, Vera Hidalgo M, Vázquez Sulleiro M, Navío C, Pariente F, Pérez EM, Lorenzo E (2021) A MoS2 platform and thionine-carbon nanodots for sensitive and selective detection of pathogens. Biosens Bioelectron 189:113375. https://doi.org/10.1016/j.bios.2021.113375
Li S, Liu JL, Chen ZT, Lu YL, Low SS, Zhu LH, Cheng C, He Y, Chen QM, Su B, Liu QJ (2019) Electrogenerated chemiluminescence on smartphone with graphene quantum dots nanocomposites for Escherichia Coli detection. Sensor Actuator B: Chem 297:126811. https://doi.org/10.1016/j.snb.2019.126811
Zheng XT, Ananthanarayanan A, Luo KQ, Chen P (2015) Glowing graphene quantum dots and carbon dots: properties, syntheses, and biological applications. Small 11(14):1620–1636. https://doi.org/10.1002/smll.201402648
Chandra S, Chowdhuri AR, Mahto TK, Samui A, Sk S (2016) One-step synthesis of amikacin modified fluorescent carbon dots for the detection of Gram-negative bacteria like Escherichia coli. RSC Adv 6(76):72471–72478. https://doi.org/10.1039/C6RA15778E
Putri FAR, Mudasir M, Morita K, Suherman S (2019) Microwave-assisted synthesis of amikacin modified N, S co-doped carbon dots for Escherichia coli detection. Chemosensors 7(4):61. https://doi.org/10.3390/chemosensors7040061
Chandra S, Mahto TK, Chowdhuri AR, Das B, Sk S (2017) One step synthesis of functionalized carbon dots for the ultrasensitive detection of Escherichia coli and iron (III). Sensor Actuator B: Chem 245:835–844. https://doi.org/10.1016/j.snb.2017.02.017
Zhong D, Zhuo Y, Feng YJ, Yang XM (2015) Employing carbon dots modified with vancomycin for assaying Gram-positive bacteria like Staphylococcus aureus. Biosens Bioelectron 74:546–553. https://doi.org/10.1016/j.bios.2015.07.015
Gao Z, Yang DZ, Wan Y, Yang YL (2020) One-step synthesis of carbon dots for selective bacterial inactivation and bacterial differentiation. Anal Bioanal Chem 412(4):871–880. https://doi.org/10.1007/s00216-019-02293-0
Wang N, Wang YT, Guo TT, Yang T, Chen ML, Wang JH (2016) Green preparation of carbon dots with papaya as carbon source for effective fluorescent sensing of iron (III) and Escherichia coli. Biosens Bioelectron 85:68–75. https://doi.org/10.1016/j.bios.2016.04.089
Lai IPJ, Harroun SG, Chen SY, Unnikrishnan B, Li YJ, Huang CC (2016) Solid-state synthesis of self-functional carbon quantum dots for detection of bacteria and tumor cells. Sensor Actuator B: Chem 228:465–470. https://doi.org/10.1016/j.snb.2016.01.062
Weng CI, Chang HT, Lin CH, Shen YW, Unnikrishnan B, Li YJ, Huang CC (2015) One-step synthesis of biofunctional carbon quantum dots for bacterial labeling. Biosens Bioelectron 68:1–6. https://doi.org/10.1016/j.bios.2014.12.028
Choi CA, Mazrad ZAI, Lee G, In I, Lee KD, Park SY (2018) Boronate-based fluorescent carbon dot for rapid and selectively bacterial sensing by luminescence off/on system. J Pharmaceut Biomed 159:1–10. https://doi.org/10.1016/j.jpba.2018.06.043
Zhang LX, Zhang ZS, Gao ZW, Xie Y, Shu S, Ke YE, Wang Y, Deng B, Yu RJ, Geng HL (2020) Facile synthesis of N, B-co-doped carbon dots with the gram-scale yield for detection of iron (III) and E. coli. Nanotechnology 31(39):395702. https://doi.org/10.1088/1361-6528/ab9b4c
Wang JL, Teng JY, Jia T, Shu Y (2018) Detection of yeast Saccharomyces cerevisiae with ionic liquid mediated carbon dots. Talanta 178:818–824. https://doi.org/10.1016/j.talanta.2017.10.029
Kumari M, Chaudhary S (2020) Modulating the physicochemical and biological properties of carbon dots synthesised from plastic waste for effective sensing of E. coli. Colloid Surface B 196:111333. https://doi.org/10.1016/j.colsurfb.2020.111333
Jo HJ, Robby AI, Kim SG, Lee G, Lee BC, Park SY (2021) Reusable biosensor-based polymer dot-coated electrode surface for wireless detection of bacterial contamination. Sensor Actuator B: Chem 346:130503. https://doi.org/10.1016/j.snb.2021.130503
Mazrad ZAI, Choi CA, Kwon YM, In I, Lee KD, Park SY (2017) Design of surface-coatable NIR-responsive fluorescent nanoparticles with PEI passivation for bacterial detection and killing. ACS Appl Mater Inter 9(38):33317–33326. https://doi.org/10.1021/acsami.7b10688
Pathak A, Venugopal P, Nair BG, Suneesh PV, SatheeshBabu TG (2020) Facile pH-sensitive optical detection of pathogenic bacteria and cell imaging using multi-emissive nitrogen-doped carbon dots. MicroChem J 159:105324. https://doi.org/10.1016/j.microc.2020.105324
Suherman S, Audio Haryanto N, Tri Wahyuni E, Ilmi M, Morita K, Oki Y (2019) Carbon dots modification for Escherichia coli detection: variation of colistin sulphate concentration. Orient J Chem 35(1):49–55. https://doi.org/10.13005/ojc/350105
Nandi S, Ritenberg M, Jelinek R (2015) Bacterial detection with amphiphilic carbon dots. Analyst 140(12):4232–4237. https://doi.org/10.1039/C5AN00471C
Zhao WB, Wang RT, Liu KK, Du MR, Wang Y, Wang YQ, Zhou R, Liang YC, Ma RN, Sui LZ, Lou Q, Hou L, Shan CX (2021) Near-infrared carbon nanodots for effective identification and inactivation of Gram-positive bacteria. Nano Res 15(3):1699–1708. https://doi.org/10.1007/s12274-021-3818-9
Yang JJ, Zhang XD, Ma YH, Gao G, Chen XK, Jia HR, Li YH, Chen Z, Wu FG (2016) Carbon dot-based platform for simultaneous bacterial distinguishment and antibacterial applications. ACS Appl Mater Inter 8(47):32170–32181. https://doi.org/10.1021/acsami.6b10398
Yang JJ, Gao G, Zhang XD, Ma YH, Chen XK, Wu FG (2019) One-step synthesis of carbon dots with bacterial contact-enhanced fluorescence emission: fast Gram-type identification and selective Gram-positive bacterial inactivation. Carbon 146:827–839. https://doi.org/10.1016/j.carbon.2019.02.040
Fu L, Chen QM, Jia L (2022) Carbon dots and gold nanoclusters assisted construction of a ratiometric fluorescent biosensor for detection of Gram-negative bacteria. Food Chem 374:131750. https://doi.org/10.1016/j.foodchem.2021.131750
Hiremath SD, Bhosle AA, Nayse A, Biswas S, Biswas M, Bhasikuttan AC, Banerjee M, Chatterjee A (2021) A redox-coupled carbon dots-MnO2 nanosheets based sensory platform for label-free and sensitive detection of E. coli. Sensor Actuator B: Chem 339:129918. https://doi.org/10.1016/j.snb.2021.129918
Bhattacharya S, Nandi S, Jelinek R (2017) Carbon-dot–hydrogel for enzyme-mediated bacterial detection. RSC Adv 7(2):588–594. https://doi.org/10.1039/C6RA25148J
Zhao MY, Gao X, Tao ZH, Wang XK, Lin XD, Wang S, Liu YQ (2020) Sugar-metabolism-triggered pathogenic bacteria identification based on pH-sensitive fluorescent carbon dots. Sensor Actuator B: Chem 316:128063. https://doi.org/10.1016/j.snb.2020.128063
Yang QL, Farooq U, Chen W, Ullah MW, Wang SQ (2019) Fluorimetric detection of single pathogenic bacterium in milk and sewage water using pH-sensitive fluorescent carbon dots and MALDI-TOF MS. Microorganisms 8(1):53. https://doi.org/10.3390/microorganisms8010053
Das R, Singh N (2019) Exploring electrochemistry of carbon nanodots and its application in noninvasive bacterial growth monitoring. Biosens Bioelectron 144:111640. https://doi.org/10.1016/j.bios.2019.111640
Chen YX, Jiang SM, Hu YC, Gong JM (2021) Enhanced electrochemiluminescence bioassay triggered by intracellular leakage for detection of Escherichia coli. Biosens Bioelectron 194:113575. https://doi.org/10.1016/j.bios.2021.113575
Liu WJ, Gu H, Liu WK, Lv CY, Du JJ, Fan JL, Peng XJ (2022) NIR-emitting carbon dots for discriminative imaging and photo-inactivation of pathogenic bacteria. Chem Eng J 450:137384. https://doi.org/10.1016/j.cej.2022.137384
Zhao B, Cui FY, Xu Y, Zhang XF, Li ZQ (2017) Study on interaction between mannose and bacteria using glycosylated carbon quantum dots. J Instrum Anal 36(11):1318–1324. https://doi.org/10.3969/j.issn.1004-4957.2017.11.005
Ahmadian-Fard-Fini S, Ghanbari D, Amiri O, Salavati-Niasari M (2021) Green sonochemistry assisted synthesis of hollow magnetic and photoluminescent MgFe2O4-carbon dot nanocomposite as a sensor for toxic Ni(II), Cd(II) and Hg(II) ions and bacteria. RSC Adv 11(37):22805–22811. https://doi.org/10.1039/D1RA02458B
Robby AI, Kim SG, Lee UH, In I, Lee G, Park SY (2021) Wireless electrochemical and luminescent detection of bacteria based on surface-coated CsWO3-immobilized fluorescent carbon dots with photothermal ablation of bacteria. Chem Eng J 403:126351. https://doi.org/10.1016/j.cej.2020.126351
Ahmadian-Fard-Fini S, Ghanbari D, Salavati Niasari M (2019) Photoluminescence carbon dot as a sensor for detecting of Pseudomonas aeruginosa bacteria: hydrothermal synthesis of magnetic hollow NiFe2O4-carbon dots nanocomposite material. Compos Part B: Eng 161:564–577. https://doi.org/10.1016/j.compositesb.2018.12.131
Ahmadian-Fard-Fini S, Salavati Niasari M, Ghanbari D (2018) Hydrothermal green synthesis of magnetic Fe3O4-carbon dots by lemon and grape fruit extracts and as a photoluminescence sensor for detecting of E. coli bacteria. Spectrochim Acta Part A 203:481–493. https://doi.org/10.1016/j.saa.2018.06.021
Roh SG, Robby AI, Phuong PTM, In I, Park SY (2019) Photoluminescence-tunable fluorescent carbon dots-deposited silver nanoparticle for detection and killing of bacteria. Mat Sci Eng C 97:613–623. https://doi.org/10.1016/j.msec.2018.12.070
Jia HR, Zhu YX, Chen Z, Wu FG (2017) Cholesterol-assisted bacterial cell surface engineering for photodynamic inactivation of Gram-positive and Gram-negative bacteria. ACS Appl Mater Interfaces 9:15943–15951. https://doi.org/10.1021/acsami.7b02562
Chu XH, Wu F, Sun BH, Zhang M, Song SJ, Zhang P, Wang YL, Zhang QC, Zhou NL, Shen J (2020) Genipin cross-linked carbon dots for antimicrobial, bioimaging and bacterial discrimination. Colloid Surface B 190:110930. https://doi.org/10.1016/j.colsurfb.2020.110930
Zhou CC, Xu WH, Zhang PB, Jiang MJ, Chen YC, Kwok RTK, Lee MMS, Shan GG, Qi RL, Zhou X, Lam JWY, Wang S, Tang BZ (2019) Engineering sensor arrays using aggregation-induced emission luminogens for pathogen identification. Adv Funct Mater 29(4):1805986. https://doi.org/10.1002/adfm.201805986
Yan CR, Wang CL, Hou TT, Guan P, Qiao YB, Guo LL, Teng YG, Hu XL, Wu H (2021) Lasting tracking and rapid discrimination of live Gram-positive bacteria by peptidoglycan-targeting carbon quantum dots. ACS Appl Mater Inter 13(1):1277–1287. https://doi.org/10.1021/acsami.0c19651
Bhaisare ML, Gedda G, Khan MS, Wu HF (2016) Fluorimetric detection of pathogenic bacteria using magnetic carbon dots. Anal Chim Acta 920:63–71. https://doi.org/10.1016/j.aca.2016.02.025
Yan R, Shou ZX, Chen J, Wu H, Zhao Y, Qiu L, Jiang PJ, Mou XZ, Wang JH, Li YQ (2018) On-off-on gold nanocluster-based fluorescent probe for rapid Escherichia coli differentiation, detection and bactericide screening. ACS Sustainable Chem Eng 6(4):4504–4509. https://doi.org/10.1021/acssuschemeng.8b00112
Wang XH, Shan MY, Zhang SK, Chen X, Liu WT, Chen JZ, Liu XY (2022) Stimuli-responsive antibacterial materials: molecular structures, design principles, and biomedical applications. Adv Sci 9(13):2104843. https://doi.org/10.1002/advs.202104843
Zheng LB, Qi P, Zhang D (2019) Identification of bacteria by a fluorescence sensor array based on three kinds of receptors functionalized carbon dots. Sensor Actuator B: Chem 286:206–213. https://doi.org/10.1016/j.snb.2019.01.147
Wang SJ, Zhang YQ, Zhuo P, Hu QS, Chen ZQ, Zhou L (2020) Identification of eight pathogenic microorganisms by single concentration-dependent multicolor carbon dots. J Mater Chem B 8(27):5877–5882. https://doi.org/10.1039/D0TB00834F
Shauloff N, Morag A, Yaniv K, Singh S, Malishev R, Paz-Tal O, Rokach L, Jelinek R (2021) Sniffing bacteria with a carbon-dot artificial nose. Nano-Micro Lett 13(1):112. https://doi.org/10.1007/s40820-021-00610-w
Funding
This work was financially supported by the Open Project of Priority Disciplinary Construction at Fujian Agriculture and Forestry University (722022003) and Natural Science Foundation of Fujian Province of China (No. 2023J01453).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, L., Fang, M., Liu, W. et al. Recent advances and perspectives of functionalized carbon dots in bacteria sensing. Microchim Acta 190, 363 (2023). https://doi.org/10.1007/s00604-023-05938-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-023-05938-1