Abstract
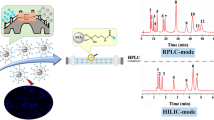
Facing the trends of green chemistry, this work tries to find a novel material for per aqueous liquid chromatography (PALC) aiming to reduce the consumption of hazardous reagents. As a kind of green nanomaterials, the chromatographic performance of carbon quantum dots (CQDs) in PALC was rarely studied. Here, hydrophilic CQDs were prepared by a simple hydrothermal method using citric acid and ethylenediamine as carbon sources. The synthesized CQDs with functional groups of amino, carboxyl, and hydroxyl were decorated on silica gels forming a novel Si-CQDs stationary phase. This Si-CQDs column possesses the typical retention feature of PALC. Compounds with different polarities including hydrophobic pesticides, polar sulfonamides, β-adrenoceptor blockers and agonists, as well as hydrophilic nucleosides and bases obtained satisfactory separation on this Si-CQDs column under PALC mode, even better resolution than in hydrophilic interaction liquid chromatography (HILIC) mode. A mixture of four sulfonamides can be separated within 6 min using a mobile phase containing only 5% acetonitrile, and the resolution achieves 2.39, 2.13, and 1.83 with an average column efficiency of 1400. For certain compounds, this Si-CQDs column showed better separation performance than commercial SiO2 column, NH2 column, and C18 column. The retention mechanism includes hydrophobic and electrostatic interactions due to the multifunctional groups of CQDs. This Si-CQDs column achieved the rapid detection of residual sulfonamides in milk with simplified sample pretreatment process and the detection of atenolol in commercial atenolol tablets. The developed Si-CQDs column has great prospects in low-cost and environmentally friendly separation and analysis.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
High-performance liquid chromatography (HPLC) has been widely used in pharmaceutical analysis, food analysis, environmental analysis, etc. The wide use of HPLC also results in a large number of organic solvent wastes. The consumption of organic solvents, especially toxic and dangerous chemicals, is a worldwide problem that has significant influence on human health and environment. It is a wake-up call for chemical industry including all laboratories that innovations are needed to reduce the use of hazardous chemicals. Undoubtedly, “green” HPLC using nontoxic mobile phases becomes an important issue and the hot topic [1,2,3].
Per aqueous liquid chromatography (PALC) named by Sandra et al. is a kind of green LC mode, in which mobile phases containing high percentage of water (70–100%) are used [4]. PALC is a complementary separation mode for reversed-phase liquid chromatography (RPLC) and is expected to be an alternative to hydrophilic interaction chromatography (HILIC) since a comparable retention as HILIC for polar compounds can be achieved under PALC [5,6,7]. PALC is a relatively new technique, covering the last decade. Special stationary phases capable of withstanding high percentage of water are needed in PALC. According to our best knowledge, there are limited types of stationary phases for PALC which is far less than that in RPLC and HILIC. So far, polysaccharide-modified silica [6, 7], ionic liquid bridged periodic mesoporous organosilicas [8], ethylene bridged hybrid silica [9], carbon nanoparticles-functionalized silica [10], congo red-derivatized silica [11], porous organic cage embedded C18 amide silica [12], and Au nanoparticles-decorated SO3H-cofunctionalized silica [13] stationary phases have been developed for PALC. Research into the synthesis of novel stationary phases with applicability in PALC and separation of polar and hydrophilic compounds will be the main issue of this method [1].
Development of advanced functional materials provides more choices for the construction of PALC stationary phases. Carbon quantum dots (CQDs) are a member of carbon nanomaterials discovered in 2004 [14]. Due to the simple and green synthesis method, low toxicity, good dispersibility, adsorption performance, and photoluminescence stability, CQDs have received great attention in various research fields such as biological imaging, fluorescence and phosphorescent sensing, photovoltaic devices, anti-counterfeiting, and encryption [15, 16]. CQDs also presented good separation performance in HILIC, RPLC, and mixed-mode chromatography [17]. However, the separation performance and application potential of CQDs in PALC has been scarcely explored.
Here, CQDs were modified on silica gels forming a novel CQDs-functionalized silica (Si-CQDs) stationary phase. Using CQDs as ligands achieves the one-step modification of multiple functional groups including –NH2, –OH, –COOH, and aromatic skeleton onto silica gels, which is simple. The retention behavior and separation performance of this Si-CQDs stationary phase under PALC mode was explored using nucleosides and bases, β-adrenoceptor blockers and agonists, pesticides, and sulfonamides as analytes. Its separation performance was compared with commercial C18 column, NH2 column, and SiO2 column. While maintaining the good separation efficiency as commercial columns, the use of organic solvents can be reduced greatly, indicating the developed Si-CQDs column may be a good choice for low-cost and environmentally friendly separation and analysis.
Experimental
Reagents
Silica gels (100 Å, 5 μm) were supplied by Fuji Silysia Chemical Ltd. (Japan). 3-Glycidoxypropyltrimethoxysilane (analytical grade, 98%), citric acid (analytical grade, 99%), ethylenediamine (analytical grade, 99%), formic acid (analytical grade, 98%), ammonium acetate (analytical grade, 98%), and standard chemicals (HPLC grade) were purchased from Meryer Chemical Technology Co. Ltd. (Shanghai, China). Methanol and acetonitrile (HPLC grade) were obtained from Macklin Biochemical Technology Co. Ltd. (Shanghai, China). Commercial Purospher STAR RP-18 endcapped Hibar RT (5 μm, 150 mm × 4.6 mm i.d.), Purospher STAR NH2 LiChroCART (5 μm, 150 mm × 4.6 mm i.d.), and Ascentis® Si HPLC 581,512-U (5 μm, 150 mm × 4.6 mm i.d.) columns were used as a comparison.
Instruments
The Fourier transform infrared spectroscopy (FTIR) spectra were acquired on a Thermo Scientific Nicolet iS5 Fourier transform infrared spectrometer (Thermo, USA). Transmission electron microscope (TEM) images were obtained on a JEOL JEM-2100F field emission transmission electron microscope (JEOL, Japan). Laser scanning confocal microscope (LSCM) images were obtained on a FLUOVIEW FV1200 biological confocal laser scanning microscope (OLYMPUS, Japan). Surface area and pore size analysis were carried on a NOVA 2200e surface analysis instrument (Quantachrome, USA). X-ray crystal diffraction (XRD) was performed on D8 ADVANCE diffractometer (Bruker AXS, Germany). X-ray photoelectron spectroscopy (XPS) was recorded on a VG ESCALAB 210 instrument (Thermo, USA). Thermogravimetric analysis was carried on a METTLER TOLEDO TGA2 instrument (METTLER TOLEDO, Switzerland). Elemental analysis was performed on vario EL apparatus (Elementar, Germany). Zeta potential values were obtained on Zetasizer Nano ZSE nanoparticle size analyzer (Malvern, Britain). Chromatographic analysis was performed on a HITACHI Primaide HPLC system equipped with a manual injector with 20 μL sample loop, a 1310 column oven, a 1110 pump, and a 1430 diode array detector (HITACHI, Japan).
Synthesis of CQDs
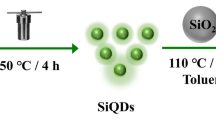
CQDs were synthesized by a simple hydrothermal method. A total of 1.0g citric acid was dissolved in 60 mL deionized water, and then 200 μL ethylenediamine was added into above solution. The mixture was transferred to a Teflon-sealed autoclave and heated at 200℃ for 4 h. After cooling to room temperature, the transparent yellow solution was dialyzed for 3 days. Finally, CQDs were obtained by freeze drying.
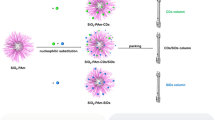
Preparation of Si-CQDs stationary phase
CQDs were bonded onto silica gels using 3-glycidoxypropyltrimethoxysilane as spacer referred to previous reports [18, 19]. For a schematic diagram of the preparation process, please see Fig. 1. The first step was to prepare epoxy groups modified silica gels (Si-epoxy). A total of 3.0g silica gels were dispersed in 75 mL anhydrous toluene, and then 1.5 mL 3-glycidoxypropyltrimethoxysilane was added into above dispersion system and heated at 100℃ for 24 h under stirring at 700 rpm. After cooling to room temperature, the products were washed using toluene, ethanol, and methanol in turn through filtration. The Si-epoxy particles were dried at 60℃ under vacuum. The second step was to prepare Si-CQDs stationary phase. In total, 0.25g CQDs, 2.5g Si-epoxy, and 100 μL triethylamine were dispersed in 100 mL methanol under stirring at 600 rpm, and the dispersion was heated at 80℃ for 12 h. The products were washed by methanol and deionized water repeatedly. Finally, the Si-CQDs stationary phase was acquired by vacuum drying at 60℃.
Column packing
The Si-CQDs particles were packed into stainless steel tubes (150 mm × 4.6 mm i.d.) at 40 MPa by a slurry packing method. Equal volume of methanol and isopropyl alcohol mixture were used as homogenate solvent and methanol was used as propulsion solvent.
Treatment of real samples
Milk called Modern Meadow was purchased from local supermarket (RT Mart, Qingdao, China). The milk was treated by a simple method without purifying through solid phase extraction column. A 5 mL milk was transferred into a 50 mL plastic centrifugal pipe, then 20 mL acetonitrile containing 0.1% formic acid was added, and the mixture was vortex-mixed for 5 min and centrifuged at 8000 rpm for 5 min. The supernatant was filtered through a filter membrane (0.22 μm), and the final volume of extract was regulated to 25 mL by acidified acetonitrile. Stock solutions of individual sulfonamides at 0.1 g L−1 were prepared by dissolving 10 mg of each sulfonamide in 100 mL of acetonitrile containing 0.1% formic acid. A total of 1.0 mL of the above solutions was mixed and further diluted to 100 mL with acidified acetonitrile to obtain standard solution of sulfonamides mixture at the concentration of 1.0 mg L−1. Milk samples spiked with sulfonamides at different concentrations were obtained by adding appropriate volumes of standard solution of sulfonamides mixture into milk.
Atenolol tablets produced by Jiangsu Huanghe Pharmaceutical Co., Ltd. (China) were purchased from Qingdao Tongfang pharmacy. Ten tablets were crushed by mortar, and 20 mg of the powder was dispersed in 200 mL methanol under ultrasonic for 30 min. Finally, the sample was filtered through nylon filter membrane (0.22 μm) to obtain the stock solution at the concentration of 0.1 g L−1. The stock solution was diluted to 25 μg L−1 by methanol for HPLC analysis.
Chromatographic parameters
The formulas of chromatographic parameters such as retention time (tR), retention factor (k), resolution (Rs), column efficiency (N), and asymmetry factor (As) are listed in Supporting Information (Eq. S1-S9). Dead time was determined from the solvent peak of methanol.
Results and discussion
Characterizations of CQDs and Si-CQDs stationary phase
The morphology of as-synthesized CQDs was characterized by TEM (Fig. 2a), from which we saw that CQDs were monodisperse spherical particles. The dimensional statistics was acquired by measuring one hundred of particles in the TEMs; the sizes of CQDs were in the range of 1.7–3.1 nm with an average size of 2.3 nm. The lattice spacing of CQDs was 0.23 nm (see the inset of Fig. 2a), which was consistent with that of (100) facet of graphite [20]. The XRD pattern measured for the CQDs showed a broad peak centered at 20.6° (Fig. 3a), which was consistent with the (002) lattice spacing of carbon-based materials [21]. Comparing the TEM images of Si-epoxy (Fig. 2b) and Si-CQDs (Fig. 2c), the surface of silica gels become rough with adhered particles after modification of CQDs. The LSCM image of Si-CQDs (Fig. 2d) also showed bright blue fluorescence, indicating the successful modification of CQDs onto silica gels.
The specific surface area and pore size were tested before and after modification of CQDs on silica gels. After modification of CQDs onto Si-epoxy, the specific surface area increased from 294.368 to 347.587 m2/g, displaying the abundant adsorption sites of CQDs. The pore size decreased by about 2.2 nm (from 10.131 to 7.966 nm) which was in accord with the size of CQDs, demonstrating CQDs were not only decorated on the surface of silica gels, but also entered into the channels of porous silica gels. The loading amount of CQDs on silica gels was tested by thermogravimetric analysis. Comparing the thermogravimetric curves of Si-epoxy and Si-CQDs depicted in Fig. S1, the loading amount of CQDs was about 2%, meaning the content of CQDs on silica gels was about 20 mg/g. No significant weight loss was observed until the temperature exceeds 300℃, indicating the good thermostability of Si-CQDs stationary phase. Elemental analysis (Table S1) was also carried out to investigate the loading of CQDs on silica gels. The content of C% and N% increased after modification of CQDs onto Si-epoxy, which again indicated the successfully preparation of Si-CQDs stationary phase.
The chemical composition of CQDs was characterized by FTIR and XPS. XPS survey (Fig. 3b) shows the predominant elements of the prepared CQDs are C, O, and N. The high-resolution C 1 s spectra (Fig. 3c) can be divided into three components, corresponding to the signals of C–C (284.7 eV), C–O (286.0 eV), and C = O (287.5 eV). The high-resolution O 1 s spectra (Fig. 3d) can be divided into three components, corresponding to quinine O (530.8 eV), C = O (532.1 eV), and C–O (532.5 eV). The high-resolution N 1 s spectra (Fig. 3e) can be divided into two components, corresponding to pyridine N (401.5 eV) and amide N (399.9 eV). The results are consistent with the data previously reported [22,23,24]. The FTIR spectrum of CQDs shows the characteristic peak of N–H and O–H located at 3424 cm−1 and 3228 cm−1, respectively. The peaks located at 1702 cm−1, 1658 cm−1, and 1426 cm−1 can be assigned to the stretching vibration of C = O in COOH, HNCO, and COO−. The peak at 1138 cm−1 can be attributed to the stretching vibration of C–O–C. According to above discussion, we roughly conclude that CQDs have functional groups of amino, hydroxyl, amide, epoxy/ether, and carboxyl groups. The FTIR spectrum of Si-CQDs shows the characteristic peaks of carboxyl and amide groups, demonstrating the successful decoration of CQDs onto silica gels.
Chromatographic performance of Si-CQDs stationary phase
Retention behavior under PALC
The retention behavior of this Si-CQDs column with the variation of water content in mobile phase was explored. Figure 4 shows the variation of log k with the increase of water content from 50 to 100% in mobile phases. The retention time of all the tested compounds increased slightly as the water content increased from 50 to 85% and then increased dramatically when the water content further increased to 100%, exhibiting typical PALC retention behaviors [6, 10]. It seems that the retention time is extremely sensitive to any small variation of acetonitrile content in water-rich mobiles phases. This may be because acetonitrile acts as strong eluent in water-rich mobile phase system and small changes of acetonitrile content could lead to the drastic composition change of the adsorbed eluent layer onto the stationary phase and have a crucial impact on retention especially when the acetonitrile content is less than 10%.
The influence of temperature on retention was investigated using mefenamic acid, ranolazine, and labetalol hydrochloride as probes. The chromatographic process is a consecutive distribution process of solutes between the mobile phase and stationary phase when they move through a column. The equilibrium distribution constant of solute molecules between stationary phase and mobile phase is related to thermodynamic parameters including standard free energy (ΔG), enthalpy (ΔH), and entropy (ΔS). The thermodynamic variables can be obtained from the temperature-dependent retention data via the van’t Hoff equation (Eq. S5) [25]. Figure S2 shows the dependence of ln k and 1/T, and we see the ln k of solutes has good liner relationship with 1/T, which is in accord with the van’t Hoff equation. The retention time decreased when the temperature increased. The thermodynamic parameters obtained from the slope and intercept of the van’t Hoff plots are listed in Table S2. In the temperature range of 20–60℃, the ΔG for the three analytes retained on the Si-CQDs column are negative values, indicating the interaction between analytes and stationary phase is spontaneous. The ΔH are negative values, demonstrating the interaction process between analytes and stationary phase is exothermic. Kara et al. propose that when ΔH is less than 40 kJ mol−1, it should be a physical adsorption process [26]. In this case, all ΔH values are less than 40 kJ mol−1, meaning the retention of compounds on Si-CQDs stationary phase is a physical adsorption process. The ΔS values are also negative, meaning the interaction between compounds and CQDs results in reduced degrees of freedom. ΔH and ΔS can reveal intermolecular interactions empirically. When ΔH < 0, ΔS < 0, as in this case, indicating the van der Waals force and hydrogen bond interaction play a major role on the retention of Si-CQDs column [27, 28].
The effect of ionic strength on retention was explored. Naproxen, mefenamic acid, ranolazine, and labetalol hydrochloride were chosen as test compounds under the mobile phase of 90% ammonium acetate/10% acetonitrile. Figure S3 shows the variation of log k with the change of concentrations of ammonium acetate from 10 to 100 mM in mobile phases. The tested compounds have different retention behaviors with the variation of concentrations of ammonium acetate, meaning the effect of ionic strength on retention is not constant, which is related to the properties of analytes. The retention of naproxen and mefenamic acid increased with the enhance of ionic strength, while it decreased for ranolazine and labetalol hydrochloride. Naproxen and mefenamic acid are acid compounds with pKa of 4.3 and 4.2, respectively. Under the neutral mobile phase, they were negatively charged and formed electrostatic repulsion interaction with COO− in CQDs. While ranolazine and labetalol hydrochloride were positively charged due to the protonation of NH2, they thus formed electrostatic attraction with COO− in CQDs. The increase of ionic strength can weaken the electrostatic interaction between analytes and stationary phase. With the increase of salt concentration in mobile phase, the electrostatic repulsion force between naproxen (and mefenamic acid) and CQDs was reduced, thus their retention increased; the electrostatic attraction interaction between ranolazine (and labetalol hydrochloride) and CQDs was reduced, and thus their retention decreased.
The influence of pH on retention was investigated. The pH of mobile phase influences the surface charge status of both stationary phase and solutes, further influencing the electrostatic interaction and thus the retention time. Selecting naproxen, mefenamic acid, ranolazine, and labetalol hydrochloride as probes, the variation of log k with pH in the range of 3.0–7.4 was explored. As shown in Fig. S4, naproxen and mefenamic acid had similar retention behavior with the variation of pH, they achieved the strongest retention at pH of 4.0, and then the retention decreased continuously with the increase of pH. The retention of ranolazine and labetalol hydrochloride increased continuously with the increase of pH. Since –NH2 and –COOH co-existed in the surface of CQDs, zeta potential was tested to figure out the charge state of CQDs. The zeta potential values of CQDs in the pH range of 3.0–7.0 are listed in Table S3. The charge of CQDs changes from positive to negative at a turning point of pH 5.0. At the pH of 3.0 and 4.0, positive zeta potential values are obtained, meaning CQDs are positively charged and the dissociated naproxen and mefenamic acid will form electrostatic attraction interaction with CQDs. With the pH increases from 3.0 to 4.0, the dissociation degree of naproxen and mefenamic acid increases, thus forming stronger electrostatic attraction interaction and achieve stronger retention at pH 4.0. While at the pH of 5.0, 6.0, and 7.0, negative zeta potential values of CQDs are obtained, meaning CQDs are negatively charged. Dissociated naproxen and mefenamic acid form electrostatic repulsion interaction with negatively charged CQDs; thus their retention decreases continuously with the pH increase from 4.0 to 7.0. Ranolazine and labetalol hydrochloride are strong alkaline compounds and are protonated in the tested pH range. With the increase of pH, the electrostatic repulsion force decreases and the electrostatic attraction interaction enhances; thus their retention increases continuously with the pH increases from 3.0 to 7.0. The existence of electrostatic interaction well explains the retention behavior of the four drugs on the Si-CQDs column with the change of ionic strength and pH.
Separation performance under PALC
Some hydrophobic, polar, and hydrophilic compounds including pesticides, β-adrenoceptor blockers and agonists, sulfonamides, nucleosides, and bases are chosen as probes to evaluate the separation performance of this Si-CQDs column under PALC and HILIC modes. The chromatograms are shown in Fig. 5; we are surprised to find that the developed Si-CQDs column not only has separation ability for various kinds of compounds under PALC mode, but also shows better separation performance than that in HILIC mode. Within the same analysis time, the same analytes achieved better separation under PALC mode than in HILIC mode, highlighting the unique superiority and huge prospects of CQDs in PALC.
Chromatograms of nucleosides (a), β-adrenoceptor blockers and agonists (b), pesticides (c), and sulfonamides (d) on Si-CQDs column under PALC and HILIC modes. Analytes: (a) (1) uridine, (2) inosine, (3) xanthine, (4) guanosine, (5) adenine; (b) (1) formoterol, (2) nebivolol, (3) carazolol, (4) bambuterol, (5) sotalol, (6) acebutolol, (7) oxprenolol, (8) clenbuterol, (9) dopamine; (c) (1) metalaxyl, (2) triadimenol, (3) hexaconazole, (4) diniconazole; (d) (1) sulfadoxine, (2) sulfanilamide, (3) sulfapyridine, (4) sulfathiazole; mobile phase: (a) ACN/H2O (v/v), PALC: 0/100, HILIC: 75/25; (b) ACN/20-mM ammonium acetate (v/v), PALC: 10/90, HILIC: 87/13; (c) ACN/20-mM ammonium acetate (v/v), PALC: 10/90, HILIC: 95/5; (d) ACN/20-mM ammonium acetate (v/v), PALC: 5/95, HILIC: 95/5; flow rate: 1.0 mL min−1; injection volume: 20 μL; UV detection wavelength: 254 nm
The peak shape in PALC and HILIC modes was studied using sulfathiazole and adenine as probes. Chromatographic parameters including tR, k, N, and As are listed in Table S4. Under similar tR, more serious peak tailing is found in PALC mode, which may be due to the stronger adsorption interaction under PALC since most hydrophobic adsorption sites cannot be occupied by acetonitrile. We also should keep in mind that too high a water concentration can seriously affect the symmetry of the peaks because of overloading effects [29]. In fact, higher N can be achieved under PALC when k is below 2.0; however, with the prolong of tR, more and more serious peak tailing leads to the decline of N [5].
Retention mechanism
To understand the retention mechanism of this novel Si-CQDs column, relationship between k and lipophilicity expressed as octanol–water partition coefficient at the pH 7.4 (log D7.4) was established by introducing the positively and negatively charged molecular fractions as additional parameters. Log kw values were determined using 100% aqueous mobile phase. The log D7.4 data and the corresponding fractions of positive (F+), negatively (F−) are taken from refs. [30, 31] and are listed in Table S5. From the obtained regression equation (Eq. 1), it can be seen both F+ and F− terms are positive values, reflecting the increase of retention in the presence of charged species, which confirms the contribution of electrostatic interactions to retention.
To further understand the retention interactions of this Si-CQDs column, linear solvation energy relationship (LSER) model was established. The principle of LSER for understanding the retention mechanism is based on the equation listed below [32,33,34].
In this equation, capital letters represent the solute descriptors, while lower case letters represent the system constants. k is the retention factor. E is the excess molar refraction and models polarizability contributions from n and π electrons. S is the solute dipolarity/polarizability. A and B are the overall hydrogen-bond acidity and basicity of solute. V is the McGowan characteristic volume. D− and D+ represent the negatively charged and positively charged ionic compounds, respectively. c is the model intercept term and is not assigned any chemical significance.
Here, 27 model compounds were employed to construct the LSER model under PALC mode using 10% methanol/20-mM ammonium acetate as mobile phase. Solute descriptors obtained from references [32,33,34,35] and log k data are listed in Table S6. The obtained LSER equation (Eq. 3) is listed below.
If the value of a system constant is positive, it indicates that solutes are more likely retained in the stationary phase. In this chromatographic system, large and positive system constants of e, v, and D+ are obtained, meaning the corresponding interactions play significant role on retention. The e system constant presents the contribution of π-π interaction on retention [36]. In this case, the e system constant is a positive value, indicating the stationary phase forms stronger π-π interaction with analytes. The π-π retention interaction may come from the aromatic skeleton of CQDs. The large and positive v system constant represents the contribution of hydrophobic interaction on retention [36]. The d system constant is a measure of the electrostatic interaction between stationary phase and solutes. In this case, a positive d+ and a negative d− is obtained, indicating the Si-CQDs stationary phase forms strong electrostatic attraction interaction with positively charged solutes and electrostatic repulsion interaction with negatively charged solutes. The conclusion drawn from LSER is in accord with the practical retention behavior of this Si-CQDs column. After investigating the retention mechanism of this Si-CQDs column, it can be concluded that hydrophobic and electrostatic interactions make great contributions on retention.
Comparison with commercial columns
The separation performance of this Si-CQDs column was compared with popular HILIC column including SiO2 column and NH2 column as well as RPLC C18 column. The compared chromatograms are shown in Fig. 6, and chromatographic parameters including k, Rs, and N are listed in Table S7. For the separation of hydrophilic nucleosides and bases (Fig. 6a), C18 column presented relatively weak separation ability due to the inadequate retention of hydrophilic compounds. Nucleosides and bases which should be separated well on NH2 column in HILIC mode according to previous experience [37] were not completely separated under the PALC mode. The SiO2 column also did not show the good separation performance under PALC mode. Under the same mobile phase condition of PALC, the Si-CQDs column showed better separation performance for the tested hydrophilic compounds.
Chromatograms of nucleosides (a), β-adrenoceptor blockers and agonists (b), pesticides (c), and sulfonamides (d) on Si-CQDs column, commercial SiO2 column, commercial NH2 column, and commercial C18 column. Analytes: (a) (1) uridine, (2) inosine, (3) xanthine, (4) guanosine, (5) adenine; (b) (1) formoterol, (2) nebivolol, (3) carazolol, (4) bambuterol, (5) sotalol, (6) acebutolol, (7) oxprenolol, (8) clenbuterol, (9) dopamine; (c) (1) metalaxyl, (2) triadimenol, (3) hexaconazole, (4) diniconazole; (d) (1) sulfadoxine, (2) sulfanilamide, (3) sulfapyridine (4) sulfathiazole; mobile phase: (a) ACN/H2O (v/v), Si-CQDs column, SiO2 column, NH2 column: 0/100, C18 column: 5/95; (b) ACN/20-mM ammonium acetate (v/v), Si-CQDs column, SiO2 column, NH2 column: 10/90, C18 column: 25/75; (c) ACN/20-mM ammonium acetate (v/v), Si-CQDs column, SiO2 column, NH2 column: 10/90, C18 column: 60/40; (d) ACN/20-mM ammonium acetate (v/v), Si-CQDs column and SiO2 column: 5/95, NH2 column: 10/90, C18 column: 20/80; glow rate: 1.0 mL min−1; injection volume: 20 μL; UV detection wavelength: 254 nm
For the separation of β-adrenoceptor blockers and agonists (Fig. 6b), the Si-CQDs column still presented the best separation performance. The 9 medicines were not completely separated on C18 column, occurred with leading peak and tailing peak for specific compounds. Moreover, it needed higher percentage of acetonitrile in mobile phase for the separation of these medicines due to the strong hydrophobicity of C18 column. Under the same mobile phase condition as Si-CQDs column, the 9 medicines had very weak retention and were co-eluted on NH2 column, and this might be due to the electrostatic repulsion interaction between protonated amino groups in these alkaline drugs and stationary phase. The 9 medicines were not separated well on SiO2 column with the occurrence of peak tailing phenomenon, which might be due to the reason that pure SiO2 column is not proper for separating alkaline compounds [38].
For the separation of pesticides and sulfonamides (Fig. 6c, d), C18 column showed the best separation performance with the best peak shape, but the separation was not under green LC mode since mobile phase needed more acetonitrile. Under the same mobile phase condition of PALC, the four pesticides achieved better separation on Si-CQDs column than SiO2 column and NH2 column. Four sulfonamides obtained relatively good separation on all the four columns. It should be noted that the retention order of analytes is different on the four columns, and this can be attributed to the different retention mechanism of C18 column, SiO2 column, NH2 column, and Si-CQDs column since they have different functional groups. The better separation performance of Si-CQDs column may come from the multifunctional groups of CQDs and the synergistic effect of multiple retention interactions.
From comparing chromatograms and chromatographic parameters, it can be found that not all columns are suitable for PALC mode. SiO2 column and NH2 column are excellent columns for HILIC under the condition of high percentage of organic solvent; however, their column efficiency and separation performance reduced under PALC mode with high percentage of water. Therefore, under the background of green chemistry, it is necessary to find and construct novel materials to develop stationary phases with applicability in PALC. Though this Si-CQDs column presented good resolution, its column efficiency under PALC mode was still lower than commercial C18 column, which should be further improved in the follow-up work. Table 1 summarizes the reported columns employed for pharmaceutical analysis. Comprehensively analyzing the number of drugs that can be completely separated, separation time, and mobile phase condition, the developed Si-CQDs column possesses the advantage that while maintaining the satisfactory separation efficiency, the use of organic solvents can be reduced greatly, which has great prospects in low-cost separation and analysis.
Column stability
Column stability is a key index to evaluate the performance of a chromatographic column. Here, we focus on the stability of this Si-CQDs column in PALC mode since CQDs-based columns have been proved to have good stability under RPLC and HILIC [37, 47]. Figure S5 shows the chromatograms of model compounds separated on this Si-CQDs column every other period of time after long-term flush by mobile phase containing high proportion of water. No obvious separation performance degradation is observed, indicating the good stability of the developed Si-CQDs column under PALC mode. The stability of this column exposed to 100% water was also tested by selecting nucleosides as probes. The tR and N had no significant change after continuously washing by water over 96 h (Fig. S6), and the relative standard deviation (RSD) of tR and N was below 0.61% and 0.47%, respectively. In fact, this column has been used for about 4 months in our lab with more than 1000 injections, and no significant performance reduce is found, indicating this Si-CQDs column meets the requirement for routine laboratory work.
Repeatability and reproducibility
The repeatability and reproducibility of the Si-CQDs column were tested using nucleosides as probes. The RSDs of k, peak area (A), and N are shown in Table S8. The RSDs of k are in the range of 0.27–0.46% (n = 10, run-to-run), 0.38–0.70% (n = 3, day-to-day), 3.6–5.4% (n = 3, column-to-column), and 5.2–7.2% (n = 3, batch-to-batch), showing the practicability of the proposed column and the effectiveness of the preparation method. Though the batch-to-batch reproducibility of the Si-CQDs column is acceptable, it is lower than small molecule modified columns, which may be due to the heterogeneity including size and surface state of as-synthesized CQDs.
Real sample analysis
Detection of residual sulfonamides in milk
The applicability of the developed Si-CQDs column for real sample analysis was tested by detecting residual sulfonamides in milk. Sulfonamides belong to synthetic broad-spectrum antibiotics; residual sulfonamides in animal derived-food will accumulate in the human body through the food chain and do great harm to human health [48, 49]. The European Union stipulates that the maximum residue limit (MRL) of sulfonamides (all substances belonging to the sulfonamide group) is 100 μg/L in milk. Here, a green HPLC method based on the novel Si-CQDs column is developed for simultaneous detection of residual sulfamerazine, sulfisoxazole, and sulfadimethoxine in milk. Generally, the sample pretreatment process of milk includes extraction, degreasing, and purifying by solid phase extraction column [50], which is not conducive to rapid detection. In this case, we simplified the pretreatment process of milk, hoping to establish a rapid detection method. The milk was extracted by acidified acetonitrile and then was injected in HPLC system for analysis directly after centrifugation and filtration. The chromatograms of milk extract without and with spiked sulfonamides are shown in Fig. 7; sulfamerazine, sulfisoxazole, and sulfadimethoxine can be separated from interferents under the optimized condition within 15 min. The three sulfonamides were not detected in the milk. The chromatographic peak area had good linear relationship with the concentrations of added sulfonamides in the range of 1–20 μg/L. The fitted linear equation for determining sulfamerazine, sulfisoxazole, and sulfadimethoxine was A = 15682c + 7643 (r = 0.9990, LOD = 0.77 μg/L), A = 18501c + 25,633 (r = 0.9997, LOD = 0.73 μg/L), and A = 12375c + 10,634 (r = 0.9998, LOD = 0.94 μg/L), respectively. The LOD was calculated based on the 3σ/m criterion (where σ is the standard deviation of the blank and m is the slope of the calibration plot). The spiked recoveries (Table S9) were in the range of 87.5–108%, with RSD less than 7.9%, demonstrating the developed method based on this Si-CQDs column was reliable.
a Chromatograms of milk extracts without and with added sulfamerazine, sulfisoxazole, and sulfadimethoxine. b Linear relationship between peak area and concentrations of sulfamerazine, sulfisoxazole, and sulfadimethoxine; mobile phase: ACN/20-mM ammonium acetate (10/90, v/v); flow rate: 1.0 mL min−1; injection volume: 20 μL; UV detection wavelength: 254 nm
Detection of atenolol tablets
The developed Si-CQDs column was used to detect atenolol in commercial atenolol tablets. The detection method was compared with a reference method based on RPLC using C18 column [51]. Figure S7 shows the chromatograms of atenolol standard and real sample on C18 column and Si-CQDs column. In the similar analysis time, atenolol can be detected on this Si-CQDs column under mobile phase containing only 10% methanol, while 40% methanol are needed using traditional reversed-phase C18 column. As shown in Fig. S7b, standard curves were established on C18 column and Si-CQDs column, respectively. Good linear relationship between the peak area and the concentrations of atenolol in the range of 5–100 μg/L is achieved. The linear equation for determining atenolol was A = 2025c + 5426 (R2 = 0.9994, LOD = 4.4 μg/L) based on the developed Si-CQDs column, and the linear equation of A = 2558c-558 (R2 = 0.9975, LOD = 3.5 μg/L) was obtained on reversed-phase C18 column. One same real sample was detected on both C18 column and Si-CQDs column, the sample content was 20.4 μg/L determined by the developed method, and a very similar result of 19.7 μg/L was obtained by a reference method based on C18 column, which further verified the practicability and reliability of this Si-CQDs column.
Conclusions
This work described the preparation and application of Si-CQDs stationary phase for PALC. Compounds with different polarity including hydrophobic pesticides, polar sulfonamides, β-adrenoceptor blockers and agonists, hydrophilic nucleosides, and bases all obtained good separation under mobile phase containing low percentage of organic solvent, demonstrating the wide application scope of this Si-CQDs column. This Si-CQDs column showed better separation performance under PALC mode than in HILIC mode and even presented better separation performance than commercial SiO2 column, NH2 column, and C18 column for certain compounds. The good separation performance of this Si-CQDs column can be attributed to the synergistic effect of multiple retention interactions of CQDs such as hydrophobic and electrostatic interactions. Despite all this, the column efficiency of the developed Si-CQDs column under PALC mode should be further improved in the follow-up work. This Si-CQDs column was successfully applied for the rapid detection of residual sulfonamides in milk and the detection of atenolol in commercial atenolol tablets, which verified the practicability of this Si-CQDs column in pharmaceutical analysis. With the progress in the synthetic technology of CQDs, the homogeneity of CQDs is hoping to be improved and the limitation of nanoparticles-based stationary phases in commercialization may be broken.
References
Dembek M, Bocian S (2020) Pure water as a mobile phase in liquid chromatography techniques. TrAC Trends Anal Chem 123:115793. https://doi.org/10.1016/j.trac.2019.115793
Plotka J, Tobiszewski M, Sulej AM, Kupska M, Gorecki T, Namiesnik J (2013) Green chromatography. J Chromatogr A 1307:1–20. https://doi.org/10.1016/j.chroma.2013.07.099
Shaaban H, Gorecki T (2015) Current trends in green liquid chromatography for the analysis of pharmaceutically active compounds in the environmental water compartments. Talanta 132:739–752. https://doi.org/10.1016/j.talanta.2014.09.050
dos Santos Pereira A, David F, Vanhoenacker G, Sandra P (2009) The acetonitrile shortage: is reversed HILIC with water an alternative for the analysis of highly polar ionizable solutes? J Sep Sci 32:2001–2007. https://doi.org/10.1002/jssc.200900272
Gritti F, Pereira AD, Sandra P, Guiochon G (2010) Efficiency of the same neat silica column in hydrophilic interaction chromatography and per aqueous liquid chromatography. J Chromatogr A 1217:683–688. https://doi.org/10.1016/j.chroma.2009.12.004
Li Y, Li J, Chen T, Liu X, Zhang H (2011) Covalently bonded polysaccharide-modified stationary phase for per aqueous liquid chromatography and hydrophilic interaction chromatography. J Chromatogr A 1218:1503–1508. https://doi.org/10.1016/j.chroma.2011.01.044
Chen T, Zhu L, Lu H, Song G, Li Y, Zhou H, Li P, Zhu W, Xu H, Shao L (2017) Preparation and application of covalently bonded polysaccharide-modified stationary phase for per aqueous liquid chromatography. Anal Chim Acta 964:195–202. https://doi.org/10.1016/j.aca.2017.02.013
Chen T, Yang X, Wang S, Song G, Zhou H, Shen W, Gao L (2021) A new ionic liquid bridged periodic mesoporous organosilicas stationary phase for per aqueous liquid chromatography and its application in the detection of biogenic amines. Talanta 235:122795. https://doi.org/10.1016/j.talanta.2021.122795
Orentiene A, Olsauskaite V, Vickackaite V, Padarauskas A (2011) Retention behaviour of imidazolium ionic liquid cations on 1.7 μm ethylene bridged hybrid silica column using acetonitrile-rich and water-rich mobile phases. J Chromatogr A 1218:6884–6891. https://doi.org/10.1016/j.chroma.2011.08.034
Li YY, Xu L, Chen T, Liu XY, Xu ZG, Zhang HX (2012) Carbon nanoparticles from corn stalk soot and its novel application as stationary phase of hydrophilic interaction chromatography and per aqueous liquid chromatography. Anal Chim Acta 726:102–108. https://doi.org/10.1016/j.aca.2012.03.032
Zhang Y, Zhang Y, Wang G, Chen W, He P, Wang Q (2016) Synthesis and characterization of a multimode stationary phase: Congo red derivatized silica in nano-flow HPLC. Analyst 141:1083–1090. https://doi.org/10.1039/c5an02021b
Zhang XM, Li HY, Zhang L, Kong FY, Fan DH, Wang W (2018) Porous organic cage embedded C18 amide silica stationary phase for high performance liquid chromatography. Anal Sci 34:445–451. https://doi.org/10.2116/analsci.17P473
Chen T, Xu L, Song GS, Li Y, Xu H, Zhou HB, Xiao Z, Li P (2021) Preparation and application of Au nanoparticles-decorated SO3H-cofunctionalized silica stationary phase for per aqueous liquid chromatography. Microchem J 164:105985. https://doi.org/10.1016/j.microc.2021.105985
Xu X, Ray R, Gu Y, Ploehn HJ, Gearheart L, Raker K, Scrivens WA (2004) Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J Am Chem Soc 126:12736–12737. https://doi.org/10.1021/ja040082h
Lim SY, Shen W, Gao Z (2015) Carbon quantum dots and their applications. Chem Soc Rev 44:362–381. https://doi.org/10.1039/c4cs00269e
Caglayan MO, Mindivan F (2020) Sahin S (2020) Sensor and bioimaging studies based on carbon quantum dots: the green chemistry approach. Crit Rev Anal Chem. https://doi.org/10.1080/10408347.2020.1828029
Chen J, Gong Z, Tang W, Row KH, Qiu H (2021) Carbon dots in sample preparation and chromatographic separation: recent advances and future prospects. TrAC Trends Anal Chem 134:116135. https://doi.org/10.1016/j.trac.2020.116135
Zhang H, Qiao X, Cai T, Chen J, Li Z, Qiu H (2017) Preparation and characterization of carbon dot-decorated silica stationary phase in deep eutectic solvents for hydrophilic interaction chromatography. Anal Bioanal Chem 409:2401–2410. https://doi.org/10.1007/s00216-017-0187-z
Cai T, Zhang H, Chen J, Li Z, Qiu H (2019) Polyethyleneimine-functionalized carbon dots and their precursor co-immobilized on silica for hydrophilic interaction chromatography. J Chromatogr A 1597:142–148. https://doi.org/10.1016/j.chroma.2019.03.026
Dong Y, Wang R, Li H, Shao J, Chi Y, Lin X, Chen G (2012) Polyamine-functionalized carbon quantum dots for chemical sensing. Carbon 50:2810–2815. https://doi.org/10.1016/j.carbon.2012.02.046
Liu Y, Xue JS, Zheng T, Dahn JR (1996) Mechanism of lithium insertion in hard carbons prepared by pyrolysis of epoxy resins. Carbon 34:193–200. https://doi.org/10.1016/0008-6223(96)00177-7
Fan Y, Cheng H, Zhou C, Xie X, Liu Y, Dai L, Zhang J, Qu L (2012) Honeycomb architecture of carbon quantum dots: a new efficient substrate to support gold for stronger sers. Nanoscale 4:1776–1781. https://doi.org/10.1039/c2nr12015a
Swain AK, Li D, Bahadur D (2013) UV-assisted production of ferromagnetic graphitic quantum dots from graphite. Carbon 57:346–356. https://doi.org/10.1016/j.carbon.2013.01.082
Vinci JC, Colón LA (2013) Surface chemical composition of chromatographically fractionated graphite nanofiber-derived carbon dots. Microchem J 110:660–664. https://doi.org/10.1016/j.microc.2013.08.002
Cho D, Park S, Hong J, Chang T (2003) Retention mechanism of poly(ethylene oxide) in reversed-phase and normal-phase liquid chromatography. J Chromatogr A 986:191–198. https://doi.org/10.1016/s0021-9673(02)01953-2
Kara M, Yuzer H, Sabah E, Celik MS (2003) Adsorption of cobalt from aqueous solutions onto sepiolite. Water Res 37:224–232. https://doi.org/10.1016/S0043-1354(02)00265-8
Ross PD, Subramanian S (1981) Thermodynamics of protein association reactions: forces contributing to stability. Biochemistry 20:3096–3102. https://doi.org/10.1021/bi00514a017
Wang Z, Chen J, Sun Q, Peijnenburg WJGM (2011) C60-DOM interactions and effects on C60 apparent solubility: a molecular mechanics and density functional theory study. Environ Int 37:1078–1082. https://doi.org/10.1016/j.envint.2011.02.016
Gritti F, dos Santos Pereira A, Sandra P, Guiochon G (2009) Comparison of the adsorption mechanisms of pyridine in hydrophilic interaction chromatography and in reversed-phase aqueous liquid chromatography. J Chromatogr A 1216:8496–8504. https://doi.org/10.1016/j.chroma.2009.10.009
Tsopelas F, Malaki N, Vallianatou T, Chrysanthakopoulos M, Vrakas D, Ochsenkuhn-Petropoulou M, Tsantili-Kakoulidou A (2015) Insight into the retention mechanism on immobilized artificial membrane chromatography using two stationary phases. J Chromatogr A 1396:25–33. https://doi.org/10.1016/j.chroma.2015.03.060
Vrakas D, Giaginis C, Tsantili-Kakoulidou A (2008) Electrostatic interactions and ionization effect in immobilized artificial membrane retention. A comparative study with octanol-water partitioning. J Chromatogr A 1187:67–78. https://doi.org/10.1016/j.chroma.2008.01.079
Chirita R-I, West C, Zubrzycki S, Finaru A-L, Elfakir C (2011) Investigations on the chromatographic behaviour of zwitterionic stationary phases used in hydrophilic interaction chromatography. J Chromatogr A 1218:5939–5963. https://doi.org/10.1016/j.chroma.2011.04.002
Abraham MH, Acree WE Jr, Fahr A, Liu X (2013) Analysis of immobilized artificial membrane retention factors for both neutral and ionic species. J Chromatogr A 1298:44–49. https://doi.org/10.1016/j.chroma.2013.05.007
Taraba L, Krizek T, Hodek O, Kalikova K, Coufal P (2017) Characterization of polyaniline-coated stationary phases by using the linear solvation energy relationship in the hydrophilic interaction liquid chromatography mode using capillary liquid chromatography. J Sep Sci 40:677–687. https://doi.org/10.1002/jssc.201600785
Schuster G, Lindner W (2013) Comparative characterization of hydrophilic interaction liquid chromatography columns by linear solvation energy relationships. J Chromatogr A 1273:73–94. https://doi.org/10.1016/j.chroma.2012.11.075
Zhang M, Chen J, Gu T, Qiu H, Jiang S (2014) Novel imidazolium-embedded and imidazolium-spaced octadecyl stationary phases for reversed phase liquid chromatography. Talanta 126:177–184. https://doi.org/10.1016/j.talanta.2014.03.057
Wu Q, Hou X, Zhang X, Li H, Zhao L, Lv H (2021) Amphipathic carbon quantum dots-functionalized silica stationary phase for reversed phase/hydrophilic interaction chromatography. Talanta 226:122148. https://doi.org/10.1016/j.talanta.2021.122148
Engelhardt H, Low H, Gotzinger W (1991) Chromatographic characterization of silica-based reversed phases. J Chromatogr 544:371–379. https://doi.org/10.1016/S0021-9673(01)83996-0
Elhenawee M, Hashem H, Ibrahim AE (2013) Comparison between calixarene and conventional HPLC-stationary phases concerning with separation of antihypertensive drugs. J Liq Chromatogr Relat Technol 37:1–25. https://doi.org/10.1080/10826076.2012.723092
Ferreira CC, Gama MR, da Silva GS, Almeida WP, Collins CH, Jardim I (2018) Synthesis and evaluation of a pentafluorobenzamide stationary phase for HPLC separations in the reversed phase and hydrophilic interaction modes. J Sep Sci 41:3855–3862. https://doi.org/10.1002/jssc.201800522
Iverson CD, Lucy CA (2014) Aniline-modified porous graphitic carbon for hydrophilic interaction and attenuated reverse phase liquid chromatography. J Chromatogr A 1373:17–24. https://doi.org/10.1016/j.chroma.2014.11.003
Yu A, Peng D, Hu K, Cao A, Chang J, Wu Y, Zhang S (2013) A new 4-ferrocenylbenzoyl chloride-bonded stationary phase for high performance liquid chromatography. J Chromatogr A 1283:75–81. https://doi.org/10.1016/j.chroma.2013.01.090
Yang B, Liu H, Chen J, Guan M, Qiu H (2016) Preparation and evaluation of 2-methylimidazolium-functionalized silica as a mixed-mode stationary phase for hydrophilic interaction and anion-exchange chromatography. J Chromatogr A 1468:79–85. https://doi.org/10.1016/j.chroma.2016.09.021
Goraieb K, Collins CH (2013) Evaluation of a doubly zirconized silica-based stationary phase for HPLC. Chromatographia 76:899–908. https://doi.org/10.1007/s10337-013-2499-2
He L, Zhang M, Zhao W, Liu J, Jiang X, Zhang S, Qu L (2012) A new 14-membered tetraazamacrocycle-bonded silica stationary phase for reversed-phase high-performance liquid chromatography. Talanta 89:433–440. https://doi.org/10.1016/j.talanta.2011.12.057
Fu YY, Yang CX, Yan XP (2013) Fabrication of ZIF-8@SiO2 core-shell microspheres as the stationary phase for high-performance liquid chromatography. Chem Eur J 19:13484–13491. https://doi.org/10.1002/chem.201301461
Yuan N, Chen J, Cai T, Li Z, Guan M, Zhao L, Qiu H (2020) Glucose-based carbon dots-modified silica stationary phase for hydrophilic interaction chromatography. J Chromatogr A 1619:460930. https://doi.org/10.1016/j.chroma.2020.460930
Arroyo-Manzanares N, Gamiz-Gracia L, Garcia-Campana AM (2014) Alternative sample treatments for the determination of sulfonamides in milk by HPLC with fluorescence detection. Food Chem 143:459–464. https://doi.org/10.1016/j.foodchem.2013.08.008
Nebot C, Regal P, Miranda JM, Fente C, Cepeda A (2013) Rapid method for quantification of nine sulfonamides in bovine milk using HPLC/MS/MS and without using SPE. Food Chem 141:2294–2299. https://doi.org/10.1016/j.foodchem.2013.04.099
Bitas D, Kabir A, Locatelli M, Samanidou V (2018) Food sample preparation for the determination of sulfonamides by high-performance liquid chromatography: state-of-the-art. Separations 5:31. https://doi.org/10.3390/separations5020031
Bhatia NM, Gurav SB, Jadhav SD, Bhatia MS (2012) RP-HPLC method for simultaneous estimation of atorvastatin calcium, losartan potassium, atenolol, and aspirin from tablet dosage form and plasma. J Liq Chromatogr Relat Technol 35:428–443. https://doi.org/10.1080/10826076.2011.601495
Funding
This work was supported by the Natural Science Foundation of Shandong Province (No. ZR2020QB087); the National Natural Science Foundation of China (No. 22176109); the High-Level Talents Scientific Research Foundation of Qingdao Agricultural University (No. 6651120016); and the Support Plan on Science and Technology for Youth Innovation of Universities in Shandong Province (No. 2019KJM002).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wu, Q., Song, J., Wang, Y. et al. Carbon quantum dots–functionalized silica stationary phase for pharmaceutical analysis by a green liquid chromatography mode. Microchim Acta 189, 175 (2022). https://doi.org/10.1007/s00604-022-05291-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-022-05291-9