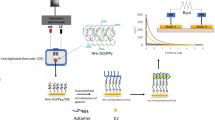
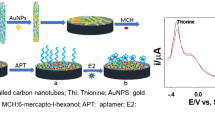
Abstract
An electrochemical aptasensor is reported for the sensitive and specific monitoring of 17β-estradiol (E2) based on the modification of electrodeposited poly(3,4-ethylenedioxythiophene) (PEDOT)-graphene oxide (GO) coupled with Au@Pt nanocrystals (Au@Pt). With excellent conductivity, chemical stability and active sites, the PEDOT-GO nanocomposite film was firstly in situ polymerized on the glassy carbon electrode by cyclic voltammetry. Subsequently, one-step synthesized Au@Pt were decorated on the conductive polymer, providing a platform for immobilizing the aptamer and enhancing the detecting sensitivity. With the addition of E2, since the interfacial electron transfer process was retarded by the E2-aptamer complex, the differential pulse voltammetry signal decreased gradually. Under optimum conditions, the calibration curve of E2 exhibited a linear range between 0.1 pM and 1 nM, with a low detection limit (S/N = 3) of 0.08 pM. The developed aptasensor showed admiring selectivity, stability, and reproducibility. It was tested in human serum, lake water and tap water samples after low-cost and simple pretreatment. Consequently, the developed platform could provide a new design thought for ultrasensitive detection of E2 in clinical and environmental samples.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
17β-Estradiol (E2), one of the most significant endocrine-disrupting chemicals (EDCs), could enter exogenously and be enriched in the body with the strongest estrogenic effect. Also, E2 could interfere with the homeostasis, agonistic behavior, and growth of the offspring health or eliminate the natural hormones in the body, leading to various diseases [1]. Chronic exposure to E2 even in extremely low concentrations as low as 1 ng L−1 can disorganize the activity of the endocrine system [2]. When E2 in water reaches 10−12 mol L−1, male fish may be feminized [3]. As a consequence, the development of accurate, fast, and sensitive analytical methods for E2 detection in aquatic samples and clinical samples is imperative.
In recent years, instrumental analytical approaches, like high-performance liquid chromatography (HPLC), liquid chromatography-mass spectrometry (LC–MS), and gas chromatography-mass spectrometry (GC–MS), have been widely utilized in E2 detection with satisfactory accuracy and stability [4,5,6,7]. In the analysis of E2, derivatization is often required due to the polarity and low volatility [8]; therefore, most of them are environmental unfriendly and time-consuming and have strict requirement. Moreover, immunological techniques such as enzyme-linked immunosorbent assay (ELISA) are relatively unstable, because of which are generally affected by site conditions, enzymatic activity, and protein modification [9].
Aptamers, a kind of short DNA or RNA fragments, are screened randomly from an oligonucleotide library by systematic evolution of ligands by exponential enrichment (SELEX) system. Aptamers are able to spatially couple target substance with high specificity and affinity [10]. With low-cost, easy synthesis and preservation, high resistance against denaturation, and low toxicity, aptamers have been new alternatives for bio-receptors except antibodies in the construction of biosensors [11]. Among different aptasensors, electrochemical aptasensors consisting of biological ingredient and electrode transducer have obtained great attention for the biomarker detection researches, owing to the high sensitivity, low-cost, and easy to miniaturize.
Poly(3,4-ethyldioxythiophene) (PEDOT) is a kind of conductive polymer with remarkable redox reversibility, biocompatibility, and electrochemical properties [12]. Consequently, PEDOT has been widely used as decorated conductive substrate on the electrode in the electrochemistry field. However, pristine PEDOT has physical and chemical limitations like the process ability, mechanical and thermochemical stability, and its electrochemical performances are not outstanding. With the development of nanomaterials and nanotechnology, graphene oxide (GO) has been utilized as the binder to construct PEDOT-based conducting polymer composites, because the resulting composites not only improve the electrochemical activity of PEDOT but also mitigate the defects of GO [13, 14]. Large amounts of the oxygen-containing functional groups shown on the GO sheets and the high mechanical strength of the graphene are both able to enhance the low cycling stability of PEDOT. In turn, the low conductivity of GO resulting from its largely disrupted sp2 hybridized network could be improved when PEDOT is integrate with it [15].
Recently, bimetallic nanomaterials have been developed as sensing substrates for the attachment of bio-receptors. Compared with single metal, bimetallic nanomaterials display superior properties thanks to the strain and electronic effects of the bimetals. Among them, Au@Pt nanocrystals (Au@Pt), with calliandra-like shape and roughly porous surfaces, could afford prominent improvement in the surface area of the electrodes and electrocatalytic performance [16]. Alloying of Pt with Au can tune the d-band center position on Pt, weaken the adsorption of poisoning intermediates to the metal active sites, and promote the electrochemical durability [17]. Nicely, the conductive polymers are ideal substrates for the uniform deposition of metallic nanoparticles. The combinations of metal and conducting polymers have the properties of each component and the new properties resulting from the synergistic effect [18]. Besides, the physical adsorption between PEDOT-GO and Au and the covalent affinity between S (well arranged in PEDOT) and Au make the PEDOT-GO/Au@Pt nanocomposite film being very mechanically stable for electrode modification [19].
Herein, the electrochemical aptasensor based on PEDOT-GO coupled with Au@Pt modification was proposed (Scheme 1) for highly selective detection of E2. The PEDOT-GO nanocomposite was firstly designed via the in situ polymerization of PEDOT doped with GO by cyclic voltammetry (CV) on the glassy carbon electrodes (GCE). Then the L-proline bio-inspired synthesis of Au@Pt was decorated on the PEDOE-GO/GCE as sensing platform for the interaction with the E2 aptamers. After that, the Au@Pt surface was passivated by 6-mercapto-1-hexanol (MCH) for limiting non-grafted aptamer adsorption. Thus, a simple and label-free electrochemical aptasensor was fabricated for E2 detection. When exposed to E2 solution, the current signals resulted from the [Fe (CN)6]3−/4− redox probe decreased with increasing E2 concentration. Real sample analysis was carried out by using human serum, lake water, and tap water samples. The developed aptasensor proved to be a robust electrochemical sensing platform for E2 detection while signifying huge potential applications in various EDCs detection.
Materials and methods
Materials and apparatus
The detailed information of materials and instruments are available in the Supporting Information.
Preparation of Au@Pt
Based on the previously reported work [20], the synthesis method of Au@Pt was as follows: added 6.17 mL of 24.3 mM HAuCl4 solution, 3.89 mL of 38.62 mM H2PtCl6 solution and 0.303 g L-proline into 79 mL double-distilled water under continuous stirring, keeping the final volume 9.0 mL. After using 1.0 mM NaOH solution to adjust the pH of the solution to 11, 10 mL of 100 mM freshly prepared ascorbic acid (AA) was added into the mixture dropwise at room temperature and continued stirring in the dark for 10 min. The as-formed nanocrystals were collected by centrifugation at 9000–10,000 rpm for 10–15 min and then washed with ultrapure water and ethanol. The solid Au@Pt were ultrasonically dispersed in ultrapure water with scattered concentration of 2.0 mg·mL−1.
Fabrication of the electrochemical aptasensor
First of all, the GCE was thoroughly cleaned, and the detailed steps are available in the Supporting Information. The modification of GCE was presented as follows: the PEDOT-GO/GCE was fabricated by the electrodeposition on the electrode surface under CV in 5 mL aqueous solution containing 1.0 mg·mL−1 GO and 10 mM EDOT. The CVs were conducted with scan rate at 0.1 V s−1, between the applied potentials: − 1.5 V to + 1.1 V for 15 cycles. The PEDOT-GO/GCE was then gently washed with double-distilled water and dried at 50 ℃ for 15 min. Next, we dropped 6 μL of the as-prepared 2.0 mg·mL−1 Au@Pt solution onto the PEDOT-GO/GCE and dried at 50 ℃ for 15 min. After casting 6 μL of 2.0 μM aptamer onto the surface of Au@Pt/PEDOT-GO/GCE, the electrode was incubated at 4 ℃ overnight, ensuring the aptamers were sufficiently integrated with Au@Pt. For blocking the remaining active sites and preventing non-specific adsorption on the detecting area, the obtained Apt/Au@Pt/PEDOT-GO/GCE was immersed in 1.0 mM MCH solution at room temperature for 0.5 h. After being rinsed to remove extra MCH, the obtained electrode was ready for further use.
Electrochemical measurement of detecting E2
The prepared MCH/Apt/Au@Pt/PEDOT-GO/GCE electrode was incubated with different concentrations of E2 for 30 min, and the differential pulse voltammetry (DPV) signals were collected in 5 mL 10 mM phosphate-buffered saline (PBS) (pH = 7.4, containing 13.7 M NaCl, 0.27 M KCl, 1 M Na2HPO4, and 0.2 M KH2PO4) with 10 mM K3[Fe (CN)]6/K4[Fe (CN)6] as redox probe. The DPV peak currents were used as the analytical signal in all of the measurements.
Real sample pretreatment before E2 detection
Human serum samples were collected from healthy female volunteers. Complete ethical approval had been obtained, and all the female volunteers gave written informed consent. Firstly, the extracted venous blood samples were centrifuged within 2 h (4℃, 15 min, 3000 rpm). After centrifugation, the supernatant serum was removed by a pipetting gun and dispensed into the EP tubes. The tubes were sealed with the parafilm and stored at − 80 °C. Tap water samples were obtained from the living areas in Wuhan, Hubei. Lake water samples were obtained from Qin Lake (Wuhan, China). The small particles were removed through ordinary filter paper and membrane filtration with an aperture of 0.22 μm. Subsequently, human serum, lake water, and tap water samples were 100-fold diluted with 10 mM PBS. All samples were, respectively, spiked with a series of concentrations of E2 standards (0.50, 5.00, 50.00 pM) before performing the pretreatment. More details were presented in the Supporting Information.
Results and discussion
The morphologic characterization of prepared aptasensor
Scanning electron microscopy (SEM) was performed in order to study the surface morphology of the surface. Figure 1A shows the surface of the GO/GCE. As shown in Fig. 1B, the PEDOT film is closely assembled to form a relatively rough surface morphology, exhibiting an unevenly distributed granular-like structure on the GCE surface. This is owing to rapid monomer initiation and polymer chain growth at a relatively high polymerization potential. From Fig. 1C, it could be seen that PEDOT polymers combined with GO flakes forming the wrinkling paper-like structures; such an open structure leads to large surface area and provides more active sites for further attachment [21]. The introduction of the GO into PEDOT was based on the role of the GO as dopant which could balance the positive charges in the backbone of the PEDOT, resulting in the nanocomposite-modified electrode, exhibiting significantly enhanced catalytic activity and abundant reactive functional groups [22, 23].
The morphology of the as-obtained Au@Pt was further investigated by transmission electron microscopy (TEM). Clearly, the as-prepared Au@Pt exhibit the well-distributed and interconnect chain-like structures (Fig. 1D), and the medium-magnification TEM image (Fig. 1E) suggests the branched hierarchical chains are composed of numerous branches nanochains. The high-resolution TEM (HR-TEM) image (Fig. 1F) reveals many visible well-resolved lattice fringes. The inter-planar spacing distances are determined to be 0.234 and 0.227 nm from the marked positions, which are bounded by the (111) facets of face centered cubic (fcc) Au@Pt alloy (0.22 nm), certifying the successful formation [24]. The selected area electron diffraction (SAED) pattern (Fig. S1A) was also obtained for phase analysis, and the energy-dispersive X-ray spectroscopy (EDS) measurement was performed to evaluate the crystal structure and compositional distribution (Fig. S1B). More details are available in the Supporting Information.
The electrochemical characterization of the prepared aptasensor
CV measurements are generally utilized to characterize the fabrication processes of the electrochemical biosensors. From curve a to curve b in Fig. 2A, the peak currents increased prominently; this enhancement was likely due to the large surface area, high conductivity of PEDOT-GO composite film, and the strong adhesion between it and the electrode surface which could accelerate the electron transfer. For Au@Pt/PEDOT-GO/GCE (curve c), the current signals increased further, indicating the Au@Pt were immobilized on the PEDOT-GO/GCE surface successfully. When attached with Au@Pt, the electrical performance of the modified electrode was improved. Because the Au@Pt multi-dendritic structure came into being, the high surface area thus enhanced electrocatalytic performance. The subsequent attachment of aptamers induced the decline of the anode peak current and of the cathode peak current (curve d), owing to the fact that the aptamers contain abundant negatively charged phosphate groups which would hinder the electron transfer kinetics for the [Fe (CN)6]3−/4− redox prove. Subsequently, MCH blocked other non-binding sites on the electrode and prevented the transport of electroactive ions on the electrode surface, so the electrochemical signal decreased at this time (curve e). When E2 was captured by the MCH/Apt/Au@Pt/PEDOT-GO/GCE, the current signals became lower (curve f), indicating the effective interaction between E2 and the aptasensor. This is because the formation of the Apt-E2 complex would hinder the efficiency of electron transfer, meanwhile.
A Cyclic voltammetry and B electrochemical impedance spectroscopy responses for bare GCE (a), PEDOT-GO/GCE (b), PEDOT-GO/Au@Pt/GCE (c), PEDOT-GO/Au@Pt/Apt/GCE (d), PEDOT-GO/Au@Pt/Apt/MCH/GCE (e), and PEDOT-GO/Au@Pt/Apt/MCH/E2/GCE (f) recorded at 10 mM K3[Fe (CN)]6/K4[Fe (CN)6] (1:1) in 10 mM PBS (pH = 7.4)
The results of electrochemical impedance spectroscopy (EIS) are in good line with the CV data. From Fig. 2B, in comparison with bare GCE (curve a), the Rct of PEDOT-GO/GCE significantly decreased (curve b), which implies that PEDOT-GO composite film is a kind of excellent electric conducting material and can enhance the conductivity of the electrode. After modification with Au@Pt, the Rct of the electrode surface became relatively smaller, the Nyquist diameter of the high-frequency region further reduced (curve c), since Au@Pt own superior electronic conductivity, and greatly enhanced specific area, which is suitable for immobilizing bio-receptors. Then the Rct values increased step by step after the immobilization of aptamer (curve d), MCH (curve e), and E2 (curve f), which is related to the improving repulsion forces between electrodes surface and redox probe and the blocking properties of MCH as mentioned above. Besides, this phenomenon is also ascribed to the formation of the “insulating layer” with biological material that severely impedes the electron transfer [25]. In conclusion, EIS and the CV results strongly confirmed the efficient construction of the electrochemical aptasensor.
The optimization of experimental conditions
In order to optimize the electro-polymerization processes of EDOT on the GCE surface and choose the supporting electrolytes with the best performance, three kinds of supporting electrolytes were tested by CV technique: 10 mM PBS solution (pH = 7.4) containing10 mM EDOT, 10 mM PBS solution (pH = 7.4) containing 10 mM EDOT and 1.0 mg mL−1 GO, and an aqueous solution containing 10 mM EDOT and 1.0 mg mL−1 GO, respectively. As shown in Fig. 3, with the increase of scanning cycles, the oxidation potential gradually stabilized at around + 1.1 V, and the charging current gradually increased, suggesting the successful electro-polymerization of EDOT on the GCE surface. In Fig. 3A and B, the polymers in the PBS and PBS containing GO gradually lost their electrochemical activity, leading to the result that the oxidation current at + 1.1 V decreased. With each voltage sweep, the initial oxidation potential shifted first negatively and then positively. Only when electro-polymerization was conducted in aqueous solution containing EDOT and GO (Fig. 3C), with the increase of scanning cycles, the current amplitude grew up, and the initial oxidation potentials continued to shift negatively, which suggests PEDOT-GO gradually accumulated on the electrode surface and formed a tightly doped stable composite film. Hence, this synergistic in situ preparation method was selected as the optimal choice. During the electro-polymerization process, the GO with abundant carboxyl groups and huge surface area was incorporated into the PEDOT conductive polymer as negatively charged doping agents, balancing the positive charge on the polymer backbone and providing additional reactive functional groups for the further deposition of EDOT. On the other hand, the disrupted sp2 bonding networks of PEDOT favorably improved the poor electrical conductivity of GO. Eventually, the PEDOT-GO composite film formed strong adhesion strength with the electrode through electrostatic interaction and π–π stacking [26]. Besides, the optimal volume of Au@Pt is 6 μL, and the optimal incubation time of the aptamer is 30 min; more details were presenting in the Supporting Information.
Cyclic voltammograms of the 1st, 5th, 10th, and 15th on the bare GCE in 10 mM PBS (pH = 7.4) containing 10 mM EDOT (A), 10 mM PBS (pH = 7.4) containing 10 mM EDOT and 1.0 mg mL−1 GO (B), and aqueous solution containing 10 mM EDOT and 1.0 mg mL−1 GO (C), respectively, within a scanning range from − 1.5 to + 1.1 V at the scan rate of 100 mV s−1
The determination of E2
The analytical performance of the aptasensor was evaluated by DPV in the PBS containing E2 at different concentrations under the optimized conditions. As depicted in Fig. 4A, with the augment of the concentration of E2, the peak currents decreased. The oxidation peak currents ∆I (IP–I0, IP, peak current in presence; I0, peak current when there is no E2) are proportional to the negative logarithm of the E2 concentrations from 10−13 to 10−9 M. The linear regression equation is expressed as ∆I (μA) = − 17.47 lg c (M) + 360.6 (R2 = 0.9969); each point is an average of three parallel determination results. The low detection limit (LOD) was calculated to be 0.08 pM (S/N = 3). Compared with the other previously reported aptasensors for E2 detection, the proposed aptasensor shows good LOD and linear range (Table 1). The ultralow detection limit may be attributed to the following reasons: (i) the construction of PEDOT-GO composite membrane with electrocatalytic activity and strong biological compatibility provided a large specific surface area and bonding strength for the subsequent bonding of complexes; (ii) the combination of various nanomaterials produced synergistic electrochemical electron transfer enhancement; and (iii) the bimetallic Au@Pt nanocomposite synthesis combined the excellent electrochemical characteristics of two kinds of single metal, affording a unique porous structure that is favorable for aptamer binding through the Au–S interaction.
A Differential pulse voltammetry responses at different concentrations of E2 (0, 10−13 M, 10−12 M, 10−11 M, 10−10 M, 10−9 M) recorded in 5 mL of 10 mM PBS containing 10 mM K3[Fe (CN)]6/K4[Fe (CN)6] (+ 0.8 V to − 0.4 V vs. Ag/AgCl). B The corresponding calibration curves. Error bar = relative standard deviation (RSD) (n = 3)
Specificity, stability and reproductivity
To assess the specificity of Au@Pt/PEDOT-GO-based electrochemical aptasensor, the structurally similar compounds (bisphenol A, estrone, estriol, ethinylestradiol) were chosen as analogues of E2 for the selective recognition study. The DPV current responses of the aptasenor were recorded toward 10−11 M E2 and 10−12 M bisphenol A, 10−11 M E2 and 10−12 M estrone, 10−11 M E2 and 10−12 M estriol, 10−11 M E2 and 10−12 M ethinylestradiol, and 10–11 M E2. As displayed in Fig. 5A, the peak currents of different analogues and E2 were close to E2 alone with a different value of less than 3%, reflecting that the other analogues could not induce significant signals. This excellent specificity of the electrochemical biosensor for detecting E2 is mostly due to the high specificity of the aptamer.
Stability and reproducibility both play a vital role for the extent of practical applications of electrochemical aptasensor. The prepared aptasensor was stored at 4 °C for 12 days to detect the same concentration of E2 (10−9 M) every 2 days. As shown in Fig. 5B, the signal responses independently remained stable with the stability rates calculated above 88.2% after 11 days, suggesting that the aptasensor had good stability. Four different batches of aptasensors were evaluated by detecting the same concentration of E2 and calculating the inter-assay RSD for investigating the reproducibility (Fig. 5C). The RSD of four batch response values was less than 5%, demonstrating the aptasensor owning prominent reproducibility.
The E2 measurement of real samples
To evaluate the applicability and accuracy of the aptasensor, the detection of spiked human serum, lake water, and tap water samples with E2 at 0.50, 5.00, and 50.00 pM was carried out by the developed aptasensor. The E2 concentration of real samples was quantitated by HPLC method beforehand as the method comparison. The results of E2 recoveries in the real samples were exhibited in Table 2. The recoveries of E2 are situated in the range of 96.68–105.28%, 97.79–102.74%, and 92.19–109.53% for human serum, lake water, and tap water samples, respectively, with RSD lower than 4%. Also, it can be seen that the results of HPLC are in good agreement with this assay, suggesting the accuracy of our assay. However, the electrochemical aptasensor can’t be reused for detection, because the amount of unbound aptamer left after a single use is uncertain; the electrodes need to be completely polished, cleaned, and redecorated before the next detection. Even so, there is no doubt that the constructed aptasensor still has great potential in complex samples, indicating a broader application prospect.
Conclusions
A novel and label-free electrochemical aptasensor based on the PEDOT-GO polymer and bimetallic nanomaterials has been successfully developed for E2 detection and been tested in diluted real samples: human serum, lake water, and tap water samples with good recoveries. The GO-doped conductive polymer PEDOT nanocomposites were electropolymerized on the GCE surface and showed excellent conductivity and large surface area, giving rise to signal amplification. Au@Pt with multi-dendritic structure were synthesized for immobilizing the aptamers and accelerating electron transfer reaction. With the synergistic effect from Au@Pt/PEDOT-GO composites improving electrocatalytic performance significantly, the GO-PEDOT/Au@Pt modified GCE has a good electrochemical response to E2 with a low LOD and a wide linear range. In spite of shortcomings such as disposable and high detection cost, this method still exhibits promising applications in the development of miscellaneous sensing system for biomarker detection in body fluid and environmental water.
References
Nesan D, Kurrasch DM (2020) Annual review of physiology gestational exposure to common endocrine disrupting chemicals and their impact on neurodevelopment and behavior. Annu Rev Physiol 82:177–202. https://doi.org/10.1146/annurev-physiol-021119
Liu M, Ke H, Sun C, Wang G, Wang Y, Zhao G (2019) A simple and highly selective electrochemical label-free aptasensor of 17β-estradiol based on signal amplification of bi-functional graphene. Talanta 194:266–272. https://doi.org/10.1016/j.talanta.2018.10.035
Nezami A, Nosrati R, Golichenari B, Rezaee R, Chatzidakis GI, Tsatsakis AM, Karimi G (2017) Nanomaterial-based aptasensors and bioaffinity sensors for quantitative detection of 17β-estradiol. TrAC Trend Anal Chem 94:95–105. https://doi.org/10.1016/j.trac.2017.07.003
Yang R, Liu J, Song D, Zhu A, Xu W, Wang H, Long F (2019) Reusable chemiluminescent fiber optic aptasensor for the determination of 17β-estradiol in water samples. Microchim Acta 186. https://doi.org/10.1007/s00604-019-3813-y
Liu Y, Li B, Yao Y, Yang B, Tian T, Miao Y, Liu B (2021) An electrochemiluminescence sensor for 17β-estradiol detection based on resonance energy transfer in α-FeOOH@CdS/Ag NCs. Talanta 221. https://doi.org/10.1016/j.talanta.2020.121479
Yao X, Wang Z, Dou L, Zhao B, He Y, Wang J, Sun J, Li T, Zhang D (2019) An innovative immunochromatography assay for highly sensitive detection of 17Β-estradiol based on an indirect probe strategy. Sensors Actuators B Chem 289:48–55. https://doi.org/10.1016/j.snb.2019.03.078
Yao L, Li Y, Cheng K, Pan D, Xu J, Chen W (2019) Determination of 17β-estradiol by surface-enhanced Raman spectroscopy merged with hybridization chain reaction amplification on Au@Ag core-shell nanoparticles. Microchim Acta 186. https://doi.org/10.1007/s00604-018-3114-x
Pu H, Huang Z, Sun DW, Fu H (2019) Recent advances in the detection of 17β-estradiol in food matrices: a review. Crit Rev Food Sci 9:2144–2157. https://doi.org/10.1080/10408398.2019.1611539
Zhang JJ, Cao JT, Shi GF, Huang KJ, Liu YM, Chen YH (2014) Label-free and sensitive electrochemiluminescence aptasensor for the determination of 17β-estradiol based on a competitive assay with cDNA amplification. Anal Methods 6:6796–6801. https://doi.org/10.1039/c4ay01147c
Ye S, Ye R, Shi Y, Qiu B, Guo L, Huang D, Lin Z, Chen G (2017) Highly sensitive aptamer based on electrochemiluminescence biosensor for label-free detection of bisphenol A. Anal Bioanal Chem 409:7145–7151. https://doi.org/10.1007/s00216-017-0673-3
Pasinszki T, Krebsz M, Tung TT, Losic D (2017) Carbon nanomaterial based biosensors for non-invasive detection of cancer and disease biomarkers for clinical diagnosis. Sensors (Switzerland) 17. https://doi.org/10.3390/s17081919
Meng L, Turner APF, Mak WC (2020) Tunable 3D nanofibrous and bio-functionalised PEDOT network explored as a conducting polymer-based biosensor. Biosens Bioelectron 159. https://doi.org/10.1016/j.bios.2020.112181
Zahed MA, Barman SC, Das PS, Sharifuzzaman M, Yoon HS, Yoon SH, Park JY (2020) Highly flexible and conductive poly (3, 4-ethylene dioxythiophene)-poly (styrene sulfonate) anchored 3-dimensional porous graphene network-based electrochemical biosensor for glucose and pH detection in human perspiration. Biosens Bioelectron 160. https://doi.org/10.1016/j.bios.2020.112220
Li Z, Yin J, Gao C, Sheng L, Meng A (2019) A glassy carbon electrode modified with graphene oxide, poly(3,4-ethylenedioxythiophene), an antifouling peptide and an aptamer for ultrasensitive detection of adenosine triphosphate. Microchim Acta 186. https://doi.org/10.1007/s00604-018-3211-x
Ma J, Yuan S, Yang S, Lu H, Li Y (2018) Poly(3,4-ethylenedioxythiophene)/reduced graphene oxide composites as counter electrodes for high efficiency dye-sensitized solar cells. Appl Surf Sci 440:8–15. https://doi.org/10.1016/j.apsusc.2018.01.100
Manivannan S, Kang I, Seo Y, Jin HE, Lee SW, Kim K (2017) M13 virus-incorporated biotemplates on electrode surfaces to nucleate metal nanostructures by electrodeposition. ACS Appl Mater Inter 9:32965–32976
Xu W, Qin Z, Hao Y, He Q, Chen S, Zhang Z, Peng D, Wen H, Chen J, Qiu J, Li C (2018) A signal-decreased electrochemical immunosensor for the sensitive detection of LAG-3 protein based on a hollow nanobox-MOFs/AuPt alloy. Biosens Bioelectron 113:148–156. https://doi.org/10.1016/j.bios.2018.05.010
Ge Y, Jamal R, Zhang R, Zhang W, Yu Z, Yan Y, Liu Y, Abdiryim T (2020) Electrochemical synthesis of multilayered PEDOT/PEDOT-SH/Au nanocomposites for electrochemical sensing of nitrite. Microchim Acta 187. https://doi.org/10.1007/s00604-020-4211-1
Rastgoo-Lahrood A, Martsinovich N, Lischka M, Eichhorn J, Szabelski P, Nieckarz D, Strunskus T, Das K, Schmittel M, Heckl WM, Lackinger M (2016) From Au-thiolate chains to thioether Sierpiński triangles: the versatile surface chemistry of 1,3,5-tris(4-mercaptophenyl) benzene on Au (111). ACS Nano 10:10901–10911. https://doi.org/10.1021/acsnano.6b05470
Weng X, Liu Y, Xue Y, Wang AJ, Wu L, Feng JJ (2017) L-Proline bio-inspired synthesis of AuPt nanocalliandras as sensing platform for label-free electrochemical immunoassay of carbohydrate antigen 19–9. Sensors Actuators B Chem 250:61–68. https://doi.org/10.1016/j.snb.2017.04.156
Fan L, Zhao G, Shi H, Liu M (2015) A simple and label-free aptasensor based on nickel hexacyanoferrate nanoparticles as signal probe for highly sensitive detection of 17β-estradiol. Biosens Bioelectron 68:303–309. https://doi.org/10.1016/j.bios.2015.01.015
Nabilah Azman NH, Lim HN, Sulaiman Y (2016) Effect of electropolymerization potential on the preparation of PEDOT/graphene oxide hybrid material for supercapacitor application. Electrochim Acta 188:785–792. https://doi.org/10.1016/j.electacta.2015.12.019
Hui N, Wang W, Xu G, Luo X (2015) Graphene oxide doped poly(3,4-ethylenedioxythiophene) modified with copper nanoparticles for high performance nonenzymatic sensing of glucose. J Mater Chem B 3:556–561. https://doi.org/10.1039/c4tb01831a
Wang AJ, Ju KJ, Zhang QL, Song P, Wei J, Feng JJ (2016) Folic acid bio-inspired route for facile synthesis of AuPt nanodendrites as enhanced electrocatalysts for methanol and ethanol oxidation reactions. J Power Sources 326:227–234. https://doi.org/10.1016/j.jpowsour.2016.06.115
Chen Y, Ge XY, Cen SY, Wang AJ, Luo X, Feng J.J (2020) Ultrasensitive dual-signal ratiometric electrochemical aptasensor for neuron-specific enolase based on Au nanoparticles@Pd nanoclusters-poly(bismarck brown Y) and dendritic AuPt nanoassemblies. Sensors Actuators B Chem 311. https://doi.org/10.1016/j.snb.2020.127931
Li D, Liu M, Zhan Y, Su Q, Zhang Y, Zhang D (2020) Electrodeposited poly(3,4-ethylenedioxythiophene) doped with graphene oxide for the simultaneous voltammetric determination of ascorbic acid, dopamine and uric acid. Microchim Acta 187. https://doi.org/10.1007/s00604-019-4083-4
Yao X, Gao J, Yan K et al (2020) Ratiometric self-powered sensor for 17β-estradiol detection based on a dual-channel photocatalytic fuel cell. Anal Chem 92:8026–8030. https://doi.org/10.1021/acs.analchem.0c01543
Zhu JH, Wang M, Tu LH et al. Nanosheets-assembled hollow CdIn2S4 microspheres-based photoelectrochemical and fluorescent dual-mode aptasensor for highly sensitive assay of 17β-estradiol based on magnetic separation and enzyme catalytic amplification. Sensors Actuators B Chem 202:347. https://doi.org/10.1016/j.snb.2021.130553
Qwab C, Pzab C, Hpab C, Dwsabc D (2022) A fluorescence aptasensor based on carbon quantum dots and magnetic Fe3O4 nanoparticles for highly sensitive detection of 17β-estradiol. Food Chem 373:131591. https://doi.org/10.1016/j.foodchem.2021.131591
Nezami A, Nosrati R, Golichenari B, Rezaee R, Chatzidakis GI, Tsatsakis AM, Karimi G (2017) Nanomaterial-based aptasensors and bioaffinity sensors for quantitative detection of 17β-estradiol. TrAC Trends Anal Chem 94:95–105. https://doi.org/10.1016/j.trac.2017.07.003
Ren S, Li Q, Li Y, Li S, Han T, Wang J, Peng Y, Bai J, Ning B, Gao Z (2019) Upconversion fluorescent aptasensor for bisphenol A and 17β-estradiol based on a nanohybrid composed of black phosphorus and gold, and making use of signal amplification via DNA tetrahedrons. Microchim Acta 186. https://doi.org/10.1007/s00604-019-3266-3
Sha H, Yan B (2021) Design of a ratiometric fluorescence sensor based on metal organic frameworks and Ru(bpy)32+-doped silica composites for 17β-estradiol detection. J Colloid Interface Sci 583:50–57. https://doi.org/10.1016/j.jcis.2020.09.030
Funding
This work was supported by the National Natural Science Foundation of China (No. 81973097), the Key Research and Development Project of Hubei Province (No. 2020BCB022), the Outstanding Young and Middle-Aged Technology Innovation Team Project of Hubei Provincial Department of Education (No. T2020003), and the Opening Project of Key Laboratory of Optoelectronic Chemical Materials and Devices of Ministry of Education of Jianghan University (No. JDGD-202018). CNRS France is acknowledged for support through IRN MAREES # 0876.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhao, Z., Chen, H., Cheng, Y. et al. Electrochemical aptasensor based on electrodeposited poly(3,4-ethylenedioxythiophene)-graphene oxide coupled with Au@Pt nanocrystals for the detection of 17β-estradiol. Microchim Acta 189, 178 (2022). https://doi.org/10.1007/s00604-022-05274-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-022-05274-w