Abstract
A triple recognition voltammetric method for the determination of brain natriuretic peptide (BNP) is described. Gold nanoparticles (AuNPs) and magnetic nanoparticles (MagNPs), sized 26 and 310 nm, respectively, were synthesized and characterized by transmission electron microscopy (TEM), FT-IR, dynamic light scattering (DLS), and Z-potential measurements. Antibody-modified MagNPs and methylene blue–labeled aptamer (Apt-MB)–modified AuNPs were used as an identifier, a signal reporter, and an amplifier, respectively. In the presence of BNP, the magnetic gold nanocomposite is formed through cascade conjugation via specific interaction. It then hybridized with complementary DNA (cDNA) on the interface, thereby amplifying the current signal of Apt-MB and increasing the selectivity of the immunoassay. Results obtained demonstrate the development of a highly selective method with a detection limit of 0.56 pg mL−1 and a linear response over the concentration range 1–10,000 pg mL−1. The standard deviation of the method is < 6% while the recovery ranged from 92.2 to 104.2%.

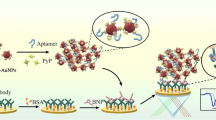
Schematic representation of triple recognition electrochemical immunosensor based on two functionalized nanoparticles (antibody-modified magnetic nanoparticle (MNP-Ab) and aptamer-modified gold nanoparticle (AuNPs-Apt)) for determination of brain natriuretic peptide (BNP).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular disease (CVD) is the leading cause of death in most countries around the world, which can eventually lead to acute heart failure (HF) and possible death [1, 2]. Brain natriuretic peptide (BNP), as a biologically active natural hormone, is a quantitative marker of HF owing to allow risk stratification for emergency department patients for medical judgment [3,4,5]. Therefore, it is highly accurate for diagnosing HF. Some methods for detecting BNP, such as conventional spectroscopy [6], surface plasmon resonance (SPR) [7, 8], and electrochemical method [9], have been developed to provide various possibilities for clinical diagnosis of CVD.
The enzyme-linked immunosorbent assay (ELISA) based on antigen-antibody interaction has been widely used in various analytical fields since its development to replace radioimmunoassay, which is not only highly specific but also capable of detecting ultralow concentrations of antigen [10, 11]. However, relying solely on an antibody to bind antigen to detect a target has certain downsides. In addition to variations in antibodies produced in different batches, it may be challenging to result in specific monoclonal antibodies, particularly against non-immunogenic molecules, which are prone to false positives in the assay [12]. Therefore, in a variety of different options, it is an ideal approach to combine the modular aptamer with a specific antibody instead of the target capture agent. On the one hand, DNA aptamers have high intrinsic chemical stability and can bind to recognition molecules with high specificity and high affinity [13, 14]. On the other hand, aptamers are easily modified and labeled by various reporter molecules and functional groups, so that the interface or nanoparticles can be easily functionalized [15, 16], improving the availability of the detection means. The dual recognition of aptamers and antibodies to targets provides a more direct and effective mode [7, 17].
Taking advantage of the distinctive physical and chemical properties of nanoparticles, many studies on functionalized nanoparticles have been reported in biomolecular detection [18,19,20]. Among various types of nanoparticles, magnetic nanoparticles (MagNPs) have attracted more and more attention by using external static magnetic fields to effectively separate analytes in complex media [21, 22]. This exceptional advantage of MagNPs simplifies the cumbersome and complex traditional experimental approach compared with classical purification ways which are particularly complicated and time-consuming. However, most of the MagNPs exhibit weak electrical conductivity. Thus, the sensitivity is limited in electrochemical amperometric applications. The combination of gold nanoparticles (AuNPs) to MagNPs not only can ensure the rapid pre-enrichment, but also can improve the electrical conduction of poor conductors, thanks to magnetic properties of MagNPs and the superior conductivity of AuNPs. Therefore, magnetic gold nanocomposite can substantially improve the sensitivity of electrochemical sensing.
Previous studies have reported that the dissociation constant (Kd) value of the antibody against the antigen is 0.33 nM [23], and the Kd value of the aptamer to the target is 12.5 nM toward BNP [24], showing excellent individual binding ability. In this study, a triple recognition strategy based on target-induced magnetic gold nanocomposite was proposed. Two kinds of nanoparticles are used to construct electrochemical method. First, MagNPs modified with anti-BNP are combined with the target BNP to form MagNP-Ab@BNP. Then, MagNP-Ab@BNP is separated from the complex environment by a permanent magnet to avoid the degradation of BNP by neutral endopeptidase. Apt-MB, which is modified on AuNPs, specifically binds the C-terminal of BNP in the MagNP-Ab@BNP complex, and a large nanocomposite is formed by cascade conjugation in this process. After magnetic separation, the nanocomposite in solution can be captured and fixed on the surface of gold electrode (GE) by hybridization between Apt and cDNA. The contents of BNP are quantitatively determined by collecting the electrochemical signals of the MB molecules on AuNP-Apt-MB (Fig. 1). This method reveals high sensitivity and wide dynamic range of detection, demonstrating practical possibilities in clinical testing applications.
Experimental section
Reagents and apparatuses
N-Hydroxysulfosuccinimide (NHS), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC), dithiothreitol (DTT), mercaptoundecanoic acid (MUA), bovine hemoglobin (BHb), myoglobin (Mb), ascorbic acid (AA), 6-mercapto-1-hexanol (MCH), sodium citrate, and hydrogen tetrachloroaurate trihydrate (HAuCl4·3H2O) were purchased from Sigma-Aldrich (Shanghai, China, http://www.aladdin-e.bioon.com.cn/). Fe3O4 magnetic nanoparticles obtained from AMI Life Science Inc. (AMO-Mag, Gimpo, Korea, http://amilifesciences.com/). BSA was purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China, http://www.reagent.com.cn). BNP and anti-BNP were obtained from Sangon Biotech Co., Ltd. (Shanghai, China, https://www.sangon.com/). All of the above chemical reagents are of analytical grade. DNA sequences were synthesized by Sangon Biotech. Co., Ltd. (Shanghai, China, https://www.sangon.com/) and listed below. Apt-MB: 5′-HS-TTT TTT TAA ACG CTC AAA GGA CAG AGG GTG CGT AGG AAG GGT ATT CGA CAG GAG GCT CAC A-MB-3′; complementary DNA (cDNA): 5′-SH-TGT GAG CCT CCT GTC GAA TAC CCT TCC TAC GCA CCC TCT GTC CTT TGA GCG TTT AAA AAA A-3′. All the solutions are from deionized water (DW) of Millipore purified water system (> 18 mΩ cm, Millipore, USA).
Electrochemical measurements were performed using an Autolab PGSTAT128N system (Eco Chemise B.V., The Netherlands). The UV-vis spectra were recorded on a Shimadzu UV-2450 spectrophotometer. Z-potential and dynamic light scattering (DLS) were performed from Malvern Nano Zetasizer (MAL1202556). Transmission electron microscopic (TEM) image was recorded from a JEM-2010F microscope (Japan). Fourier transform infrared (FT-IR) spectra were generated by an FT-IR spectroscopy (Bruker 70, Germany) instrumentation.
Preparation of AuNP-apt-MB and MagNP-Mb
AuNPs were synthesized in a one-pot process. The colloidal solution of AuNP was synthesized by classical citrate reduction of HAuCl4·3H2O [25]. Specific experimental details and the modification of Apt-MB were presented in the Supporting Information. Details on the preparation of MagNPs and modification of antibodies were provided in the Supporting Information.
Preparation of functionalized nanoconjugate
Initially, 100 μL of MagNP-Ab was mixed with BNP solution in centrifuge tube and stirred slightly for 20 min at 37 °C. Then MagNPs were suspended in 100 μL phosphate-buffered saline (PBS) after washing. Next, 200 μL excess of the AuNP-Apt-MB was added to the tube and reacted for 30 min at 37 °C with gentle shaking. After washing with PBS for three times, the MagNP-Ab@BNP@AuNP-Apt-MB nanoconjugate was collected by a permanent magnet under the centrifuge tube and then resuspended in 100 μL PBS.
Electrode surface modification
First, the electrode was polished using fine-grained sandpaper (5000) to regenerate GE surface, and polished with 0.3 and 0.05 μm polishing powders. Then, the piranha solution (30% H2O2:H2SO4 = 1:3) was covered on the surface of GE for 5 min to eliminate organic and inorganic substances that might be adsorbed on the electrode surface. After the electrode was washed with DW, cyclic voltammetry (CV) activation (20 cycles, 0–1.6 V) was carried out in a 0.5-M H2SO4 solution to remove the oxide film on the surface of the electrode. Finally, the electrode was washed gently with DW and dried with nitrogen.
A total of 90 μL of DTT (1 mM) was mixed with 10 μL cDNA (1 μM) and reacted in the dark for 1 h, which converted disulfide bonds into sulfhydryl groups and facilitated the fixation of nucleic acids on GE surface. A total of 50 μL DNA were modified electrode surface for 2 h, and the unbound DNA was removed by washing with DW for three times. Next, the cDNA-functionalized GE was placed in 200 μL MCH (0.5 mM) solution for 1 h to obtain a regular array of DNA monolayer. MCH used here was a good blocking agent, which not only backfilled the Au substrate but also resisted non-specific binding. Then, 50 μL nanocomposite was used to modify the surface of GE for 30 min. At this point, all the reactants had been modified to the surface of the electrode.
Electrochemical measurements
Electrochemical measurements were performed with a three-electrode system (CE, SCE, and WE). Electrochemical measurement methods included CV, differential pulse voltammetry (DPV), and electrochemical impedance spectroscopy (EIS). CV and EIS of the biosensor were treated in 8 mL [Fe (CN)6]3−/4− solution. The scanning potential of CV was − 0.1 to 0.5 V, and the scanning rate was set to 100 mV/s. The EIS had a frequency range of 0.1 Hz to 100 kHz. DPV was carried out in PBS with a potential of − 0.6 to 0.1 V, a pulse amplitude of 50 mV, a pulse width of 50 ms, and a pulse period of 0.1 s. Each concentration measured was repeated three times, and the average was taken as the result.
Application to real samples
Human serum samples were collected from the First Affiliated Hospital of Zhengzhou University (Zhengzhou, China) and they used without any pre-treatment. Recovery experiments were performed at BNP serum samples spiked with 1 pg mL−1, 10 pg mL−1, 100 pg mL−1, 1 ng mL−1, and 10 ng mL−1.
Results and discussion
Characterization of materials
The formation of MagNPs and AuNPs were confirmed by TEM imaging and DLS (Fig. 2 a and b). The TEM image from Fig. 2a clearly shows that the size of the core-shell MagNPs is 320 nm, and the black near-spherical Fe3O4 core is surrounded by a uniform SiO2 shell of 23-nm thickness to accommodate subsequent antibody modification. The particle size distribution of MagNPs was analyzed by DLS (inset). The mean equivalent hydrodynamic radius of MagNPs is 310 nm. The synthesized AuNPs are uniform spherical and monodisperse with a diameter of 26 ± 0.5 nm. DLS (inset of Fig. 2b) reveals that AuNP particle size distribution is in a narrow range, with 78.7% of the particle sizes within 20 to 32 nm and a single peak at 26 nm (Fig. 2b). To evaluate the coupling of MagNPs to antibodies and the modification of DNA onto AuNPs, FT-IR spectroscopy was employed to analyze the IR spectra that correspond to MagNPs, MagNP-Ab, AuNPs, and AuNP-Apt-MB (Fig. 2 c and e), which were used to identify the specific functional groups of the effective components based on the peak positions in the region of infrared radiation. The strong stretching vibration of N-H located at 3385 cm−1 and the C=O of the amide bond at 3421 cm−1 demonstrate successful modification of the antibody on MagNP-Ab; in-plane bending of the C-H in functional group of the antibody also shows the coupling of the antibody to the MagNPs (Fig. 2c). As shown in Fig. 2e, the C=O bond disappears in the DNA-modified AuNPs, which is attributed to the combination of Apt-MB and AuNPs to remove the trisodium citrate as the stabilizer [26]. The presence of the -SH bond indicates the successful modification of DNA on the surface of AuNPs. In addition, we studied the assembly of AuNPs and Apt-MB by UV-vis. The maximum plasma absorption of the bare AuNPs occurred at 519 nm, as shown in Fig. 2d. The absorption peak at this wavelength corresponds to a typical AuNPs of 25 nm, which is also consistent with the visual image in the TEM. The maximum plasma absorption of the AuNPs-Apt-MB appeared at 522 nm, the covalent bond of -SH further broadened the absorption band, showing a slight red shift. Finally, the charge changed after surface modification of nanoparticles which were studied by Z-potential (Fig. 2f). Coupling MagNPs with positively charged antibodies resulted in a change in potential from − 44.7 to − 14.2 mV. DNA modification on the surface of the gold nanoparticles caused a 5.2-mV negative potential shift of the charge. These results confirm that AuNP-Apt-MB and MagNP-Ab are synthesized successfully, and both dispersion solutions have good physical stability.
TEM and DLS data (inset) of synthetic MagNPs (a) and AuNPs (b). (c) FT-IR spectra of MagNPs before (black) and after (red) antibody coupling. (d) UV-vis spectrum of AuNPs before (black) and after (red) Apt-MB modification. (e) FT-IR spectra before (black) and after (red) aptamer modification of AuNPs. (f) Z-potential before and after functionalization of nanoparticles
Electrochemical properties
Depending on the functionalization steps, Fig. 3a shows the different changes in the imaginary part of impedance (-Z″) as a function of the real part of impedance (Z′) [27, 28]. The bare GE presented a small Rct value (the diameter of semi-circular Nyquist plots) and a large redox peak of [Fe (CN)6]3−/4−, respectively, in the process, indicating that GE has a small electron transfer resistance and a strong interfacial electron transfer ability. After the surface of the electrode was modified with cDNA and MCH, respectively, the Rct value increased due to enhancive blockage of the redox probe, [Fe (CN)6]3−/4− through electrode surface. Similarly, the redox peak gradually decreased, as indicated by CV shown in Fig. 3b. Finally, the nanocomposite bonded to cDNA on the electrode surface, the Rct value was decreased while the redox peak increased. Due to the presence of AuNPs in the nanocomposite, the surface of the electrode had enhanced electron transport capabilities. The above results show that this method has been successfully constructed. The electrochemical properties of the constructed system were studied using CV, and the electrochemical signal of MB was collected in 0.1 M PBS, as shown in Fig. S1. The height of the reduction peak at − 0.3 V was used as a measure for the quantification of the target analyte.
a EIS of bare GE, cDNA/GE, MCH/cDNA/GE, nanocomposite/MCH/cDNA/GE in 5 mM [Fe (CN)6]3−/4− containing 0.1 M KCl (the experimental points (the symbols) and the fitting curve (the solid lines)). Inset: Randles equivalent circuit using a constant phase element. b CV characterization of different modified GEs in 5 mM [Fe (CN)6]3−/4− containing 0.1 M KCl
Optimization of the experimental parameters
The following parameters were optimized: (a) the concentration of BNP antibody, (b) pH of the reaction system, (c) temperature, (d) reaction time. Respective text and figures on optimizations are given in the Electronic Supporting Material (Fig. S2). In short, the following experimental conditions were found to give best results: (a) optical concentration, 20 μg mL−1; (b) pH, 7; (c) best temperature, 37 °C; (d) reaction time, 20 min.
Sensor response for BNP determination
Under the optimized conditions, the performance of the triple recognition immunoassay was evaluated with different concentrations of the target BNP solution (contains key concentrations 100 pg mL−1 and 400 pg mL−1 for determining HF disease status). There is no charge transfer during the specific binding of MagNP-Ab and BNP, as well as the coupling with subsequent AuNP-Apt-MB, but only the formation of nanocomplex, which mean that the detection of BNP depended on the oxidation signal of MB on the nanocomplex already fixed to the electrode surface. During this process, the more BNP are captured, the larger nanocomposite is formed (containing more MB), resulting in an increase in peak current. Different concentrations of BNP were detected using this detection strategy as presented in Fig. 4a, the DPV oxidation currents of MB increased proportionally with the BNP concentrations increasing, corresponding to the measurement principle and process described above. As illustrated in Fig. 4b, the results show a good linear relationship between the oxidation peak currents and logarithm of BNP concentration in the range of 1 to 104 pg mL−1. Meanwhile, the linear regression equation is Y (μA) = 0.240 lg CBNP + 0.272 (R2 = 0.996) with a low detection limit of 0.56 pg mL−1 (S/N = 3), according to the means of 3’s blank criterion, which is significantly lower than the clinical detection index (100 pg mL−1). The sensitivity of the method was found to be 176.23 μA μM−1 cm−2. Good linear range and low detection limit indicate that the triple recognition strategy has an ideal detection effect for the analyte. This method is compared with other literature methods for detecting BNP and shows good analytical performance as summarized in Table 1. The combination of MagNPs and AuNPs improves the conductivity of the complex while simplifying the pre-enrichment step. The dual targeting of antibodies and aptamers to BNP enhanced the specificity of recognition.
Analytical performances of the triple recognition strategy. a DPV responses of this method. After incubation with different concentrations of BNP. b Linear relationship for BNP determination in the range of 1 pg mL−1 to 10 ng/mL in 0.1 M PBS. Voltage range, − 0.6 to 0.1 V; pulse amplitude, 50 mV; pulse width, 50 ms; pulse period, 0.1 s. Reference electrode, saturated calomel electrode (SCE)
Selectivity and stability studies
According to the mechanism of the immunoassay strategy, this method should be ineffective against other interferers. In order to study the selectivity of our approach, four kinds of interferences (AA, BHb, Mb, BSA) were tested using this detection system. It is notable that the interferences’ concentration used here is much higher than target BNP. Obvious changes in current response are observed for target BNP compared with the interfering substances (Fig. 5a). These results demonstrate that the triple recognition strategy enables excellent selectivity for determining BNP. To research the storage stability of the assay, this immunosensors were stored in a dry environmental (4 °C) for different times, and different concentrations of BNP (100 pg mL−1 and 10 ng mL−1) were tested. It is observed that the electrochemical signal retained about 93.4% of its original value (Fig. 5b and S3), showing that this strategy has excellent stability.
Determination of BNP in serum samples
To evaluate the analytical performance of the immunoassay in real samples, we investigated the potential utility of our method for detecting BNP in clinical serum samples. Serum samples with BNP concentrations of 1 pg mL−1, 10 pg mL−1, 100 pg mL−1, 1 ng mL−1, and 10 ng mL−1 were prepared according to the procedure described in the experimental section. Three sets of parallel samples were tested for each concentration, and the results were taken as the average of the three sets of experiments. The measured sample concentration was calculated by substituting the signal value into the obtained linear regression equation (Fig. S4, R2 = 0.991). Statistical comparison and analysis show that the detection strategy has good consistency in different matrices (Fig. S5), which indicates that the detection strategy has predictable application value. As shown in Table 2, the relative standard deviation (RSD) of the detection results are less than 6.31%, and the recovery are in the range of 92.22–104.2%, indicating that the measured experimental values are well matched with the true concentration of BNP in serum samples. The great magnetic concentration property of MagNP-Ab helps to capture analyte directly in the complex biological samples without the purification process. To sum up, this strategy has potential clinical application and can be used for the detection of BNP in clinical serum samples.
Conclusions
In this work, an enhanced voltammetry immunoassay using a triple recognition system is developed for determination of BNP. MagNPs and Apt-MB-modified AuNPs are used as separable identifier and signal amplifier, respectively. The double recognition of BNP by aptamers and antibodies lead to the formation of MagNP-Ab@BNP@AuNP-Apt-MB nanocomposite, which simplifies the pre-separation procedure and enhances conductivity. This strategy exhibits excellent bioactivity and can be used to detect BNP in clinical serum samples. However, this method requires voltammetry, and electrochemical reduction is irreversible, which may limit its applicability to single assays. In general, this detection platform holds good promise for HF-related BNP detection and can be further exploited for sensing applications in disease diagnosis.
References
Sarangadharan I, Wang SL, Tai TY, Pulikkathodi AK, Hsu CP, Chiang HHK, Wang YL (2018) Risk stratification of heart failure from one drop of blood using hand-held biosensor for BNP detection. Biosens Bioelectron 107:259–265. https://doi.org/10.1016/j.bios.2018.02.036
Lin DCC, Diamandis EP, Januzzi JL, Maisel A, Jaffe AS, Clerico A (2014) Natriuretic peptides in heart failure. Clin Chem 60:1040–1046. https://doi.org/10.1373/clinchem.2014.223057
Li X, Liu L, Dong X, Zhao G, Li Y, Miao J, Fang J, Cui M, Wei Q, Cao W (2019) Dual mode competitive electrochemical immunoassay for B-type natriuretic peptide based on GS/SnO2/polyaniline-Au and ZnCo2O4/N-CNTs. Biosens Bioelectron 126:448–454. https://doi.org/10.1016/j.bios.2018.11.009
Serafín V, Torrente-Rodríguez RM, González-Cortés A, García de Frutos P, Sabaté M, Campuzano S, Yáñez-Sedeño P, Pingarróna JM (2018) An electrochemical immunosensor for brain natriuretic peptide prepared with screen-printed carbon electrodes nanostructured with gold nanoparticles grafted through aryl diazonium salt chemistry. Talanta 179:131–138. https://doi.org/10.1016/j.talanta.2017.10.063
Szunerits S, Mishyn V, Grabowska I, Boukherroub R (2019) Electrochemical cardiovascular platforms: current state of the art and beyond. Biosens Bioelectron 131:287–298. https://doi.org/10.1016/j.bios.2019.02.010
Nishikimi T, Okamoto H, Nakamura M, Ogawa N, Horii K, Nagata K, Nakagawa Y, Kinoshita H, Yamada C (2013) Direct immunochemiluminescent assay for proBNP and total BNP in human plasma proBNP and total BNP levels in normal and heart failure. PLoS One 8:e53233. https://doi.org/10.1371/journal.pone.0053233
Jang HR, Wark AW, Baek SH, Chung BH, Lee HJ (2014) Ultrasensitive and ultrawide range detection of a cardiac biomarker on a surface plasmon resonance platform. Anal Chem 86:814–819. https://doi.org/10.1021/ac4033565
Kim S, Wark AW, Lee HJ (2016) Femtomolar detection of tau proteins in undiluted plasma using surface plasmon resonance. Anal Chem 88:7793–7799. https://doi.org/10.1021/acs.analchem.6b01825
Zhao Y, Li L, Hu L, Zhang Y, Wu D, Ma H, Wei Q (2019) An electrochemiluminescence immunosensor for the N-terminal brain natriuretic peptide based on the high quenching ability of polydopamine. Microchim Acta 186:606. https://doi.org/10.1007/s00604-019-3709-x
Lim MJ, Foster GJ, Gite S, Ostendorff HP, Narod S, Rothschild KJ (2010) An ELISA-based high throughput protein truncation test for inherited breast cancer. Breast Cancer Res 12:78–79. https://doi.org/10.1186/bcr2722
Auld D, Lea W, Davis MI, Simeonov A (2013) Literature search and review: detected through a quick glance. Assay Drug Dev Techn 11:1–8. https://doi.org/10.1089/adt.2013.1101.lr
Toh SY, Citartan M, Gopinath SC, Tang TH (2015) Aptamers as a replacement for antibodies in enzyme-linked immunosorbent assay. Biosens Bioelectron 64:392–403. https://doi.org/10.1016/j.bios.2014.09.026
Wang P, Hatcher KL, Bartz JC, Chen SG, Skinner P, Sreevatsan S (2011) Selection and characterization of DNA aptamers against PrPSc. Exp Biol Med 236:466–476. https://doi.org/10.1258/ebm.2011.010323
Shamah SM, Healy JM, Cload ST (2008) Complex target SELEX. Acc Chem Res 41:130–138. https://doi.org/10.1021/ar700142z
Kim SH, Nam O, Jin E, Gu MB (2019) A new coccolith modified electrode-based biosensor using a cognate pair of aptamers with sandwich-type binding. Biosens Bioelectron 123:160–166. https://doi.org/10.1016/j.bios.2018.08.021
Lin Y, Wang X, Sun Y, Dai Y, Sun W, Zhu X, Luo C (2019) A chemiluminescent biosensor for ultrasensitive detection of adenosine based on target-responsive DNA hydrogel with Au@HKUST-1 encapsulation. Sensors Actuators B Chem 289:56–64. https://doi.org/10.1016/j.snb.2019.03.075
Zhang W, He Z, Yi L, Mao S, Li H, Lin JM (2018) A dual-functional microfluidic chip for on-line detection of interleukin-8 based on rolling circle amplification. Biosens Bioelectron 102:652–660. https://doi.org/10.1016/j.bios.2017.12.017
Yoon J, Cho SH, Seong H (2017) Multifunctional ultrasmall superparamagnetic iron oxide nanoparticles as a theranostic agent. Colloid Surface A 520:892–902. https://doi.org/10.1016/j.colsurfa.2017.02.080
Melnychuk N, Klymchenko AS (2018) DNA-functionalized dye-loaded polymeric nanoparticles: ultrabright FRET platform for amplified detection of nucleic acids. J Am Chem Soc 140:10856–10865. https://doi.org/10.1021/jacs.8b05840
Zheng L, Qi P, Zhang D (2018) A simple, rapid and cost-effective colorimetric assay based on the 4-mercaptophenylboronic acid functionalized silver nanoparticles for bacteria monitoring. Sensors Actuators B Chem 260:983–989. https://doi.org/10.1016/j.snb.2018.01.115
Lv S, Sheng J, Zhao S, Liu M, Chen L (2018) The detection of brucellosis antibody in whole serum based on the low-fouling electrochemical immunosensor fabricated with magnetic Fe3O4@Au@PEG@HA nanoparticles. Biosens Bioelectron 117:138–144. https://doi.org/10.1016/j.bios.2018.06.010
Tavallaie R, McCarroll J, Le Grand M, Ariotti N, Schuhmann W, Bakker E, Gooding JJ (2018) Nucleic acid hybridization on an electrically reconfigurable network of gold-coated magnetic nanoparticles enables microRNA detection in blood. Nat Nanotechnol 13:1066–1071. https://doi.org/10.1038/s41565-018-0232-x
Tetin SY, Ruan Q, Saldana SC, Pope MR, Chen Y, Wu H, Richardson PL (2006) Interactions of two monoclonal antibodies with BNP: high resolution epitope mapping using fluorescence correlation spectroscopy. Biochemistry 45:14155–14165. https://doi.org/10.1021/bi0607047
Wang Y, Wu J, Chen Y, Xue F, Teng J, Cao J, Chen W (2015) Magnetic microparticle-based SELEX process for the identification of highly specific aptamers of heart marker--brain natriuretic peptide. Microchim Acta 182:331–339. https://doi.org/10.1007/s00604-014-1338-y
Grabar KC, Freeman RG, Hommer MB, Natan MJ (1995) Preparation and characterization of Au colloid monolayers. Anal Chem 67:735–743. https://doi.org/10.1021/ac00100a008
Park JW, Shumaker-Parry JS (2014) Structural study of citrate layers on gold nanoparticles: role of intermolecular interactions in stabilizing nanoparticles. J Am Chem Soc 136:1907–1921. https://doi.org/10.1021/ja4097384
Huerta-Nuñez LFE, Gutierrez-Iglesias G, Martinez-Cuazitl A, Mata-Miranda MM, González-Díaz CA (2019) A biosensor capable of identifying low quantities of breast cancer cells by electrical impedance spectroscopy. Sci Rep 9:6419. https://doi.org/10.1038/s41598-019-42776-9
Fan T, Du Y, Yao Y, Wu J, Meng S, Luo J, Gao F (2018) Rolling circle amplification triggered poly adenine-gold nanoparticles production for label-free electrochemical detection of thrombin. Sensors Actuators B Chem 266:9–18. https://doi.org/10.1016/j.snb.2018.03.112
Grabowska I, Sharma N, Vasilescu A, Iancu M, Badea G, Boukherroub R, Ogale S, Szunerits S (2018) Electrochemical aptamer-based biosensors for the detection of cardiac biomarkers. ACS Omega 3:12010–12018. https://doi.org/10.1021/acsomega.8b01558
Lee I, Luo X, Huang J, Cui XT, Yun M (2012) Detection of cardiac biomarkers using single polyaniline nanowire-based conductometric biosensors. Biosensors 2:205–220. https://doi.org/10.3390/bios2020205
Xu R, Lu P, Wu B, Wang X, Pang X, Du B, Fan D, Wei Q (2018) Using SiO2/PDA-Ag NPs to dual-inhibited photoelectrochemical activity of CeO2-CdS composites fabricated a novel immunosensor for BNP ultrasensitive detection. Sensors Actuators B Chem 274:349–355. https://doi.org/10.1016/j.snb.2018.07.122
Lei YM, Xiao MM, Li YT, Xu L, Zhang H, Zhang ZY, Zhang GJ (2017) Detection of heart failure-related biomarker in whole blood with graphene field effect transistor biosensor. Biosens Bioelectron 91:1–7. https://doi.org/10.1016/j.bios.2016.12.018
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 61275085).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 872 kb)
Rights and permissions
About this article
Cite this article
Zhao, J., Zhu, ZZ., Huang, X. et al. Magnetic gold nanocomposite and aptamer assisted triple recognition electrochemical immunoassay for determination of brain natriuretic peptide. Microchim Acta 187, 231 (2020). https://doi.org/10.1007/s00604-020-4221-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-020-4221-z