Abstract
A voltammetric method is described for the determination of chloroquine (CQ) and validated simultaneously by two techniques and in three different conditions. The WS2 quantum dots (WS2 QDs) were synthesized by a hydrothermal method and then placed on reduced graphene oxide (rGO) sheets. The resulting composite material was then deposited on a glassy carbon electrode (GCE) where it showed excellent electroactivity. The modified GCE responds to chloroquine at a typical potential maximum of 1.2 V (vs. AgCl/Ag). Techniques including cyclic voltammetry and differential pulse voltammetry were tested. Features of merit include (a) a wide linear response (in the 0.5 μM to 82 μM CQ concentration range), (b) an electrochemical sensitivity of 0.143–0.90 μA·μM−1·cm−2), and a 40–120 nM limit of detection (at S/N = 3). The sensor has excellent selectivity even in the presence of potentially interfering biological compounds. Responses were tested in phosphate buffer, human serum and pharmaceutical formulations, and no cross reactivity or matrix effects were found. In all the three cases, quite satisfactory recoveries were obtained.

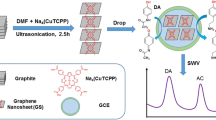
Schematic representation of the mechanism for electro-oxidation of chloroquine on a glassy carbon electrode modified with an rGO@WS2 quantum dot composite. The sensor displays enhanced electrocatalytic activity towards chloroquine. The method was validated in biological samples and pharmaceutical formulations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chloroquine (CQ) is extensively used in the treatment of sudden attacks of malaria and elimination of porphylaxis for many decades. This drug is widely used for curing rheumatoid arthritis and similar kinds of collagen diseases. Chloroquine is also used for amoebic hepatitis treatment [1,2,3]. Various strains of Plasmodium falciparum have developed resistance against CQ [4]. But due to its low cost this drug is still widely used in developing countries. It is also recommended as a strong anti-cancerous agent [5, 6]. High doses of this drug may cause several side effects like heart attack [7]. There are various analytical methods reported in the literature to monitor the concentration of drug like spectrophotometric method [8], high performance liquid chromatography with fluorescence detection [9, 10], chemiluminescence including radiostorage- and photostorage-chemiluminescence(CL) [11] and voltammetric methods [12]. Among all methods voltammetric methods got immense attention due to its fast response, less volume consumption of samples, high selectivity and sensitivity.
Graphene is an atom-thick two dimensional crystalline carbon film with sp2 hybrid carbon atoms. It reveals the unprecedented amalgamation of beneficial properties such as remarkable mechanical strength, large surface area, high thermal conductivity and extraordinary electrical properties like tremendous charge carrier mobility and conductivity [13, 14]. Due to these peculiar material properties, graphene have grabbed significant attention and proved their applicability in the fields of sensors [15, 16], catalysis [17], energy-storage [18], electronics [19] and optoelectronics [20]. The graphene oxide (GO) possesses various hydrophilic groups like hydroxyl carboxyl, and epoxy and also renders the possibility of fuctionalization using covalent or non-covalent bindings. These functional groups also allow dispersion of GO in aqueous solution [21]. Further, the conductivity of GO is found to be lesser than that of reduced graphene oxide (rGO). So, in place of GO, rGO can also be used wherever applicable [22, 23]. Utilizing the beneficial film like structure, graphene based composites by incorporation of graphene with diverse functional nanomaterials like metal compounds [24], noble metals [25], carbonaceous materials [26], polymeric materials [27] etc. have been prepared which showed enhanced mechanical, electrical, chemical, mechanical and catalytic properties. Consequently, varieties of sensors have been developed in the recent scenario depicting great stability, sensitivity and selectivity [28,29,30,31,32,33].
Apart from graphene, two dimensional analogues like transition metal dichalcogenides have also been gaining attention because of similar properties as that of graphene. 2D tungston disulfide (WS2) nanosheet is one of the members of this family. It has 2D building blocks of S-W-S layers which are weakly bound by Vander Waals forces. Because of the exclusive chemical, electronic and mechanical properties, it has been used in several fields [34,35,36]. However, if the size of WS2 is controlled, zero dimensional WS2 quantum dots can be synthesized with size in the range of 1–10 nm. Not many reports have been published on the synthesis and applications of WS2 QDs [37]. In comparison to the nanosheets, quantum dots possess larger surface area and rich exposed edges which is benign to the electrocatalytic activity towards electrochemical sensing. Also, due to the luminescent properties of the dots, they have been used in the fields of sensing, bioimaging, catalysis, electronics, hydrogen evolution reaction etc. [38, 39]. Coating of QDs over 2D materials can help to prevent the aggregation and enhance the stability of these materials. The WS2 QDs decorated rGO sheets can act as immensely active electrocatalyst to enhance electrode kinetics.
The paper describes fabrication, characterization and analytical performance of a chloroquine sensor based on the rGO@WS2 QDs modified glassy carbon electrode by simple voltammetric techniques in phosphate buffer at pH 6. WS2QDs is synthesized hydrothermally and GO was synthesized by modified Hummer’s method followed by reduction. Proposed sensor is highly reproducible, stable and sensitive for the determination of CQ in broad concentration range of 0.5 μM–82.4 μM.
Experimental section
Materials
Chloroquine phosphate was brought from Sigma, Aldrich, USA. Absolute ethanol, Na2HPO4, NaH2PO4 and distilled water were purchased from Merck, India. For electro-sensing of Chloroquine phosphate freshly prepared solution was prepared using deionised water (DI). Na2WO4. 2H2O was brought from SRL private Ltd. L-cysteine was procured from Merck, India. Conc. H2SO4, Conc. HCl, Conc. H3PO4, distilled water and Conc. H2O2 were purchased from Merck, India. All other chemicals like Hydrazine hydrate, graphite powder and KMnO4 were of analytical grade and used as such with no additional purification. Blood samples (from Volunteers) for the analysis of Chloroquine in real sample was collected from hospital, IMS, BHU, Varanasi, India.
Instrumentation
The entire electrochemical experiments were executed using Autolab (PGSTAT, 302, The Netherlands). Three electrode assembly was used for electrochemical studies. Glassy carbon electrode (GCE) was employed as a working electrode, Pt disc electrode was used as counter electrode, Ag/AgCl is applied as reference electrode.
UV-Vis. spectra were recorded with a spectrophotometer, Epoch 2 microplate reader Biotech, USA in quartz cuvette having 1 cm optical path length. Transmission electron microscopy images were obtained by using TECHNAI G2 20 TWIN (Czech Republic) run at an accelerating voltage of 200 keV on copper grid coated with carbon. Confirmation of the different functional groups present in rGO@WS2 QDs were done by FT-IR measurements using Nicolet 380 FT-IR spectrometer in the transmission mode (Thermo Nicolet Corporation, USA), which was equipped with a beam splitter and DTGS KBr detector.
Preparation of WS2 QDs
WS2 QDs were synthesized through hydrothermal method in a single step. Sodium tungstate dihydrate (Na2 WO4·2H2O) (0.25 g) and L-cysteine (HO2CCH (NH2) CH2SH) (0.5 g) (1:2 w/w) were mixed in DI water separately and both the solutions were constantly stirred for 10–15 min. Further the two solutions were uniformly mixed, again followed by continuous stirring for 15 min and temperature was maintained at 40 °C. In the next step, 12 N concentrated HCl was added in required amount to set pH at 3.0. To initiate hydrothermal process, the above mixture was poured into stainless steel lined Teflon autoclave of 100 mL capacity. Initially temperature was set at 120 °C for 2–3 h and further 190 °C for 46 h. Then reaction mixture was left in the autoclave for attaining room temperature. Finally yellow colloidal solution of WS2 was obtained which was poured into dialysis bag of retained molecular weight 2000 Da. Then the dialyzed bag was left in ideal condition for 3–4 days to get WS2-QDs as the final product (Fig. 1).
Preparation of rGO
Graphene oxide (GO) was synthesized with graphite powder using well known improved Hummer’s method [40] with some modification. To start with 1.5 g of graphite powder was pre-oxidized with equal amount of K2S2O8, and P2O5 (5 g each) in 40 ml of 98% H2SO4 at 80 °C for 4 h. Then suspension obtained was washed for 4–5 times by deionized water (DI) kept at 50 °C in vacuum oven. In the next step, pre-oxidized graphite was further oxidised by a mixture of concentrated H2SO4 and H3PO4 in 180: 13 volume ratio under continuous stirring. 9 g of KMnO4 was added after 5 min in the above reaction mixture. Temperature was maintained at 50 °C and the stirring was continued for 15 h. Then the reaction was stopped and left to cool down to R.T. 200 ml ice was added to reaction mixture, followed by the addition of 1.5 mL of 30% H2O2. A U.S. standard testing sieve (pore size 30 μm) was used to separate fine material from the mixture. Thus obtained filtrate was centrifuged for 3 h at 5000 rpm. Sediment material was washed a number of times with DI water, HCl (30%) and ethanol and separated by centrifugation. A suspension was made in 100 mL ether with the concluding sediment and filtered by PTFE membrane (pore size = 0.45 μm).
Afterwards, to obtain rGO, the resultant suspension was sonicated for 2 h more. At R.T., hydrazine hydrate was added drop by drop in the exfoliated GO suspension in 9: 7 ratio and then reduced the mixture at 100 °C for 1 h. Resulting precipitate was filtered with cellulose filter paper and washing was done with 1 M HCl and DI until pH was 7. In the final step, filtrate was dried at R.T. for 24 h to get rGO.
Preparation of the rGO@WS2 quantum dot composite
rGO@WS2QDs composite is prepared by mixing both WS2 QDs and rGO in 1:1 ratio by volume using probe sonicator for 2 h at R.T.
Preparation of modified electrode
A glassy carbon electrode (GCE) with a geometrical surface area of 0.07 cm2 was used as a substrate for the modification with rGO@WS2 QDs nanostructures. Before modification, GCE was cleaned on a wet polishing pad with 0.05 μm alumina slurry. Then it was washed 3–4 times with DI and further sonicated in DI for 2 min for complete surface cleaning. Then, as-prepared suspension of rGO@WS2 QDs composite was drop casted over this cleaned and polished GCE surface and allowed to dry at ambient temperature for 5–6 h and then used for all electrochemical measurements.
Results and discussions
Characterization of nanomaterials by FT-IR and UV-visible spectroscopy
The interaction between WS2 QDs and rGO was acquired with FT-IR spectroscopy and shown in fig. S1 of ESI. FT-IR spectra were analysed for all three samples in order to justify the composite formation The FT-IR spectra of WS2 QDs, rGO and rGO@WS2 QDs have been presented in the fig. S1. A broad and strong peak at 3130–3500 cm−1 is attributed to the -O-H and -N-H stretching. A strong peak at 1635 cm−1 is assigned to the stretching vibration of -C=O of the carboxylic group [41]. FT-IR of rGO was same as earlier works reported in literature and exhibiting different types of possible functional groups where 3435 cm−1 correspond to O-H stretching vibration, 1635 cm−1 showed -C=O stretching, -C=C stretching vibration at 1654 cm−1, -C-OH deformation vibration at 1384 cm−1 and a low intensity broad band at 1040 cm−1 for -C-O(epoxide) vibration and -C-OH vibration of -COOH groups [42]. The characteristics peaks of both WS2 QDs and rGO are observed in the rGO@WS2 QDs sample that elucidate the successful decoration of WS2 QDs over rGO sheets. On comparison, the difference in the -O-H stretching vibration and the peak deviation towards longer wavelength are ascribed to the interaction among WS2 QDs and -OH groups of rGO.
Further UV-Vis. absorption spectrum of WS2 QDs, rGO and rGO@WS2 QDs has been investigated and shown in fig. S2 of ESI. WS2 QDs. showed two absorption peak at 262 nm and 364 nm which was observed because of its excitonic feature. Absorption peak of rGO was found at 265 nm which endorsed the π- π* transition in the doubly bonded carbon atoms. Further the interaction of rGO@WS2 QDs was observed because of the quenching of peaks at 261 nm and 326 nm [40, 43].
Structural characterisation and Elemental analysis of nanomaterials by TEM, EDAX and EDAX mapping
HRTEM was used to unravel the atomic structure of rGO, WS2 QDs and the composite. Specimens for TEM were prepared using dilute samples by drop-casting method onto the carbon-coated TEM grids and left over for complete drying for 24 h in vacuum. TEM images of rGO (Fig. 2a) reveal the sheet like morphology. Single- or few-layered nanosheets of rGO are observed with plenty of wrinkles. Intrinsic nature of graphene is recommended by corrugation and scrolling. The water soluble WS2 QDs were characterized by TEM. The TEM image demonstrates the monodispersity of WS2 QDs (Fig. 2b). Its structure is further verified by SAED pattern which reveals the polycrystalline nature of WS2 QDs. Further TEM image of rGO@WS2 QDs (Fig. 2c) reveals that the prepared composite has the signature of the quantum dots distributed over nanosheets. In addition, EDAX technique was used to investigate the presence of different elements in rGO, WS2 QDs and rGO@WS2 QDs composite (Fig. S3). EDAX spectra of rGO@WS2 QDs (Fig. S3c) display the signals for Carbon, Oxygen, Tungston and Sulphur which prove their existence in the synthesized composite. EDAX mapping was also performed to confirm the constant distribution of all elements in rGO@WS2 QDs composite as shown in Fig.3.
Comparative study of different modified electrode towards CQ detection
To investigate the potential application of synthesized rGO, WS2 QDs and rGO@WS2 QDs composite, Glassy carbon electrode was chosen and modified by different materials. Performance of different modified GCEs (WS2 QDs modified GCE, rGO modified GCE and rGO@WS2 QDs modified GCE) were thoroughly investigated for CQ detection by CV (Fig. 4). It is observed that in all cases CQ oxidation occurs almost at same potential but displays different current values. The highest current response is obtained in case of rGO@WS2 QDs modified GCE as compared to WS2 QDs modified GCE and rGO modified GCE. This happens reasonably due to synergistic interaction between both the components.
Further CVs of Chloroquine were recorded at a series of scan rates ranging from 10 mV/s to 100 mV/s in 0.1 M phosphate buffer at bare GCE (Fig. 5a) and rGO@WS2 QDs modified GCE (Fig. 5b). It was revealed that in both the cases, the peak current increased on increasing the scan rate. According to Randles-Sevcik equation [44].
where ip is the anodic peak current, n is the number of exchanged electrons, A is the surface area of electrode (cm2), C is the bulk concentration (mol cm−3), ʋ is the scan rate (V/s) and D is the diffusion coefficient. From Eq. (1), current shows direct relation with the concentration as well as square root of the scan rate. Keeping the CQ concentration same, the anodic peak current ip was plotted against the square root of different scan rate for the bare as well as modified GCE and straight line was found in both the cases. Figure 5c shows that the anodic peak current increases linearly with the square root of the scan rate, indicating that this electrochemical process is a diffusion controlled process. The curve suggests the faster electron transfer for rGO@WS2 QDs modified GCE as compared to bare GCE. This is attributed to nano dimensional structure that enhances the accessible interface in between the electrolytes and electrode, facilitating the electrochemical reaction over the surface of the electrode.
Electrochemical sensing of CQ by cyclic voltammetry (CV) and differential pulse voltammetry (DPV)
After optimisation of various experimental conditions, electrochemical trace level detection of chloroquine phosphate was performed via CV and DPV techniques at rGO@WS2 QDs composite modified nanostructured electrode in 0.1 M phosphate buffer (pH = 6). The rGO@WS2 QDs modified GCE responds quickly towards oxidation of CQ on serial addition from 0.5 μM to 82.4 μM concentration. As the concentration of CQ increases, anodic peak current of CQ also increases gradually which is clearly depicted in its corresponding calibration plot shown with each CV and DPV plots (Fig. S4A, S4B and Fig. 6a, b). On addition of higher concentration, peak potential is shifted towards higher values which suggests that oxidation of CQ is a diffusion controlled process. Electrochemical sensing of CQ is performed in the linear range from 0.5 μM to 82.4 μM concentration with regression coefficient in the order of 0.97. The LOD and sensitivity is found to be 40 nM and 0.800 μAμM−1 cm−2 respectively through DPV (Fig. 6a, b). The excellent sensitivity towards CQ detection is obtained due to larger surface area, high electroactivity and connected structures of rGO@WS2 QDs composite through various functionalities.
After successful detection of CQ at rGO@WS2 QDs modified GCE in 0.1 M phosphate buffer (pH 6), sensing was performed in human serum in order to investigate any major complications that might be related to test real samples by both CV and DPV (Fig. S5A, S5B and Fig.7a, b). Blood samples of a healthy and adult person was collected and blood cells were removed through centrifugation. The obtained serum was diluted five times using phosphate buffer. After dilution, pH of buffer was checked and there was no change in pH. Same sensing behaviour is obtained at rGO@WS2 QDs modified GCE in serum from 0.5 μM to 82.4 μM concentration with linear calibration. The calibration plot obtained offers limit of detection (LOD) 120 nM with 0.900 μAμM−1 cm−2 sensitivity at S/N: 3 (Fig. 7a, b). The same sensing behaviour in human serum also confirms the validity of proposed sensor in other biological fluids.
Chloroquine phosphate is a potent drug against malaria. So, strategy of its sensing cannot be completed before its detection in pharmaceutical formulations. For this purpose, tablets of CQ were purchased from a medical shop. A tablet of CQ was powdered using mortar and pestle and finely ground. Versatility of CQ detection was explored by acquiring same experimental parameters as employed in ideal sensing. Same voltammetric response was obtained at rGO@WS2 QDs modified GCE on successive addition of tablet solution with linear calibration range of 0.5 μM to 82.4 μM concentration in both CV and DPV (Fig. S6A, S6B and Fig.8a, b). The obtained calibration plot offers good LOD as 80 nM and sensitivity of 0.143 μAμM−1 cm−2 at S/N: 3 by DPV (Fig.8a, b).
As seen from both CV and DPV curves, Chloroquine is oxidized in 1.20 V - 1.25 V potential range, which was shown in the form of oxidation peak. It is found that this oxidation process was irreversible, as no cathodic peak was found in CV. A plausible electro-sensing mechanism for Chloroquine involves its electro-oxidation over rGO@WS2 QDs modified GCE as shown in Fig. 9. The synthesized material may probably augment the conductivity of electrode and facilitate the process of electron transfer, thereby improving the analytical selectivity and sensitivity towards the detection of the particular analyte. Also, high electro-catalytic efficiency of rGO@WS2 QDs facilitates a superior corridor for electro-oxidation performance of choloroquine [40]. Thus anodic peak shown in each DPV or CV may be ascribed to the irreversible oxidation of the N-heterocyclic nitrogen of the aminoquinoline moiety and the nitrogen of alkylamino side chain grouping of chloroquine molecule [12, 45, 46].
Interference study
It is well known that human blood serum has many other biocomponents viz. carbohydrates, amino acids, lipids, salts etc., which can interfere in desired drug detection and hinder the selectivity of proposed electrochemical sensor or biosensor. Thus, we have performed experiments with some common molecules present in human blood serum. Results described in Fig. 10, for D-glucose, Creatine hydrate, L-ascorbic acid, uric acid, L-cysteine and urea, showed very less or negligible interference even at 10 times higher concentration of each interferent (500 μl of 1 mM stock) with respect to concentration of choloroquine (50 μl of 1 mM stock).
Reproducibility and stability study
Developed sensor can be used 2–3 times due to less change in current values as shown in Fig. 11a. For a particular concentration of drug it is observed that developed sensor showed retention of current upto 97%. After 3 h of time interval, only 2–3% current is decreased which shows that our developed sensor shows reliabe stability or can be reused. On the other hand interday stability investigation of developed sensor shows that it is adequately stable upto 10 days. More than 90% current is retained as displayed in Fig. 11b. The electrode is highly stable towards CQ sensing and can be applied for many days with good accuracy.
Our developed sensor is highly sensitive towards detection of CQ in buffer, real samples and in pharmaceutical formulations without any interference. The electrode shows reliable stability and it can be compared with other developed sensors reported earlier in literature in Table 1.
Conclusions
We developed a sensitive 2D composite-modified GCE for electrochemical detection of anti malarial drug, Chloroquine phosphate by two techniques, CV and DPV simultaneously. The composite serves as a nano-mediator for the rapid transfer of electron to the electrode from the redox centre. The rGO@WS2 QDs modified GCE is highly sensitive towards detection of CQ from 0.5 μM to 82.4 μM concentration range with 40 nM limit of detection (LOD) at signal to noise ratio (S/N): 3. Detection of CQ is also performed in real samples (human serum) as well as in pharmaceutical formulations. Our developed sensing platform is highly sensitive and reliable for developing electro-sensing devices using screen printed electrodes and open new avenues in the field of electro-sensing.
References
Tracy JW, Webster LT, Brunton L, Parker K, Lazo J, Buxton I (1996) Goodman and Gilman’s the pharmacological basis of therapeutics, D. Blumenthal (Eds.), McGraw-Hill, New York, , pp. 965
Weingarten HL, Cherry EJA (1981) A chloroquine fatality. Clin Toxicol 18:959–963. https://doi.org/10.3109/15563658108990324
Thapliyal N, Chiwunze TE, Karpoormath R, Goyal RN, Patel H, Cherukupalli S (2016) Reasearch progress in electroanalytical techniques for detremination of antimalarial drugs in pharmaceutical and biological samples. RSC Adv 6:57580–57680. https://doi.org/10.1039/c6ra05025e
Saad B, Zin ZM, Jab MS, Rehman IA, Saleh MI, Mahsufi S (2005)Flow-through chloroquine sensor and its applications in pharmaceutical analysis. Anal Sci 21:521–524. https://doi.org/10.2116/analsci.21.521
Savarino A, Trani LD, Donatelli I, Cauda R, Cassone A (2006) New insights into the antiviral effects of chloroquine. Lancet Infect Dis 6:67–69. https://doi.org/10.1016/S1473-3099(06)70361-9
Sotelo J, Brice˜no E, L’opez-Gonz’alez MA (2006) Adding chloroquine to conventional treatment for Glibostoma Multiforme: a randomized, double-blind, placebo-controlled trial. Ann Intern Med 144:337–343. https://doi.org/10.7326/003-4819-144-5-200603070-00008
Muhm M, Stimpfl T, Malzer R, Mortinger H, Binder R, Vycudilik W, Berzlanovich A, Bauer G, Laggner AN (1996) Suicidal chloroquine poisoning: clinical course, autopsy findings, and chemical analysis. J Forensic Sci 41:1077–1079. https://doi.org/10.1520/JFS14054J
Qarah AS N, Basavaiah K, Swamy N (2017) Sensitive and selective extraction-free spectrophotometric assay of chloroquine phosphate in pharmaceuticals based on ion-pair reaction with Bromocresol green and Bromocresol purple. Pharm Anal Acta 8(3):539–566. https://doi.org/10.4172/2153-2435.1000539
Ducharme J, Farinotti R (1997) Rapid and simple method to determine chloroquine and its desethylated metabolites in human microsomes by high-performance liquid chromatography with fluorescence detection. J Chromatogr B 698:243–250. https://doi.org/10.1016/S0378-4347(97)00273-9
Croes K, McCarthy PT, Flanagan RJ (1994) Simple and rapid HPLC of quinine, Hydrochloroquine, chloroquine, and Desethylchloroquine in serum, whole blood, and filter paper-adsorbed dry blood. J Anal Toxicol 18:255–260. https://doi.org/10.1093/jat/18.5.255
Papadopoulos K, Triantis T, Dimotikali D, Nikokavouras J (2000)Radiostorage- and photostoragechemiluminescence: analytical prospects. Anal Chim Acta 423:239–245. https://doi.org/10.1016/S0003-2670(00)01118-1
Radi A (2005) Accumulation and trace measurement of chloroquine drug at DNA-modified carbon paste electrode. Talanta 65:271–275. https://doi.org/10.1016/j.talanta.2004.05.024
Jiao L, Zhang L, Wang X, Diankov G, Dai H (2009) Narrow graphene nanoribbons from carbon nanotubes. Nature 458:877–880. https://doi.org/10.1038/nature07919
Park S, Ruoff RS (2009) Chemical methods for the production of graphenes. Nat Nanotechnol 4:217–224. https://doi.org/10.1038/nnano.2009.581
Ding J, Sun W, Wei G, Su Z (2015) Cuprous oxide microspheres on graphene nanosheets: an enhanced material for non- enzymatic electrochemical detection of H2O2 and glucose. RSC Adv 5:35338–35345. https://doi.org/10.1039/C5RA04164C
Zhang P, Lu X, Huang Y, Deng J, Zhang L, Ding F, Su Z, Wei G, Schmidt OG (2015) MoS2 nanosheets decorated with gold nanoparticles for rechargeable LiO2 batteries. J Mater Chem A 3:14562–14566. https://doi.org/10.1039/C5TA02945G
Zhu S, Wang J, Fan W (2015)Graphene-based catalysis for biomass conversion. Catal Sci Technol 5:3845–3858. https://doi.org/10.1039/C5CY00339C
Kim KS, Zhao Y, Jang H, Lee SY, Kim JM, Kim KS, Ahn J-H, Kim P, Choi J-Y, Hong H (2009) Large scale pattern growth of graphene films for stretchable transparent electrodes. Nature 457:706–710. https://doi.org/10.1038/nature07719
Neto AHC, Guinea F, Peres NMR, Novoselov KS, Geim AK (2009) The electronic properties of graphene. Rev Mod Phys 81:109–162. https://doi.org/10.1103/RevModPhys.81.109
Liu Z, Liu Q, Huang Y, Ma Y, Yin S, Zhang X, Sun W, Chen Y (2008) Organic photovoltaic devices based on a novel acceptor material: graphene. Adv Mater 20:3924–3930. https://doi.org/10.1002/adma.200800366
Xiong D, Li X, Shan H, Zhao Y, Dong L, Xu H, Zhang X, Li D, Sun X (2015) Oxygencontaining functional groups enhancing electrochemical performance of porous reduced graphene oxide cathode in lithium ion batteries. Electrochim Acta 174:762–769. https://doi.org/10.1016/j.electacta.2015.06.041
Becerril HA, Mao J, Liu Z, Stoltenberg RM, Bao Z, Chen Y (2008) Evaluation of solution processed reduced graphene oxide films as transparent conductors. ACS Nano 2:463–470. https://doi.org/10.1021/nn700375n
Shin H-J, Kim KK, Benayad A, Yoon S-M, Park HK, Jung I-S, Jin MH, Jeong H-K, Kim JM, Choi J-Y, Lee YH (2009) Efficient reduction of graphite oxide by sodium borohydride and its effect on electrical conductance. Adv Funct Mater 19:1987–1992. https://doi.org/10.1002/adfm.200900167
Yin S, Men X, Sun H, She P, Zhang W, Wu C, Qin W, Chen X (2015) Enhanced photocurrent generation of bio-inspiredgrapheme/ZnO comppsite films. J Mater Chem A 3:12016–12022. https://doi.org/10.1039/C5TA02297E
Du Y, Zhao Y, Qu Y, Chen CH, Chen CM, Chuang CH, Zhu Y (2014) Enhanced light-matter interaction of grapheme-glod nanoparticle hybrid films for high performance SERS detection. J Mater Chem C 2:4683–4691. https://doi.org/10.1039/C4TC00353E
Zhao X, Zhang P, Chen Y, Su Z, Wei G (2015) Recent advances in the fabrication and structure-specific applications of grapheme-based inorganic hybrid membranes. Nanoscale 7:5080–5093. https://doi.org/10.1039/C5NR00084J
Kumar A, Prakash R (2014) Graphene sheets modified with Polyindole for electrochemical detection of dopamine. J Nanosci Nanotechnol 14:2501–2506. https://doi.org/10.1166/jnn.2014.8549
Salavagione HJ, Díez-Pascual AM, Lázaro E, Vera S, Gómez-Fatou MA (2014) Chemical sensors based on polymer composites with carbon nanotubes and grapheme: the role of the polymer. J Mater Chem A 2:14289–14328. https://doi.org/10.1039/C4TA02159B
Liu Y, Wang R, Zhu Y, Li R, Zhang J (2015) Photochemical sensing of catechol based on CdS-DNA-pristine grapheme nanocomposite film. Sensors Actuators B Chem 210:355–361. https://doi.org/10.1016/j.snb.2014.12.124
Srivastava M, Nirala NR, Srivastava SK, Prakash R (2018) A comparative study of Aptasensor vs Immunosensor for label-free PSA Cancer detection on GQDs-AuNRs modified screen-printed electrodes. Sci Rep 8:1923–1933. https://doi.org/10.1038/s41598-018-19733-z
Chen M, Su H, Mao L, Guo M, Tang J (2018) Highly sensitive electrochemical DNA sensor based on the use of three-dimensional nitrogen-doped graphene. Microchim Acta 185:51–59. https://doi.org/10.1007/s00604-017-2588-2
Xing L, Ma Z (2016) A glassy carbon electrode modified with nanocomposite consisiting of MoS2 and reduced graphene oxide for electrochemical simultaneous determination of ascorbic acid, dopamine, and uric acid. Microchim Acta 183:257–263. https://doi.org/10.1007/s00604-015-1648-8
Dadkhah S, Ziaei E, Mehdinia A, Kayyal TB, Jabbari A (2016) A glassy carbon electrode modified with amino-functionalized grahene oxide and molecularly imprinted polymer for electrochemical sensing of bisphenol a. Microchim Acta 183:1933–1941. https://doi.org/10.1007/s00604-016-1824-5
Yan X, Dai Y, Chen M, Wang J, Ren J, Wang Q, Wu Y, Ye N, Wang Y, Cheng X (2017) WS2 nanosheets decorated by ag nanoparticles with different content and uniform distribution for enhanced electrochemical properties. J Nanopart Res 19(5):165–173. https://doi.org/10.1007/s11051-017-3865-z
Zhang XH, Tan H, Fan Z, Ge MZ, Ye X, Xue MQ (2017) Synthesis and electrochemical performance of ultrathin WS2 nanosheets. Chalcogenide Lett 14(10):419–423
Zhou Y, Sui C, Yin H, Wang Y, Wang M, Ai S (2018) Tungsten disulfide (WS2) nanosheet-based photoelectrochemical aptasensing of chloramphenicol. Microchim Acta 185(10):453–460. https://doi.org/10.1007/s00604-018-2970-8
Gopalakrishnan D, Damien D, Shaijumon MM (2014) MoS2 Quatum dot-interspersed exfoliated MoS2 Nanosheets. ACS Nano 8:5297–5303. https://doi.org/10.1021/nn501479e
Yang Z-C, Wang M, Yong AM, Wong SY, Zhang X-H, Tan H, Chang AY, Li X, Wang J (2011) Intrinsically fluorescent carbon dots with tunable emission derived from hydrothermal treatment of glucose in the presence of monopotassium phosphate. Chem Commun 47:11615–11617. https://doi.org/10.1039/C1CC14860E
Yang Y, Cui J, Zheng M, Hu C, Tan S, Xiao Y, Yang Q, Liu Y (2012)One-step synthesis of amino-functionalized fluorescent carbon nanoparticles by hydrothermal carbonization of chitosan. Chem Commun 48:380–382. https://doi.org/10.1039/C1CC15678K
Tiwari P, Nirala NR, Prakash R (2018) Determination of the anti-HIV drug Nevirapine using electroactive 2D material Pd@rGO decorated with MoS2 quantum dots. Chemistry Select 3:5341–5347. https://doi.org/10.1002/slct.201702250
Zhao X, He D, Wang Y, Fu C (2018) Facile fabrication of tungsten disulfide quantum dots (WS2 QDs) as effective probes for fluorescence detection of dopamine (DA). Mater Chem Phys 207:130–134. https://doi.org/10.1016/j.matchemphys.2017.12.045
Singh MK, Pandey RK, Prakash R (2017)High-performance photo detector based on hydrothermally grown SnO2nanowire/reduced graphene oxide (rGO) hybrid material. Org Electron 50:359–366. https://doi.org/10.1016/j.orgel.2017.08.016
Yan Y, Zhang C, Gu W, Ding C, Li X, Xian Y (2016) Facile synthesis of water-soluble WS2 quantum dots for turn-on fluorescent measurement of lipoic acid. J Phys Chem C 120:12170–12177. https://doi.org/10.1021/acs.jpcc.6b01868
Rafati AA, Afraz A (2014) Amperometric sensing of anti-HIV drug zidovudine on ag nanofilm-multiwalled carbon nanotubes modified glassy carbon electrode. Mater Sci Eng C 39:105–112. https://doi.org/10.1016/j.msec.2014.02.037
Mathur NC, Goyal RN, Malik WU (1990) Oxidation of 2-aminoquinoline at a stationary pyrolytic graphite electrode. Anal Chim Acta 230:91–93. https://doi.org/10.1016/S0003-2670(00)82765-8
Lama K, Stephen J, Van W, William EG (2017)One-electron oxidation of chloroquine, cymanquine, and related aminoquinolines in nonaqueous media. J Electroanal Chem 799:531–537. https://doi.org/10.1016/j.jelechem.2017.06.041
Mashhadizadeh MH, Akbarian M (2009) Voltammetric determination of some anti-malarial drugs using a carbon paste electrode modified with Cu(OH)2 nano-wire. Talanta. 78:1440–1445. https://doi.org/10.1016/j.talanta.2009.02.040
Acknowledgements
The first author (MS) is grateful to DST, New Delhi (SR/WOS-A/CS-52/2018) for fellowship. The authors are also thankful to Prof. D. Dash, Institute of Medical Science, BHU,Varanasi, for the help extended towards availing blood serum and CIF, IIT (BHU) Varanasi for providing various characterization facilities.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests. Monika Srivastava and Preeti Tiwari with Vineet have carried out experiments and are responsible for raw data if required. Human blood serum was collected from blood donors of the institute using our institute hospital (Institute of Medical Sciences, BHU, Varanasi) facility following the methods in accordance with relevant guidelines and regulations. All experimental protocols were approved by the Biochemistry lab of the Institute of Medical Sciences, BHU, Varanasi and further, it is sanctioned by institutional committee (Institute Ethical Committee) and consent was obtained from all subjects.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
It includes FT-IR Spectroscopy, UV-Vis spectroscopy, Energy dispersive X-Ray spectroscopy of WS2 QDs, rGO and rGO@WS2 QDs. It further covers CV responses of rGO@WS2 QDs modified GCE towards electro-oxidation of CQ in buffer, blood serum and in pharmaceutical formulation. (DOC 1030 kb)
Rights and permissions
About this article
Cite this article
Srivastava, M., Tiwari, P., Mall, V.K. et al. Voltammetric determination of the antimalarial drug chloroquine using a glassy carbon electrode modified with reduced graphene oxide on WS2 quantum dots. Microchim Acta 186, 415 (2019). https://doi.org/10.1007/s00604-019-3525-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3525-3