Abstract
A method is described for sensitive and selective detection of iodine by using a paper strip modified with silver triangular nanoplates (AgTNPs). It is based on the extraction of iodine from a solution into a flow of air via dynamic gas extraction and transferring it through a reactive paper modified with AgTNPs. The interaction of AgTNPs with iodine results in a color change from blue to white. This can be visually detected and monitored by digital colorimetry. The dynamic gas extraction is highly selective for volatile compounds so that a sample pretreatment is minimal. Due to the sensitivity of AgTNPs for iodine, the limit of its detection is as low as 7 μg L−1, and the analytical range is of 20–200 μg L−1. The method also was applied in a new approach for determination of organic compounds that can interact with iodine. The organic compound is exposed to an excess of iodine, and this is followed by detection of residual iodine as described above. The method was applied to the determination of ascorbic acid, caffeine and the drug metamizole.

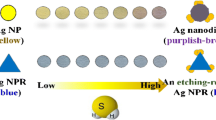
Schematic representation of a procedure of organic iodine-interacting compounds (Org.) determination. It is based on their iodination followed by gas extraction of the residual iodine, its interaction with silver triangular nanoplates and colorimetric detection with a scanner.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gold and silver nanoparticles have found various applications in analytical chemistry due to their surface plasmon resonance (SPR) often also called localized surface plasmon resonance (LSPR). It is a basis for the development of optical sensors with nanoparticles both in colloid solution and on solid supports [1,2,3,4,5,6,7,8,9,10].
The shape of the nanoparticles essentially influences upon their optical and chemical properties. It determines their possible analytical applications. A good example is silver triangular nanoplates (AgTNPs). Due to their sharp corner plane shape AgTNPs exhibit an intense SPR band in a long-wave part of the visible spectral region and are assumed to be a sensitive and easily prepared optical probe in chemical analysis [11,12,13,14,15,16].
In spite of such advantages as high sensitivity, ease of the analytical response detection, tunability of the optical and analytical parameters, there are some limitations associated with these nanoparticles. One of them is a decreased selectivity caused by aggregative instability and shape transformations induced by some species, e.g. chloride, bromide, and iodide anions [17,18,19,20]. These limitations hinder application of AgTNPs in some fields of chemical analysis.
Our previous works [21, 22] showed that sensing in a gas phase is more selective. Spatial separation of nanoparticles from a solution by a “gaseous membrane” is an effective tool to overcome above mentioned limitations. In this sense, good opportunities are provided by nanocomposites, where nanoparticles are deposited on solid supports [23].
The aim of this study was to develop a selective and sensitive colorimetric method to detect iodine based on using paper modified with AgTNPs and dynamic gas extraction and to assess analytical possibilities of this detection method for determination of iodine-interacting organic compounds.
Materials and methods
Reagents and instruments
The following reagents were used in this study: silver nitrate (PZTsM-Vtormet), sodium citrate (Sigma-Aldrich, www.sigmaaldrich.com), polyvinylpyrrolidone (Mw = 58,000 g mol−1, Acros Organics, www.acros.com), hydrogen peroxide (30 wt.% in H2O, Sigma-Aldrich, www.sigmaaldrich.com), sodium borohydride (Acros Organics, www.acros.com), sulfuric acid (Aquatest, www.atest-rostov.ru), hydrochloric acid (Aquatest, www.atest-rostov.ru), ethanol (Sigma-Aldrich, www.sigmaaldrich.com), ascorbic acid (Aquatest, www.atest-rostov.ru), caffeine (Aquatest, www.atest-rostov.ru), potassium iodide (Aquatest, www.atest-rostov.ru), starch (Aquatest, www.atest-rostov.ru), potassium iodate (Aquatest, www.atest-rostov.ru), sodium thiosulfate (Aquatest, www.atest-rostov.ru). All the reagents were at least of analytical grade. Working solutions of the substances were prepared by dissolving their weighed portions or aliquots and dilution in deionized water obtained using Millipore Simplicity water purification system (Merk Millipore, www.merckmillipore.com). For preparation of paper test strips modified with AgTNPs, the paper Whatman Grade 113 (Whatman International Ltd) was used. Iodine g L−1 stock solution was prepared from 0.1 g L−1 commercial solution. The concentration of iodine was daily established by iodometric titration. Working standard iodine solutions were prepared by dilution of the stock solution immediately before use.
Absorption spectra of solutions were recorded by SF-103 spectrophotometer (Akvilon, Russia, www.akvilon.su), diffuse reflectance measurements were carried out using Eye-One Pro mini-spectrophotometer (X-Rite, www.xrite.com) on a white base. The measured diffuse reflectance coefficient values (R) were recalculated in terms of the Kubelka–Munk function (F) by the formula F = (1–R)2/(2R). Paper strips were scanned using Canon CanoScan LiDE 210 (Canon, www.canon.ru) on a white background with the resolution of 300 ppi. The scanned images were processed in Adobe Photoshop 7.0 graphical editor by averaging RGB color coordinates of individual pixels as follows. A round zone was selected on the image of a test-strip where a gas stream passed, using Elliptical Marquee Tool. Then, Image – Histogram tool was selected from the main toolbar. In the appearing tab, mean values of R, G and B color coordinates were found.
Scanning electron microscopic studies of paper test strips microstructure were carried out using scanning electron microscope Jeol JSM 7100 F (Jeol, Japan) at the accelerating voltage of 3–7 kV. Recording SEM images was performed with a detector of back reflected electrons at the low vacuum operation mode.
A commercial-grade simulator based on the finite-difference time-domain method was used to perform the calculations of the AgTNP spectrum (FDTD Solutions, Lumerical Inc., Canada).
Dynamic gas extraction was processed using a setup proposed earlier [24, 25] and represented in Fig. 1. It included a glass vessel for analyzed solution (1) closed with a rubber stopper (2), a test strips holder (3), an air micro-compressor (4) connected via a polymer hose (5) with a glass bubbler (6) sealed in the vessel.
Preparation of silver triangular nanoplates (AgTNPs)
Synthesis of AgTNPs was performed as described elsewhere [26, 27] with minor modifications. Glassware used in the synthesis was pre-washed with freshly prepared “aqua regia”, thoroughly rinsed with distilled water and air-dried. A 0.5 mL portion of 0.01 M silver nitrate aqueous solution was diluted with 4.1 mL of deionized water. Then 2.3 mL of 1%w/w sodium citrate aqueous solution, 0.6 mL of 20 g L−1 polyvinylpyrrolidone aqueous solution and 1.2 mL of 3%w/w hydrogen peroxide aqueous solution were successively added under vigorous stirring. Then 1.0 mL of a freshly prepared 0.1 M sodium borohydride aqueous solution was added drop by drop to the solution under stirring. Mixture got pale yellow-green color, which half an hour later abruptly changed to intense emerald-green and then to blue-violet. The stirring was stopped. The AgTNPs colloidal solution was stable for at least 1 month when stored at room temperature. The final concentration of AgTNPs in the solution was 56 μg mL−1 (0.52 mmol L−1 in terms of silver atoms), which was calculated based on the introduced silver amount assuming quantitative yield of the product.
Preparation of paper test strips modified with AgTNPs
Deposition of AgTNPs onto the paper was performed as described earlier [28]. Briefly, a 1.5 mL portion of AgTNPs solution, prepared as described above, was placed into a Petri dish. A Whatman Grade 113 paper disk was placed into the dish to cover the solution portion. This was accompanied by rapid and quite uniform distribution of the solution across the paper. Then the Petri dish was placed on an electric heater and dried in air at ~ 80 °C. The operation was repeated for 3 times, which corresponds to the amount of deposited AgTNPs 0.55 mg g−1 of paper. The modified paper was cut into test strips and fitted inside the test strips holder. The content of AgTNPs on the paper was calculated based on the added total amount of AgTNPs solution.
Calibration plot
To construct the calibration plot, series of iodine solutions prepared from the iodine 0.01 g L−1 stock solution were used. Different aliquots of 0.1; 0.2; 0.5; 1.0; 1.5; and 2.0 mL were pipetted into a 100 mL flask and diluted up to the mark with distilled water. The final solutions had the concentrations of 0.01; 0.025; 0.05; 0.1; and 0.2 mg L−1. These solutions were successively placed into the reaction glass vessel (Fig. 1). Then 2 mL of concentrated sulfuric acid was added, the neck of the vessel was closed tightly with the stopper carrying the test strips holder with the AgTNPs modified test strip attached. The air micro-compressor was turned on and the air was bubbled through the solution with the volume velocity of 2.8–3.0 L min−1 during 20 min. Then the test strip was withdrawn and scanned. The saved image file was analyzed in terms of R,G,B-color coordinates.
Determination of iodine-interacting organic compounds
Metamizole sodium in pharmaceutical formulations “Analgin” and “Baralgin M”
Tablets of the formulations were ground in a porcelain mortar, a sample portion of 0.03 g was taken, placed in a 200 mL flask, and diluted up to the mark with water. For the analysis, 0.1 mL of the resulting solution was taken, placed into a 100 mL flask, and diluted up to the mark with water. The solution was placed into the glass vessel of the dynamic gas extraction setup, then 2 mL of concentrated sulfuric acid, and 2 mL of 0.01 g L−1 iodine solution were added. The residual iodine concentration was then determined as described above.
Alternatively determination of metamizole sodium in the pharmaceuticals was carried out by a titrimetric method according to [29]. To do this, 0.15 g of a pharmaceutical formulation was placed in a dry flask; 20 mL of 96%v/v ethanol, 5 mL of 0.01 M hydrochloric acid were added and immediately titrated with 0.05 M iodine solution under stirring until a yellow color that did not disappear during 30 s.
Ascorbic acid
A portion of ascorbic acid of 0.035 g was placed into a 1000 mL flask, dissolved, and diluted up to the mark with water. 0.1 mL of the resulting solution was taken, placed into the glass vessel of the dynamic gas extraction setup, then 2 mL of concentrated sulfuric acid, and 2 mL of 0.01 g L−1 iodine solution were added. The residual iodine concentration was then determined as described above.
Alternatively ascorbic acid was determined according to [30]. To do this, 10 tablets were ground and a weighed portion of the powder containing ~0.1 g of ascorbic acid was taken. The sample was placed into a 100 mL volumetric flask, and dissolved in 10 mL of distilled water and 10 mL of 2%w/w hydrochloric acid solution under shaking for 10 min. The volume of the resulting solution was adjusted to the mark with water, stirred and filtered. The first 10 mL of the filtrate was discarded. Then 10.0 mL of the resulting solution were placed into a 100 mL flask, 1 mL of 2%w/w hydrochloric acid solution, 0.5 mL of 1%w/w potassium iodide solution, 2 mL of 0.5%w/w starch solution, 10 mL of water were added. The mixture was titrated with 0.00167 M solution of potassium iodate until appearance of a persistent light blue color.
Caffeine
A portion of caffeine of 0.03 g was dissolved in 50 mL of hot water in a 500 mL volumetric flask, and the solution was dissolved up to the mark. 0.1 mL of the resulting solution was taken, placed into the glass vessel of the dynamic gas extraction setup, then 2 mL of concentrated sulfuric acid, and 2 mL of 0.01 g L−1 iodine solution were added. The residual iodine concentration was then determined as described above. The same procedure was used for the analysis of a pharmaceutical formulation and samples of coffee.
Alternatively the determination was made by iodometric titration [31]. Caffeine was dissolved in 30 mL of hot water, then 10 mL of 15%w/w sulfuric acid solution and 20 mL of 0.1 N iodine solution were added. The solution was dissolved up to the mark in a 100 mL volumetric flask, thoroughly mixed, allowed to stand for 15 min and filtered into a dry flask. Excess of iodine in an aliquot part of the solution was titrated with 0.05 N sodium thiosulfate.
Determination of caffeine in coffee was carried out with preliminary extraction by chloroform according to [32] (see Supplementary Material).
Results and discussion
It is well known that interaction with iodine, as a soft oxidant, is utilized for determination of many organic compounds. However, classical procedures based on this interaction, e.g. titration, photometry, are usually either insufficiently sensitive or face interferences in complex matrices. The solutions of these problems may be found using modern sensitive reagents for detection of iodine and separation of the analyte from interfering compounds respectively.
Principle of the detection method
A principle behind the approach is based on dynamic extraction of iodine from solution into an air flow with simultaneous detection by AgTNPs attached to a test-strip spatially separated from interfering ions and other non-volatile compounds by an air barrier. It can be realized using a setup represented in Fig. 1. The dynamic regime of the setup operation enables simultaneous detection of iodine by the sensing material and easy control of sensitivity by changing the operation time.
The air stream extracts iodine from the acidified solution and carries it to the paper modified with AgTNPs. The reaction of iodine with immobilized AgTNPs results in their oxidation and appearing a white spot on the test strip. The decrease of the color intensity, which is monitored by ordinary scanner, can be correlated with the concentration of iodine in the solution.
Characterization of silver triangular nanoplates (AgTNPs) and paper modified with AgTNPs
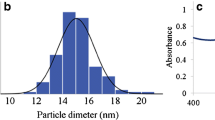
The AgTNPs and the paper modified with AgTNPs were characterized by spectrophotometry, diffuse reflectance spectroscopy and scanning electron microscopy. Figure 2a represents a spectrum of AgTNPs in aqueous solution. These nanoparticles exhibit an intense absorption band at 600–650 nm and a shoulder at 400–450 nm. The good correspondence of experimental and calculated spectra indicates that the AgTNPs edge length averages about 50 nm, whereas their thickness is about 4–6 nm (number of particles measured >100). The AgTNPs colloidal aqueous solution is stable and retains their spectral characteristics practically unaltered for at least 1 month.
For preparing paper modified with AgTNPs, an impregnation method has been used, which is simple, ease of control, and rapid. Moreover, the AgTNPs spectral characteristics are preserved during described above modification. As it can be seen from Fig. 2b, a diffuse reflectance spectrum of the modified paper contains an absorption band similar to the solution. On the SEM image of the modified paper (Fig. 2c), one can see AgTNPs distributed on the surface of paper fibers.
Interaction of paper modified with AgTNPs with iodine
Interaction of AgTNPs deposited on a paper strip has been studied using the dynamic gas extraction setup described above. In this setup, molecular iodine extracted from aqueous solution by air flow is transferred to the paper modified with AgTNPs. This interaction results in discoloration the blue colored paper due to oxidation of AgTNPs with iodine. A study of the discolored reactive zone with SEM showed presence of differently shaped nanoparticles attached to the paper fibers, which presumably are AgI (Fig. 3).
The color changes were monitored using ordinary scanner, by calculating corresponding RGB color coordinates using common image processing software. At fixed volume of the solution, the main parameters influencing the interaction completeness are the dynamic gas extraction presets (time of extraction and rate of air flow) and concentration of iodine in the solution. Effects of the extraction presets are described in the Supplementary Material. Based on them, the air flow rate of 2.8–3.0 L min−1 and the time of dynamic gas extraction of 20 min were set for all further experiments.
Discoloration of paper modified with AgTNPs can be monitored by calculating corresponding RGB color coordinates of a scanned image. The RGB color coordinate system was chosen as a native one for image recording devices. The interaction with iodine leads to increasing all the three color coordinates as represented in Fig. 4.
The dependence of RGB color coordinates on the concentration of iodine can be described by first order exponential equation similar to one proposed in our previous works [21, 22]: y = y0 + Ae−c/t, where y is R, G or B color coordinate, c is the concentration of an analyte, y0, A and t – regression parameters. Alternatively, these dependences can be approximated by the equation: y = y0 + A(1 − e−c/t). In this case y0 is associated with the corresponding color coordinate of the blank. The latter equation was used in this work to fit experimental data. Sensitivity coefficients were calculated as A/t [21, 22]. Optimal parameters of the fitting are represented in Table 1. One can see that the highest sensitivity coefficient was achieved using R color coordinate. Therefore, this color coordinate was used as the analytical response for construction the calibration plot. The limit of detection of iodine, which was calculated as 3s0/(A/t), where s0 – standard deviation of R color coordinate for the blank, is equal to 7 μg L−1 and the determination range is 20–200 μg L−1. These values indicate high sensitivity of the method compared to other reported in literature (Table 2).
Due to spatial separation of AgTNPs and analyzed solution, the detection of iodine is possible in the presence of high amounts of electrolytes and non-volatile compounds. It is affected by chlorine, bromine and hydrogen sulfide, which can also be extracted by air flow.
Determination of iodine-interacting organic compounds
There are many analytical procedures where iodine is added to a determined substance, followed by measuring iodine excess and calculating amount of consumed iodine to estimate concentration of the substance of interest. Selective and sensitive detection of iodine is a key step of such procedures, remarkably affecting accuracy of the analysis. The present method for detection of iodine thus can be a good basis for new approach to determine iodine-interacting organic compounds. To assess practical applicability of the approach, we perform determination of such compounds of different classes. This approach was used in the analysis of pharmaceuticals (metamizole sodium containing drugs “Analgin” and “Baralgin M”), ascorbic acid and caffeine. The excess of iodine should be within 20–200 μg L−1 range (it is desirable of ~ 100 μg L−1) above the stoichiometric amount required for interaction with a compound.
Determination of metamizole sodium is based on its oxidation with iodine according to the Scheme 1.
This reaction proceeds quantitatively with stoichiometry of 1:1. At high concentrations, the determination can be realized via titrimetry. However, at low concentrations, it is not possible because of low sensitivity of the titrimetric analysis. The results of metamizole sodium determination in pharmaceutical formulations “Analgin” and “Baralgin M” are summarized in Table 3.
Ascorbic acid is an important vitamin that should be controlled in various samples. Its iodometric determination can be realized through the reaction represented in Scheme 2.
The discussed procedure was applied to the analysis of pure pharmaceutical ascorbic acid as well as its pharmaceutical formulations. The results are given in Table 4.
To determine caffeine, its ability to form polyiodides in acidic medium was used [31]. This can be depicted by the Scheme 3.
The results of caffeine determination in formulations and coffee are represented in Table 5.
The results given in these tables indicate that the method has good precision and accuracy. It can be applied to different matrices. The main analytical characteristics of the AgTNP-based approach are compared with those reported in literature by the example of ascorbic acid as the most often determined iodine-interacting compound (Table 2). It can be seen that sensitivity of the method is very high.
To sum up, the main advantages of the gas extraction-colorimetric determination are as follows:
-
a significant increase in the sensitivity of the determination (for ascorbic acid and metamizole sodium, the determination is possible in the range of 0.5 × 10−8 to 7.5 × 10−8 mol in a sample, for caffeine – 0.25 × 10−8 to 3.8 × 10−8 mol in a sample), which makes future prospects of applying the method to biological fluids for the purposes of medicinal chemistry and pharmacokinetics;
-
lower consumption of reagents compared to iodometric titration;
-
simple sample preparation step (in contrast to spectrophotometry, the present method can be applied directly to analysis of cloudy and colored solutions).
The main limitation of the approach remains inability to determine individual iodine-interacting compounds in their mixture. To do this, one should use standard analytical procedures of sample preparation.
Conclusion
A new method combining nanoparticle-based optical detection with dynamic gas extraction of iodine has been proposed. The experimental conditions have been optimized. This method has been shown to be promising for determination of various iodine-interacting organic compounds, such as components of pharmaceutical formulations, ascorbic acid, caffeine etc. Its main advantages are high sensitivity and selectivity. This method can be easily hyphenated with modern measuring techniques, such as digital colorimetry.
References
Priyadarshini E, Pradhan N (2017) Gold nanoparticles as efficient sensors in colorimetric detection of toxic metal ions: a review. Sensors Actuators B Chem 238:888–902
Fang C, Dharmarajan R, Megharaj M, Naidu R (2017) Gold nanoparticle-based optical sensors for selected anionic contaminants. Trends Anal Chem 86:143–154
Lim M-C, Kim Y-R (2016) Analytical applications of nanomaterials in monitoring biological and chemical contaminants in food. J Microbiol Biotechnol 26:1505–1516
Nie G, Li G, Wang L, Zhang X (2016) Nanocomposites of polymer brush and inorganic nanoparticles: preparation, characterization and application. Polym Chem 7:753–769
Abalde-Cela S, Carregal-Romero S, Paulo Coelho J, Guerrero-Martínez A (2016) Recent progress on colloidal metal nanoparticles as signal enhancers in nanosensing. Adv Colloid Interf Sci 233:255–270
Terenteva EA, Apyari VV, Kochuk EV, Dmitrienko SG, Zolotov YA (2017) Use of silver nanoparticles in spectrophotometry. J Anal Chem 72:1138–1154
Choi I (2016) Recent advances in nanoplasmonic sensors for environmental detection and monitoring. J Nanosci Nanotechnol 16:4274–4283
Yue G, Su S, Li N, Shuai M, Lai X, Astruc D, Zhao P (2016) Gold nanoparticles as sensors in the colorimetric and fluorescence detection of chemical warfare agents. Coord Chem Rev 311:75–84
Apyari VV, Arkhipova VV, Dmitrienko SG, Zolotov YA (2014) Using gold nanoparticles in spectrophotometry. J Anal Chem 69:1–11
Sharma R, Ragavan KV, Thakur MS, Raghavarao KSMS (2015) Recent advances in nanoparticle based aptasensors for food contaminants. Biosens Bioelectron 74:612–627
He Y, Yu H (2015) A novel triangular silver nanoprisms-based surface plasmon resonance assay for free chlorine. Analyst 140:902–906
Zhang L, Li L (2016) Colorimetric detection of hydrogen peroxide using silver nanoparticles with three different morphologies. Anal Methods 8:6691–6695
Millstone JE, Hurst SJ, Metraux GS, Cutler JI, Mirkin CA (2009) Colloidal gold and silver triangular nanoprisms. Small 5:646–664
Zhang LL, Ma FF, Kuang YF, Cheng S, Long YF, Xiao QG (2016) Highly sensitive detection of bovine serum albumin based on the aggregation of triangular silver nanoplates. Spectrochim Acta A 154:98–102
Chen Z, Zhang C, Wu Q, Li K, Tan L (2015) Application of triangular silver nanoplates for colorimetric detection of H2O2. Sensors Actuators B Chem 220:314–317
Peng J, Yang X-H, Ling J, Liu C-J, Zhang X-Q, Cao Q-E, Ding Z-T (2014) Selective detection of mercury (II) by etching the corners of silver triangular nanoplates. Spectrosc Lett 47:549–553
Abkhalimov EV, Timofeev AA, Ershov BG (2018) Electrochemical mechanism of silver nanoprisms transformation in aqueous solutions containing the halide ions. J Nanopart Res 20:26. https://doi.org/10.1007/s11051-018-4133-6
An J, Tang B, Zheng X, Zhou J, Dong F, Xu S, Wang Y, Zhao B, Xu W (2008) Sculpturing effect of chloride ions in shape transformation from triangular to discal silver nanoplates. J Phys Chem C 112:15176–15182
Yang XH, Ling J, Peng J, Cao QE, Ding ZT, Bian LC (2013) A colorimetric method for highly sensitive and accurate detection of iodide by finding the critical color in a color change process using silver triangular nanoplates. Anal Chim Acta 798:74–81
Hsu MS, Cao YW, Wang HW, Pan YS, Lee BH, Huang CL (2010) Time-dependent surface plasmon resonance spectroscopy of silver nanoprisms in the presence of halide ions. Chem Phys Chem 11:1742–1748
Apyari VV, Gorbunova MO, Shevchenko AV, Furletov AA, Volkov PA, Garshev AV, Dmitrienko SG, Zolotov YA (2018) Towards highly selective detection using metal nanoparticles: a case of silver triangular nanoplates and chlorine. Talanta 176:406–411
Gorbunova MO, Shevchenko AV, Apyari VV, Furletov AA, Volkov PA, Garshev AV, Dmitrienko SG (2018) Selective determination of chloride ions using silver triangular nanoplates and dynamic gas extraction. Sensors Actuators B Chem 256:699–705
Katsoulidis AP, He J, Kanatzidis MG (2012) Functional monolithic polymeric organic framework aerogel as reducing and hosting media for ag nanoparticles and application in capturing of iodine vapors. Chem Mater 24:1937–1943
Gorbunova MO, Bayan EM (2017) A rapid field test method for the determination of hydrogen sulfide and sulfides in waters with gas preextraction. J Anal Chem 72:1263–1269
Gorbunova MO, Bayan EM, Shevchenko AV, Kulyaginova MS (2017) Digital colorimetric determination of chlorides in water using gas extraction and methyl orange. Analitika i kontrol’ [Analytics and Control] 21:274–280
Metraux GS, Mirkin CA (2005) Rapid thermal synthesis of silver nanoprisms with chemically tailorable thickness. Adv Mater 17:412–415
Furletov AA, Apyari VV, Garshev AV, Dmitrienko SG, Zolotov YA (2017) Triangular silver nanoplates as a spectrophotometric reagent for the determination of mercury(II). J Anal Chem 72:1203–1207
Apyari VV, Furletov AA, Garshev AV, Volkov PA, Gorbunova MO, Shevchenko AV (2017) Preparation of reagent indicator papers with silver triangular nanoplates for chemical analysis. Mosc Univ Chem Bull 72:167–173
National pharmacopeia of Russian Federation, Metamizol sodium, FS21000315
National pharmacopeia of Russian Federation, Methods for quantification of vitamins, OFS123001715
Belikov VG (2008) Pharmaceutical chemistry. MEDpress-inform, Moscow
National standard of Russian Federation, Coffee products. Procedure of caffeine mass part measurement, GOST R 51182–98
Costa GO, Feiteira FN, de M. Schuenck H, Pacheco WF (2018) Iodine determination in table salts by digital images analysis. Anal Methods 10:4463–4470
Devouge-Boyer C, Mouda S, Gueguen O, Marcotte S (2018) Determination of iodine in polyamide by inductively-coupled plasma/mass spectrometry. Talanta 189:568–572
Mequanint T, Moges G, Tessma M, Mehretu S (2012) All-solid-state iodide selective electrode for iodimetry of iodized salts and vitamin C. Orient J Chem 28:1547–1555
Gavrilenko NA, Fedan DA, Saranchina NV, Gavrilenko MA (2019) Solid phase colorimetric determination of iodine in food grade salt using polymethacrylate matrix. Food Chem 280:15–19
Novo DLR, Mello JE, Rondan FS, Henn AS, Mello PA, Mesko MF (2019) Bromine and iodine determination in human saliva: challenges in the development of an accurate method. Talanta 191:415–421
Hendawy HAM, Ibrahim AM, Hassan WS, Shalaby A, El-sayed HM (2019) Voltammetric method for simultaneous determination of ascorbic acid, paracetamol and guaifenesin using a sequential experimentation strategy. Microchem J 145:428–434
Salkić M, Selimović A, Keran H (2011) Spectrophotometric determination of l-ascorbic acid in pharmaceutical preparations using glycine as a stabilizer. Eur J Sci Res 53:193–198
Zhao Y, Zhou J, Jia Z, Huo D, Liu Q, Zhong D, Hu Y, Yang M, Bian M, Hou C (2019) In-situ growth of gold nanoparticles on a 3D-network consisting of a MoS2/rGO nanocomposite for simultaneous voltammetric determination of ascorbic acid, dopamine and uric acid. Microchim Acta 186:92. https://doi.org/10.1007/s00604-018-3222-7
Salkić M, Selimović A (2015) Spectrophotometric determination of L-ascorbic acid in pharmaceuticals based on its oxidation by potassium peroxymonosulfate and hydrogen peroxide. Croat Chem Acta 88:73–79
Yu J, Yang W, Xing S, Wang J, Han H, Zhang P, Xiang C, Zhang B (2019) Blended gold/MnO2@BSA nanoparticles for fluorometric and magnetic resonance determination of ascorbic acid. Microchim Acta 186:89. https://doi.org/10.1007/s00604-018-3205-8
Acknowledgements
This work was supported by the Russian Foundation for Basic Research [grant number 18-53-00014-Bel_a] and the Belarusian Republican Foundation for Fundamental Research [grant number F18R-237].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 52 kb)
Rights and permissions
About this article
Cite this article
Gorbunova, M.O., Baulina, A.A., Kulyaginova, M.S. et al. Dynamic gas extraction of iodine in combination with a silver triangular nanoplate-modified paper strip for colorimetric determination of iodine and of iodine-interacting compounds. Microchim Acta 186, 188 (2019). https://doi.org/10.1007/s00604-019-3300-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3300-5