Abstract
Three-dimensional Cu@Cu2O aerogels with excellent electrocatalytic activity were prepared and used as electrode matrix for constructing novel electrochemical glucose sensors. The aerogels were obtained by adding a fresh solution of NaBH4 into a mixture of CuCl2 and NaOH aqueous solutions under stirring at room temperature. The aerogels were assembled with Cu or Cu2O nanoparticles. The materials show superfine spongy-like structures with large surface-to-volume ratio, numerous active sites and good solubility. The Cu@Cu2O aerogels show highly efficient electrochemical activity toward glucose oxidation with a relatively low-onset potential (0.25 V) in 0.1 M NaOH solution. This non-enzymatic glucose sensor offers a low detection limit of 0.6 μM (S/N = 3), a high sensitivity (195 mA M−1 cm−2), and two wide linear ranges (0.001–5.2 mM, 5.2–17.1 mM) at a working voltage of 0.6 V (vs. Ag/AgCl) in alkaline solution. While in neutral pH values, the respective data are a linear analytical range from 0.1 to 10 mM; a detection limit of 54 μM (S/N = 3) and a sensitivity of 12 mA M−1 cm−2 at scan rate of 100 mV s−1. The sensor possesses high selectivity, good reproducibility and long-time stability. It was utilized to determine glucose levels in (spiked) human serum samples, and satisfactory results were obtained.

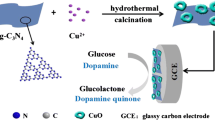
Schematic presentation of a glassy carbon electrode modified with 3D porous Cu@Cu2O aerogels. The aerogels were obtained by a reduction reaction at room temperature (Scheme 1A). The aerogel networks were used to develop a highly sensitive electrochemical sensing platform for the detection of glucose (Scheme 1B).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-enzymatic glucose sensors have attracted extensive attention owing to their low detection limit, high sensitivity and long-term storage stability [1, 2]. Great efforts have been paid to the development of non-enzymatic glucose sensors, which heavily depends on the features of an excellent electrode material. Generally, the properties of nanomaterials are highly size- and shape-dependent, thereby controlling the size and morphology of the nanomaterials is extremely important to realize the full potential of these nanomaterials. One-, two-, and three-dimensional (3D) nanostructures with high electrocatalytic activity such as metal nanoparticles (Au, Pt, Cu) [3,4,5,6], and metal oxide nanoparticles (CuO, NiO, Co3O4) [7,8,9] have been successfully used in construction of non-enzymatic electrochemical glucose sensors. However, these nanoparticles are easily aggregated or oxidized in the ambient atmosphere and have limited catalytic activities, which greatly lower the analytical performance of the sensor. Therefore, it is still a big challenge to exploit facile and green approaches to assemble nanoscale entities into macroscopic nanostructures endowed with excellent electrocatalytic activity.
Porous nanostructures are promising candidates as electrocatalysts because of their abundant active sites, large surface to volume ratio, good solubility and high stability. Among these, aerogels are specifically promising in that they possess many marvelous properties, such as ultralow densities, large surface areas, continuous porosities and adjustable structure. Aerogels have been developed and applied in many fields [10,11,12,13]. For example, the noble metal aerogels Au hydrogels [14] and multimetallic hierarchical aerogel networks Ni-PdxPty [15] have been widely developed for electrocatalytic oxidation of glucose and other small molecules. However, these noble metal nanomaterials are cost prohibitive, and therefore, restricting their large-scale applications. Copper oxide-based nanomaterials assembled with cheap metal Cu can controllably generate into various nanostructures such as nanowires [16], nanobelts [17] and nanocubes [18], which have been considering as ideal candidates for low-cost catalytic materials in the field of sensing. Nevertheless, the 3D porous aerogels assembled with copper oxide nanoparticles have rarely been reported for electrocatalytic oxidation of glucose.
Herein, 3D Cu@Cu2O aerogels as an electrocatalysts were designed using a efficient one-step reduction method (Scheme 1a). Then the Cu@Cu2O aerogels were then immobilized on glassy carbon electrode (GCE) to construct non-enzymatic glucose sensor (Scheme 1b). The 3D Cu@Cu2O aerogels displayed large surface to volume ratio, high electrical conductivity and good stability. The fabricated glucose sensor exhibited high selectivity, sensitivity, and good stability toward glucose detection in 0.1 M NaOH solution. In addition, the glucose detection performance of the prepared sensor was evaluated in a phosphate buffer at physiological condition.
Schematic presentation of a glassy carbon electrode modified with 3D porous Cu@Cu2O aerogels. The aerogels were obtained by a reduction reaction at room temperature (Scheme 1a). The aerogel networks were used to develop a highly sensitive electrochemical sensing platform for the detection of glucose (Scheme 1b)
Experimental section
Materials and reagents
Sodium borohydride (NaBH4), sodium hydroxide (NaOH), cupric chloride (CuCl2), ethanol, fructose, sucrose, maltose, dopamine-hydrochloride (DA), ascorbic acid (AA), acetaminophen (AP) and Uric acid (UA) were purchased from Sinopharm Chemical Reagent Co. Ltd. (www.crc-bj.com). Glucose and Nafion were obtained from Sigma-Aldrich (www.sigmaaldrich.com). Other chemicals and reagents are of analytical grade and prepared using the distilled water.
Apparatus
Electrochemical measurements were performed on a CHI852C electrochemistry workstation (CH Instruments Co., Shanghai, China). X-ray powder diffractions (XRD) were conducted with a D8 Advance X-Ray diffractometer from Bruker Co. (Germany). X-ray photoelectron spectroscopy (XPS) was performed on an ESCALAB250Xi spectrometer (USA). Transmission electron micrographs (TEMs) were conducted on a JEM-2100 transmission electron microscope operating at an accelerating voltage of 200 kV. High-resolution TEM (HRTEM) and EDS mapping were captured with a FEI Tecnai G2 F30 transmission electron microscopy (USA) at an acceleration voltage of 300 kV. Field-emission SEM (FESEM) used to characterize the modified electrodes were carried out on a Zeiss Supra 55 scanning electron microscope (Germany) at an acceleration voltage of 5 kV.
Preparation of Cu@Cu2O aerogels
Porous Cu@Cu2O aerogels were synthesized according to the former literature with a slight modification [19]. Schematic of the preparation of Cu@Cu2O aerogels were illustrated in Scheme 1a. Briefly, 5.0 mL CuCl2 (0.1 M) and 5.0 mL NaOH (0.2 M) were mixed into 150 mL pure water in a 250 mL three-necked flask, and stirred vigorously for 20 min under nitrogen atmosphere. Subsequently, 150 μL ethanol and 50 mg fresh NaBH4 (dissolved in 25 mL H2O) quickly added into the solution under strong stirring (20 min). After centrifuged and washed three times with ethanol and water successively, the products of porous Cu@Cu2O aerogels were obtained and dried in vacuum at 60 °C for 2 h.
Fabrication of Cu@Cu2O modified electrodes
The GCE was polished with 0.03 and 0.05 mm alumina slurry (Beuhler), respectively, and then ultrasonic cleaned with ethanol and water, and dried under nitrogen protection. Then, 2.0 mg of Cu@Cu2O nanocompostie was dispersed in water with sonication. Subsequently, 5.0 μL of the resulting suspension was added on the pretreated GCE and dried in air. Prior to measurement, 6.0 μL of 0.5% Nafion was modified on the GCE to avoid the leakage of Cu@Cu2O from the surface of the GCE.
Results and discussion
Characterization of 3D Cu@Cu2O aerogels
The morphology and structure of the Cu@Cu2O shown in Fig. 1 is captured by SEM and TEM. As shown in Fig. 1a and b, the Cu@Cu2O displays a typical three-dimensional porous nanostructure and composed of superfine spongy-like structures. The HRTEM image (Fig. 1c) shows lattice fringes with an interplanar spacing of 0.243 nm which is correspond to the (111) plane of Cu2O. The corresponding elemental mapping of Cu@Cu2O Aerogels (Fig. 1d–f) demonstrates that the Cu and O elements are homogeneously distributed within the whole nanocomposite. The measurement of EDX spectra and the XPS survey spectrum (Fig. S1) proves that the 3D Cu@Cu2O aerogels are mainly consisted of Cu and O elements.
XRD is used to further identify the crystal phase of 3D Cu@Cu2O aerogels in Fig. 2a. The diffraction peaks (2θ) at 43.3°, 50.4° and 74.1° correspond to (111), (200) and (220) planes of the Cu (JCPDS no. 04–0836), respectively. Furthermore, two diffraction peaks located at 36.4° and 61.3° are attributed to the reflection of (111) and (220) of Cu2O (JCPDS no. 78–2076), respectively. It suggests the successful formation of the Cu@Cu2O composite. XPS is used to investigate the the element information of the nanocomosite (Fig. 2b–d). As shown in Fig. 2b, the bind energies for Cu 2p are centered at around 932.39 eV and 952.25 eV, which typically represent Cu2O species, and can be assigned to Cu/Cu2O (Cu(0)/Cu(I)) [20, 21]. In addition, the presence of main peaks locate at 934.21 and 954.22 eV, together with adjacent peaks at 941.32 eV, 943.92 eV and 962.30 eV correspond to the Cu2+ [22, 23]. This can be inferred that there exists CuO or/and Cu(OH)2 species. As discussed above, the 3D porous Cu@Cu2O composite show large surface-to-volume ratio and abundant active sites. However, Cu2O is thermodynamically unstable at room temperature and ambient pressure. This explains that tiny amounts of Cu2O on the surface can be rapidly oxidized to CuO or/and Cu(OH)2 when the product is exposed to humid air [24]. The discrepant phenomena between XRD and XPS comes from their respective detection sensitivity and detection depth, the Cu2+ can be detected by the XPS, while the CuO and Cu(OH)2 can hardly be detected by XRD in the composite [24, 25]. In Fig. 2c, the binding energy peak of Auger electron spectrum locate at 569.44 eV, which is corresponding to the electron state of Cu2O [20]. Figure 2d exhibits the O 1 s XPS spectrum for the Cu@Cu2O composite. The middle peak at 531.31 eV, which can be attributed to the absorbed oxygen. The O 1 s peak is mainly at 529.82 eV indicates that oxygen is present in the Cu2O lattice, while the weak peak present at 532.79 eV is owing to the surface species in Cu@Cu2O aerogels. The result is consist with the Cu 2p XPS spectrum in Fig. 2b.
Electrochemical behavior of the glassy carbon electrode (GCE) modified with Cu@Cu2O
The electrocatalytic performances of Cu@Cu2O aerogels towards glucose oxidation were characterized by cyclic voltammetry (CV) technique in N2-saturated 0.1 M NaOH aqueous solution. As observed in Fig. 3a, no peak is observed for bare GCE (curve a), the Cu@Cu2O/GCE shows a pair of well-defined redox peaks (curve b). After adding 10 mM glucose, an obvious increase of oxidation current and decrease of the cathodic peak currents can be seen (curve c), which are typical electrocatalytical features. Furthermore, It can be seen that the oxidation current responses increase with the concentration of glucose (Fig. 3b). The oxidation potential of Cu(0) to Cu(I) and Cu(I) to Cu(II) is lower than 0.2 V [26]. However, the Cu@Cu2O/GCE toward glucose oxidation with a onset potential at 0.25 V, It suggest the glucose oxidation is corresponding to the conversion of Cu(II) to Cu(III), rather than Cu (I) or Cu (II). Thus, Cu (III) species act as an electron mediator during the glucose oxidation process [27]. The possible mechanism for oxidation of glucose by the Cu@Cu2O can be represented by following reactions: Cu(I) is first oxidized to Cu(OH)2 and then transformed to CuO. Secondly, CuO is oxidized to CuOOH, and the redox couple of CuO/CuOOH can catalyze the oxidation process. The corresponding reaction process can be shown in follwing eqs. (1)–(4) [28]:
Figure 4a shows the CVs of the Cu@Cu2O/GCE in 0.1 M NaOH solution containing 0.8 mM glucose at different scan rates. Figure 4b displays that both anodic peak and cathodic peak currents are proportional to the square root of the scan rate, indicating that this electrocatalytic process is controlled by the diffusion of glucose molecules to the electrolyte/electrode interface, thus depending on the concentration of glucose in solution.
Optimization of experimental conditions
The applied potential has a critical impact on the sensing performance of the glucose sensor. The applied potential was studied in a range from 0.45 to 0.65 V. As shown in Fig. S2, the amperometric current first ascends with the increase potential (ranging from 0.45 to 0.6 V), and then decreases after 0.65 V. Thus, for considering the detection sensitivity, the potential of 0.60 V was chosen as the optimum applied potential in the following experiments.
Analytical performance of Cu@Cu2O/GCE
Amperometric technique was used to study the analytical performance of the glucose sensor due to its high sensitivity, convective mass transport and rapid detection towards the oxidation of glucose. Figure 5a displays the typical amperometric responses of Cu@Cu2O/GCE on the successive addition of glucose into the N2-saturated stirring in NaOH solution. The corresponding calibration plot of the Cu@Cu2O/GCE sensor is described in Fig. 5b, which displays two linear response from 0.001 mM to 5.2 mM (correlation coefficient R2 = 0.9955) and 5.2 mM to 17.1 mM (correlation coefficient R2 = 0.9922). As we know that the diffusion rates have a great influence on the electrocatalytic process. The above two linear ranges can be explained by the different diffusion rates of reaction products under the different glucose concentrations. When in low concentration of glucose, the oxidation products can quickly leave away from the surface of electrode. Nevertheless, the high concentrations of glucose obstruct the diffusion of the gluconolactone. As a result, lower oxidation peak current is detected in the high concentration range and relatively higher oxidation current existed in low concentration of glucose. The limit of detection (LOD) was estimated to be 0.6 μM and the sensitivity is calculated to be 195 mA·M−1·cm−2 (S/N = 3). Moreover, the performance of this sensor compared to previous reports is shown in Table 1. It can be shown that the performance of the sensor in this work is better than most previous studies in terms of sensitivity and LOD. These results further reveal the excellent performance of 3D Cu@Cu2O aerogels as a promising material in glucose detection.
a Amperometric curve of the response of Cu@Cu2O/GCE to glucose with successive addition of glucose in 0.1 M NaOH at an applied potential of 0.6 V (vs. Ag/AgCl). Inset: i-t curve of the response of the Cu@Cu2O/GCE to glucose with successive addition of glucose of 1, 2, 3, 4, 5, 10, 20, 30, 40 and 50 μM. b Corresponding calibration plot of current versus glucose concentrations for Cu@Cu2O/GCE
Glucose sensing response of modified electrode at neutral pH
Cyclic voltammetry measurements were conducted to evaluate the electrochemical performance of Cu@Cu2O/GCE sensor for glucose detection in a phosphate buffer at physiological condition. The electrochemical response of the Cu@Cu2O electrode in presence of various concentrations of glucose in phosphate buffer at pH of 7 is shown in Fig. 6a As can be seen, the anode current and cathode current decrease as the glucose concentration increases. The anode peak in −0.5 V, −0.06 V, 0.22 V appears subsequently in the figure, representing the mutual transformation of the redox pairs of Cu(0)/Cu(I), Cu(0)/Cu(II), Cu(I)/Cu(II) during the electrochemical processes. This is similar to the reports under alkaline conditions [33]. Figure 6b shows the plot of glucose oxidation peak current vs. glucose concentration. As shown in Fig. 6b, the application of linear regression revealed that the Cu@Cu2O electrode exhibits good response toward glucose oxidation over the concentration range of 0.1 to 10 mM with the linear equation of i (μA) = −0.85 C (mM) + 55.73 and the correlation coefficient of 0.998. The sensitivity and limit of detection of the electrode for glucose detection in the 0.1 M phosphate buffer was determined to be 12 mA M−1 cm−2 and 54 μM. It can be seen that the Cu@Cu2O based glucose sensor used in this paper can work well under both alkaline and neutral phosphate buffer solutions. However, it demonstrated much lower sensitivity and narrower linear range as compared with alkaline condition. Therefore, in the following, alkaline conditions are selected for characteristics analysis of the sensor and for glucose detection in serum.
Selectivity, reproducibility and stability studies of sensor
Selectivity test is an essential parameter for glucose sensors, electroactive species such as ascorbic acid (AA), acetaminophen (AP) and uric acid (UA) usually coexist in human serum, although the concentration of these electroactive species are much lower than glucose. Some electroactive species are added into 0.1 M NaOH to investigate their influences on the current responses of the glucose sensor. Amperometric measurement was carried out on the Cu@Cu2O/GCE at 0.6 V with successive injecting 1 mM glucose, 0.1 mM AA, 0.1 mM AP, 0.1 mM UA, and 1 mM glucose into 0.1 M NaOH solution (Fig. 7a). It can be seen that only addition of glucose generated significant current signals, while the interference species showed no obvious current change. In addition, the interference responses with sugar interfering substances were also studied in Fig. S3. Above results suggests that the glucose sensor embodies good selectivity in human serum. To study the reproducibility of the developed sensor, the Cu@Cu2O/GCE was measured under ten injections of 10 μM glucose. As shown in Fig. 7b, the sensor exhibits an obvious and equivalent increase after each injection, which indicates the good repeatability of the sensor. The stability of the glucose sensor was also investigated by analysis the current responses periodically. The modified electrodes were stored at room temperature, and the current response measurements were performed with 0.1 mM glucose at an interval of every 3 days. Figure S4 displays the investigation results, the current response remains at about 92% of its initial value, demonstrating high stability of the designed glucose sensor.
a Amperometric i-t response of Cu@Cu2O/GCE in N2-saturated 0.1 M NaOH with the successive addition of 1 mM glucose (Glu), 0.1 mM ascorbic acid (AA), 0.1 mM acetaminophen (AP), 0.1 mM uric acid (UA), and a second addition of 1 mM glucose. b Current-time response of Cu@Cu2O/GCE with addition of 10 μM glucose for ten times into 0.1 M NaOH at 0.6 V
Application of the sensor in the determination of glucose in serum
The practical applicability of the glucose sensor was investigated by measuring clinical serum samples and the results were compared with the reference values providing by Yangzhou Youhao hospital. The process was evaluated with the 10 μL of human blood serum in 10 mL of 0.1 M NaOH solution and its amperometric current responses were monitored at an applied potential of 0.6 V vs. Ag/AgCl. The results were listed in Table 2 (each sample was measured five times). It shows the relative errors between two methods were no more than 5.9%, indicating the fabricated glucose sensor has a good accuracy for clinical practical samples.
Conclusions
An electrochemical sensing platform based on 3D porous Cu@Cu2O aerogels was developed for the sensitive and selective detection of glucose without the needs of natural enzymes. The aerogels show excellent electrocatalytic activity toward the glucose oxidation served as electrode matrix to construct non-enzymatic glucose sensor. The aerogels were characterized utilizing the electron microscopy, X-ray diffraction, X-ray photoelectron, energy disperse spectroscopy mapping, and cyclic voltammetry, respectively. It should be mentioned that there still exist few potential application limitations such as relatively narrow linear range and low sensitivity in physiology neutral condition. However, the constructed sensor showed high sensitivity, wide linear range, high selectivity, and good reproducibility in alkaline detection condition. The consistent results of this sensor and commercial method for the detection of human serum samples indicate the potential advantages in practical application, providing a candidate platform for rapid screening and testing of diabetes mellitus.
References
Pei YJ, Hu M, Tu FH, Tang XY, Huang W, Chen S, Li ZL, Xia Y (2018) Ultra-rapid fabrication of highly surface-roughened nanoporous gold film from AuSn alloy with improved performance for nonenzymatic glucose sensing. Biosens Bioelectron 117:758–765
Dhara K, Mahapatra DR (2018) Electrochemical nonenzymatic sensing of glucose using advanced nanomaterials. Microchim Acta 185(1):49
Karra S, Wooten M, Griffith W, Gorski W (2016) Morphology of gold nanoparticles and electrocatalysis of glucose oxidation. Electrochim Acta 218:8–14
Gao F, Zhou F, Yao Y, Zhang Y, Du L, Geng D, Wang P (2017) Ordered assembly of platinum nanoparticles on carbon nanocubes and their application in the non-enzymatic sensing of glucose. J Electroanal Chem 803:165–172
Nantaphol S, Watanabe T, Nomura N, Siangproh W, Chailapakul O, Einaga Y (2017) Bimetallic Pt-Au nanocatalysts electrochemically deposited on boron-doped diamond electrodes for nonenzymatic glucose detection. Biosens Bioelectron 98:76–82
Shi L, Zhu X, Liu T, Zhao H, Lan M (2016) Encapsulating Cu nanoparticles into metal-organic frameworks for nonenzymatic glucose sensing. Sensors Actuators B Chem 227:583–590
Sun S, Zhang X, Sun Y, Yang S, Song X, Yang Z (2013) Facile water-assisted synthesis of cupric oxide nanourchins and their application as nonenzymatic glucose biosensor. Acs Appl Mater Interfaces 5(10):4429–4437
Chen J, Xu Q, Shu Y, Hu X (2018) Synthesis of a novel Au nanoparticles decorated Ni-MOF/Ni/NiO nanocomposite and electrocatalytic performance for the detection of glucose in human serum. Talanta 184:136–142
Madhu R, Veeramani V, Chen SM, Manikandan A, Lo AY, Chueh YL (2015) Honeycomb-like porous carbon-cobalt oxide nanocomposite for high-performance enzyme less glucose sensor and supercapacitor applications. ACS Appl Mater Interfaces 7(29):15812–15820
Arachchige IU, Brock SL (2006) Sol-gel assembly of CdSe nanoparticles to form porous aerogel networks. J Am Chem Soc 128(24):7964–7971
Antonietti M, Fechler N, Fellinger TP (2014) Carbon aerogels and monoliths: control of porosity and nanoarchitecture via sol-gel routes. Chem Mater 26(1):196–210
Liu W, Herrmann AK, Bigall NC, Rodriguez P, Wen D, Oezaslan M, Schmidt TJ, Gaponik N, Eychmueller A (2015) Noble metal aerogels-synthesis, characterization, and application as electrocatalysts. Acc Chem Res 48(2):154–162
Bag S, Trikalitis PN, Chupas PJ, Armatas GS, Kanatzidis MG (2007) Porous semiconducting gels and aerogels from chalcogenide clusters. Science 317(5837):490–493
Wen D, Liu W, Haubold D, Zhu C, Oschatz M, Holzschuh M, Wolf A, Simon F, Kaskel S, Eychmueller A (2016) Gold aerogels: three-dimensional assembly of nanoparticles and their use as electrocatalytic interfaces. ACS Nano 10(2):2559–2567
Cai B, Dianat A, Huebner R, Liu W, Wen D, Benad A, Sonntag L, Gemming T, Cuniberti G, Eychmueller A (2017) Multimetallic hierarchical aerogels: shape engineering of the building blocks for efficient electrocatalysis. Adv Mater 29(11)
Zhang Y, Su L, Manuzzi D, De Los Monteros HVE, Jia W, Huo D, Hou C, Lei Y (2012) Ultrasensitive and selective non-enzymatic glucose detection using copper nanowires. Biosens Bioelectron 31(1):426–432
Huang TK, Lin KW, Tung SP, Cheng TM, Chang IC, Hsieh YZ, Lee CY, Chiu HT (2009) Glucose sensing by electrochemically grown copper nanobelt electrode. J Electroanal Chem 636(1–2):123–127
Gao Z, Liu J, Chang J, Wu D, He J, Wang K, Xu F, Jiang K (2012) Mesocrystalline Cu2O hollow nanocubes: synthesis and application in non-enzymatic amperometric detection of hydrogen peroxide and glucose. Crystengcomm 14(20):6639–6646
Ling P, Zhang Q, Cao T, Gao F (2018) Versatile three-dimensional porous Cu@Cu2O aerogel networks as electrocatalysts and mimicking peroxidases. Angew Chem Int Ed 57(23):6819–6824
Martin L, Martinez H, Poinot D, Pecquenard B, Le Cras F (2013) Comprehensive X-ray photoelectron spectroscopy study of the conversion reaction mechanism of CuO in Lithiated thin film electrodes. J Phys Chem C 117(9):4421–4430
Svintsitskiy DA, Stadnichenko AI, Demidov DV, Koscheev SV, Boronin AI (2011) Investigation of oxygen states and reactivities on a nanostructured cupric oxide surface. Appl Surf Sci 257(20):8542–8549
Ai Z, Zhang L, Lee S, Ho W (2009) Interfacial hydrothermal synthesis of Cu@Cu2O core-shell microspheres with enhanced visible-light-driven photocatalytic activity. J Phys Chem C 113(49):20896–20902
Chen F, Zhao X, Liu H, Qu J (2014) Reaction of Cu(CN)3 2− with H2O2 in water under alkaline conditions: cyanide oxidation, Cu+/Cu2+ catalysis and H2O2 decomposition. Appl Catal B Environ 158:85–90
Chusuei CC, Brookshier MA, Goodman DW (1999) Correlation of relative X-ray photoelectron spectroscopy shake-up intensity with CuO particle size. Langmuir 15(8):2806–2808
Shu J, Qiu Z, Lv S, Zhang K, Tang D (2017) Cu2+-doped SnO2 Nanograin/Polypyrrole Nanospheres with synergic enhanced properties for ultrasensitive room-temperature H2S gas sensing. Anal Chem 89(20):11135–11142
Qian Y, Ye F, Xu J, Le ZG (2012) Synthesis of cuprous oxide (Cu2O) nanoparticles/graphene composite with an excellent electrocatalytic activity towards glucose. Int J Electrochem Sci 7(10):10063–10073
Wang J, Zhang WD (2011) Fabrication of CuO nanoplatelets for highly sensitive enzyme-free determination of glucose. Electrochim Acta 56(22):7510–7516
Khan R, Ahmad R, Rai P, Jang LW, Yun JH, Yu YT, Hahn YB, Lee IH (2014) Glucose-assisted synthesis of Cu2O shuriken-like nanostructures and their application as nonenzymatic glucose biosensors. Sensors Actuators B Chem 203:471–476
Faranak F, Mansour R, Mohammad JH, Hasuck K (2018) Microwave-assisted synthesis of graphene modified CuO nanoparticles for voltammetric enzyme-free sensing of glucose at biological pH values. Microchim Acta 185(1):57
Somasekhar RC, Ilhwan P, Sungbo C (2018) Nonenzymatic determination of glucose at near neutral pH values based on the use of nafion and platinum black coated microneedle electrode array. Microchim Acta 185(5):250
Nikolaev KG, Ermakov SS, Offenhäusser A, Mourzina Y (2018) Nonenzymatic determination of glucose on electrodes prepared by directed electrochemical nanowire assembly (DENA). J Anal Chem 72(4):371–374
Thiruppathi M, Thiyagarajan N, Gopinathan M, Chang JL, Zen JM (2017) A dually functional 4-aminophenylboronic acid dimer for voltammetric detection of hypochlorite, glucose and fructose. Microchim Acta 184(10):4073–4080
Zhao YX, Li YP, He ZY, Yan ZF (2013) Facile preparation of Cu-Cu2O nanoporous nanoparticles as a potential catalyst for non-enzymatic glucose sensing. RSC Adv 3(7):2178–2181
Wang AJ, Feng JJ, Li ZH, Liao QC, Wang ZZ, Chen JR (2012) Solvothermal synthesis of Cu/Cu2O hollow microspheres for non-enzymatic amperometric glucose sensing. Crystengcomm 14(4):1289–1295
Li S, Zheng Y, Qin GW, Ren Y, Pei W, Zuo L (2011) Enzyme-free amperometric sensing of hydrogen peroxide and glucose at a hierarchical Cu2O modified electrode. Talanta 85(3):1260–1264
Acknowledgments
The funding of this work was provided by National Natural Science Foundation of China (no. 31371806 and 21575125). In addition, much thanks to The Testing Center of Yangzhou University due to all the characterizations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• The Cu@Cu2O aerogels were obtained with a one-step reduction method.
• The aerogel were firstly prepared to construct electrochemical glucose sensor.
• The electrochemical glucose sensor showed a wide linear range, a low detection limit and a high sensitivity in alkaline detection condition.
• This research provided a promising platform for sensing applications.
Electronic supplementary material
ESM 1
(DOC 311 kb)
Rights and permissions
About this article
Cite this article
Gao, Y., Yang, F., Yu, Q. et al. Three-dimensional porous Cu@Cu2O aerogels for direct voltammetric sensing of glucose. Microchim Acta 186, 192 (2019). https://doi.org/10.1007/s00604-019-3263-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3263-6