Abstract
A colorimetric assay for ATP is described that uses a strategy that combines the concept of split Mg(II)-dependent DNAzyme, split aptamer, and hybridization-induced aggregation of gold nanoparticles (AuNPs). Both ATP aptamer and Mg(II)-dependent DNAzyme are split into two fragments which are allocated to two well-designed DNA probes. The probes also possess mutually complementary stem sequences and spacer sequences. In the presence of ATP, the separated DNAzyme sequences in the two probes assemble via the synchronous recognition of ATP with two fragments of the aptamer. Then, the activated DNAzyme catalyzes multiple cycles of the cleavage of its substrate DNA sequence. The latter acts as a linker and induces the aggregation of two types of ssDNA-modified AuNP through the hybridization between the complementary sequences. Thus, the color of the AuNP solution remains red. However, in the absence of ATP, the detached aptamer cannot induce the assembly of DNAzyme to cleave the linker DNA. This results in the aggregation of AuNP and a concomitant color transition from red to purple. This ATP assay, performed at a wavelength of 530 nm, has a linear detection range that extends from 10 pM to 100 nM, with a detection limit of 5.3 pM. It was applied to the detection of ATP in human serum. Conceivably, the strategy has a wide scope in that it may be applied to the colorimetric detection of various other analytes through the split aptamer configuration.

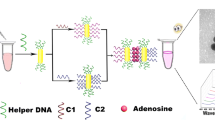
Schematic presentation of colorimetric assay for adenosine triphosphate (ATP) based on the use of a split Mg(II)-dependent DNAzyme, a split aptamer, and by exploiting the hybridization-induced aggregation of gold nanoparticles that leads to a color change from red to purple
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adenosine triphosphate (ATP) serves as the direct source of energy in life and as a mediator of extracellular signalling [1, 2]. There is sound evidence that the fluctuation in ATP level is closely related to different diseases such as cardiovascular diseases, Parkinson’s disease and some malignant tumors [3,4,5]. Therefore, it is of great significance to establish an accurate and sensitive method for ATP monitoring. Currently, multiple strategies have been designed for ATP detection, particularly focusing on developing small molecule or aptamer probes combined with fluorescence and nanotechnologies [6,7,8,9,10,11]. For example, Jiang et al. developed an colorimetric assay for ATP by using 4-Mercaptophenylboronic acid (MPBA) as a recognition element [6]. Although this method performed in a simple manner, the detecting range and limit can be further improved. Qu et al. constructed a fluorescent aptasensor for ATP based on the interaction of Tb-MOF with dispersive and aggregated AuNPs [7]. Both Cheng [8] and Luo [9] reported that a fluorometric-fluoratiometric assay making use of ATP aptamer along with carbon dots and graphene oxide. Recently, Liu et al. synthesized a multifunctional fluorescent probe for the determination of ATP [10]. Despite their excellent performance, these aptamer-based biosensors usually require complicated synthesis and are time-consuming. Therefore, high sensitive and high specific assay for ATP with low cost and easy operation is still highly desired.
DNAzyme displays high catalytic activity and exhibits several advantages such as simple synthesis, high thermal stability, low cost and ease of modification [12]. With these obvious advantages, DNAzyme makes it an ideal signal-amplifier for analysis [13]. Furthermore, to improve the specificity and affinity towards the target, the idea of splitting nucleic acid sequence into two or even multiple fragments endows diverse smart design in aptamer-based biosensors [14, 15]. The increased affinity is achieved by substantially increasing the local concentrations of the oligonucleotides via assembling the two complementary oligonucleotides to a target, which result in a greatly improved sensitivity [16,17,18].
Inspired by this, a colorimetric ATP assay operated in a synchronous mode based on the concept of split aptamer combined with DNAzyme is presented. The presence of the target induces the formation of the complete aptamer, which combines two separated DNA probes and further forms the complete structure of DNAzyme. The catalytic activity of DNAzyme can be indicated by its substrate mediated hybridization between the ssDNA modified on AuNPs, which finally can be expressed in aggregation/disaggregation of AuNPs. The color change obtained can be directly readout by the bare eyes and measured easily by UV/VIS spectrophotometry. Moreover, the colorimetric assay enables to ultrasensitive, rapid, and low cost detection of ATP and its performance satisfies the requirement for analysis of ATP in clinical diagnostics.
Experimental
Materials and reagents
Chloroauric acid (HAuCl4·3H2O) was purchased from Sinopharm Chemical Reagent Co., Ltd. (ShangHai, China, http://en.reagent.com.cn). 5′-adenosine triphosphate (ATP), trisodium citrate, three (2-chloroethyl)phosphate (TCEP), acetonitrile, were purchased from Sigma-Aldrich (https://www.sigmaaldrich.com). Other reagents were in analytical purity. The water used in the experiment was purified by Milli Q purification system (Barnstead, USA, https://www.thermofisher.com) with a resistivity greater than 18 MΩ cm. ATP was dissolved in physiological buffer (saline; 10 mM phosphate buffer containing 140 mM NaCl, 5 mM KCl and 1 mM MgCl2, pH 7.9).
The nucleic acid sequences used in the experiments were all synthesized and purified by Sango Biotech Co., Ltd. (Shanghai, China, https://www.sangon.com), which are listed below.
The probes consist of four parts: (1) the split ATP aptamer fragments (green); (2) continuous spacers (grey); (3) the short complementary regions (blue); (4) the split Mg(II)-dependent DNAzyme fragments (purple). The underlined sequences can complementarily hybridize with the linker.
The red and pink sequence can complementarily hybridize with the strand 1 and 2 modified on AuNP.
The red and pink regions are complementary to the linker. The italic regions are the interval area.
Preparation and modification of the gold nanoparticles (AuNPs)
All the glassware used in the preparation of AuNP should be soaked in aqua regia (hydrochloric acid: nitrate =3:1, v/v), and washed repeatedly by distilled water, and dried before use. Citrate-reduced AuNPs were chemically prepared according to the citrate reduction method [19]. The detail synthesis processes are shown in Electronic Supplementary Material (ESM). The average diameter of the obtained AuNP is 13 nm determined by TEM.
The nucleic acid strand 1 and strand 2 used to modify AuNP were respectively activated in 15 mM TCEP for 1 h at room temperature through the reduction of possible disulfur bond. Then the activated strand 1 and strand 2 are respectively added to the above prepared AuNP solution, make the final concentration of 3 μM. The solution was kept in the dark at room temperature for 16 h. Every half an hour, a certain amount of sodium chloride solution is added for aging for totally 6 times, making the final concentration of sodium chloride to be 0.1 M. Aging is to increase the modification amount of nucleic acid on AuNP. Keep aging for 12 h at room temperature. After centrifugation at 16,330×g for 30 min, the modified AuNP was washed for 3 times and resuspended with 10 mM Tris-HCl buffer (pH 7.4). The prepared two types of nucleic acids modified AuNPs (AuNP-1 and AuNP-2 for strand 1 modified AuNP and strand 2 modified AuNP, respectively) were then placed at 4 °C prior to use.
Colorimetric assay of ATP based on DNAzyme and AuNPs
In the step of cyclic amplification, 1 μL probe A (1 μM), 1 μL probe B (1 μM), 1 μL linker (1 μM) and 1 μL magnesium ion solution (50 mM) were firstly mixed and reacted at 90 °C for 5 min, making the sequences fully extend out. Then 1 μL ATP with different concentrations was added and incubated at 20 °C for 60 min. ATP induced the self-assemble of DNAzyme and the cleavage of the linker.
In the step of detection, 5 μL AuNP-1, 5 μL AuNP-2 and 5 μL of 2 M NaCl were added into the solution after DNAzyme catalysis, allowed to react at room temperature for 30 min. Then UV-visible spectra of the solution were recorded by an ultraviolet spectrophotometer.
Analysis of real samples
The serum from volunteers was collected by The First Affiliated Hospital of Nanjing Medical University, and informed consent was obtained for the use of human serum. The 500 μL human serum samples were pretreated using 2.0 mL of acetonitrile to deposit most of the protein inside which prevents the ATP sensing through protein-AuNPs interactions. After vortex-mixing, the sample was centrifuged at 12000 rpm for 20 min and the supernatant was diluted 10 times. Then, ATP was spiked into the diluted serum solution with a series of concentrations of 10, 100, and 1000 μM and the spiked serum samples were measured with the method, which was repeated three times. The test results were compared with each other and the average recovery was calculated.
Results and discussion
Choice of materials
Aptamers, also known as chemical antibodies, show overwhelming advantages including high selectivity, thermal stability, and convenient chemical synthesis [20]. In split aptamer based strategy, one target is simultaneously recognized by two probes. This enhances the specificity of the method [21]. In addition, DNAzymes, a kind of artificial DNA sequences, are low cost, easy to synthesize and have high catalytic efficiency [22]. The DNAzyme-based amplification strategy endows extra high sensitivity to the method [23, 24]. AuNPs are easy to prepare and chemically modify, offer a simple strategy for visual detection of the target [25]. In summary, a strategy was designed for ATP assay by taking full advantage of the split aptamer, DNAzymes and the AuNPs-based colorimetry.
The principle of the colorimetric assay
The experimental principle of the method is shown in Fig. 1. Both probe A and probe B are composed of four parts, namely split ATP aptamer area, spacer, short complementary region and split Mg(II)-dependent ribozyme. Among them, the short complementary region cannot be stably hybridized at room temperature because the melting temperature is too low, therefore the split DNAzyme sequences are separated. In the presence of ATP, the two parts of split aptamer can combine with the target at the same time, which shortens the distance of two probes, and helps the stable hybridization between the short complementary region and further formation of the complete DNAzyme structure [26]. The activated DNAzyme with magnesium ion can cleave the linker sequence, which can hybridize with strand 1 and strand 2 on the surface of AuNP-1 and AuNP-2 via the complementary nucleotides. The broken linker is unable to crosslink AuNP-1 and AuNP-2, so the solution is still red. In contrast, in the absence of ATP, the complete linker can trigger the aggregate of AuNP-1 and AuNP-2; turn the color of the solution into blue purple. The color change can be observed by the bare eye and recorded and quantified by UV-vis spectra.
Verification of the experimental feasibility
To evaluate the feasibility of the experimental design, a set of control experiments were carried out and characterized by UV-vis spectroscopy, color change and transmission electron microscopy (TEM). As shown in Fig. 2, the UV-vis absorption spectrum of the mixture of AuNP-1 and AuNP-2 (Fig. 2a, curve a) exhibits a typical absorption at 530 nm, indicating the dispersion of AuNP in the solution. This can also be characterized by TEM (Fig. 2c, image a). And the color of the mixed AuNP solution is wine red (Fig. 2b, image a). When sufficient linker sequence is added, the UV-vis absorption peak intensively decreases and a significant peak shift can be observed (Fig. 2a, curve d), indicating the aggregation of AuNPs due to the crosslink of nucleic acid-modified AuNPs via the linker sequence. This can also be proven by TEM (Fig. 2c, image d) and the color change from red to purple (Fig. 2b, image d). When all the components except ATP are present, the linker, probe A, probe B and magnesium ion are firstly incubated and then added to mix AuNPs solution. The results are extremely similar to those of only in the presence of linker (Fig. 2a, curve c; Fig. 2b, image c; Fig. 2c, image c), which means the structure of DNAzyme cannot be formed and the linker sequence cannot be cleaved. However, when the target molecule ATP is incubated with the linker, probe A, probe B and magnesium ion at 20 °C for 60 min, and then added into the AuNPs mixture, the UV-vis absorption peak only slightly decreases compared to that of the AuNPs mixture, whereas much higher than that of in the absence of ATP (Fig. 2a, curve b). The color of the solution is still red (Fig. 2b, image b) and AuNPs in TEM image shows their dispersion state (Fig. 2c, image b). Therefore, ATP can induce the self-assembly of the two probes to form the complete DNAzyme structure, which cleaves its substrate linker in the presence of magnesium ion, resulting in disaggregation of AuNPs.
Verification of the feasibility (a) UV-vis spectra, (b) Photographs of the reaction solution and (c) TEM images of AuNPs recorded in the presence of the mixture of two AuNPs (a); ATP, two probes, magnesium ion, linker and two AuNPs (b); two probes, magnesium ion, linker and two AuNPs (c); and linker with two AuNPs (d)
Optimization of method
To achieve the best sensing performance, the following parameters were optimized: (a) concentration of linker; (b) incubation temperature; (c) incubation time. As shown in Fig. S1a, 40 nM is known to be an appropriate concentration. At this linker concentration, even when a few linkers are cloven by the DNAzymes, the cross-linking of AuNPs and the color of the test solution will be affected. Thus, 40 nM linker was selected for the further experiment. Furthermore, incubation time on the sensor performance was investigated. The absorbance was monitored at the certain time intervals. As shown in Fig. S1b, the absorbance was gradually increased along with the prolongation of incubation time, and at 60 min it reached a plateau. Thus, 60 min is the optimal reaction time for the reaction. Finally, Fig. S1c shows the relationship between the absorbance of the AuNPs and the incubation temperature. When incubation temperature is 20 °C, the system exhibited the best sensing performance. The following experimental conditions were found to give best results: (a) Best linker concentration is 40 nM; (b) Incubation temperature 20 °C, and (c) Reaction time 60 min.
Colorimetric assay of ATP
Under the optimized conditions, the quantitative detection of ATP was carried out, and the results are shown in Fig. 3. With the increase of the concentration of ATP, the absorbance at 530 nm of the reaction solution gradually increases (Fig. 3a). A linear relationship between the absorbance and the logarithm of the concentration of ATP exists in the range from 10 pM to 100 nM (Fig. 3b, inset), with the low detection limit of 5.3 pM. The LOD was determined by extrapolating the concentration from the signal equal to background signal plus 3SD of the background signal.
a UV-vis absorption spectra of the reaction solution in the presence of different concentration of ATP; b The relationship between the absorbance at 530 nm and logarithm ATP concentration; Inset shows the linear relationship between the absorbance of AuNPs at 530 nm and the concentration of ATP from 10 pM to 100 nM (Error bars represent one standard deviation for three measurements). Conditions: concentration of the linker (40 nM), incubation temperature (20 °C) and incubation time (60 min)
The specificity of the experimental protocol was further studied. Through the detection of four kinds of nucleoside three phosphate ATP, CTP, GTP and UTP, we found that even the concentration of control molecules were ten times of that of ATP, they cannot induce the self-assembly of ribozyme and cut the linker chain, resulting in the complete crosslink of AuNPs and much lowered absorbance at 530 nm (Fig. 4). These results show that only ATP can bind with the two split aptamer sequences and lead to a series of subsequent reaction. Thus the method is highly selective for ATP.
Application to real samples
In order to investigate the practical application of the method in clinical analysis, it was applied for the quantification of ATP in serum samples using standard addition methods, and the colorimetric analyses were carried out using the same steps described above. As shown in Table 1, the recoveries were achieved with a satisfying result from 99.8 to 103% with RSD of 4.1 to 5.7%. The good recovery showed that the colorimetric method has the feasibility and reliability for real sample analysis.
Limitations of assay
The method has several limitations. Owing to the nondirective aggregation of the AuNPs, the color of the solution fades away within a few hours, and the duration time of the experiment should be fixed. Moreover, the color change of AuNPs from red to blue is not obvious as expected, quantitative assay for ATP more rely on UV/VIS spectrophotometry.
Conclusion
A colorimetric sensor is presented for the highly sensitive and selective detection of ATP. By taking full advantage of the split aptamer, DNAzyme and the AuNP-based colorimetry, the concentration of ATP can be easily determined with the bare eye or a UV-vis spectrometer. Under the optimal conditions, an excellent liner response to the ATP concentration in the range from 10 pM to 100 nM, with a detection limit of 5.3 pM, which is much lower than the previous reports. The method was successfully applied for the determination of ATP in human serum. However, the aggregation of the AuNPs is time-dependent and the color change of the solution is nodirective, which may restrict accurate target determination. Based on the split aptamer strategy, the method has a wide scope in colorimetric determination of various other analytes.
References
Tedsana W, Tuntulani T, Ngeontae W (2013) A highly selective turn-on ATP fluorescence sensor based on unmodified cysteamine capped CdS quantum dots. Anal Chim Acta 783(11):6573
Bell PD, Komlosi P, Zhang ZR (2009) ATP as a mediator of macula densa cell signalling. Purinergic Signal 5(4):461–471
Bell CJ, Manfredi G, Griffiths EJ, Rutter GA (2007) Luciferase expression for ATP imaging: application to cardiac myocytes. Methods Cell Biol 80:341–352
Gourine AV, Llaudet E, Dale N, Spyer KM (2005) ATP is a mediator of chemosensory transduction in the central nervous system. Nature 436(7047):108–111
Moreno-Sánchez R, Marín-Hernández A, Saavedra E, Pardo JP, Ralph SJ, Rodríguez-Enríquez S (2014) Who controls the ATP supply in cancer cells? Biochemistry lessons to understand cancer energy metabolism. Int J Biochem Cell Biol 50(1):10–23
Jiang G, Zhu W, Xin S, Lei X, Li X, Rui W, Liu C, Zhou X (2017) Colorimetric and visual determination of adenosine triphosphate using a boronic acid as the recognition element, and based on the deaggregation of gold nanoparticles. Microchim Acta 184(11):4305–4312
Qu F, Sun C, Lv X, You J (2018) A terbium-based metal-organic framework@gold nanoparticle system as a fluorometric probe for aptamer based determination of adenosine triphosphate. Microchim Acta 185(8):359
Cheng X, Cen Y, Xu G, Wei F, Shi M, Xu X, Sohail M, Hu Q (2018) Aptamer based fluorometric determination of ATP by exploiting the FRET between carbon dots and graphene oxide. Microchim Acta 185(2):144
Luo J, Shen X, Li B, Li X, Zhou X (2018) Signal amplification by strand displacement in a carbon dot based fluorometric assay for ATP. Microchim Acta 185(8):392
Liu X, Lin B, Yu Y, Cao Y, Guo M (2018) A multifunctional probe based on the use of labeled aptamer and magnetic nanoparticles for fluorometric determination of adenosine 5′-triphosphate. Microchim Acta 185(4):243
Kurdi RE, Patra D (2018) Nanosensing of ATP by fluorescence recovery after surface energy transfer between rhodamine B and curcubit[7]uril-capped gold nanoparticles. Microchim Acta 185(7):349
Jing L, Yi L (2000) A highly sensitive and selective catalytic DNA biosensor for Lead ions. Jamchemsoc 122(42):10466–10467
Mu Q, Liu G, Yang D, Kou X, Cao N, Tang Y, Miao P (2018) Ultrasensitive detection of DNA based on exonuclease III-assisted recycling amplification and DNAzyme motor. Bioconjug Chem 29:3527–3531
Zuo X, Xiao Y, Plaxco KW (2009) High specificity, electrochemical sandwich assays based on single aptamer sequences and suitable for the direct detection of small-molecule targets in blood and other complex matrices. J Am Chem Soc 131(20):6944–6945
Liu J, Bai W, Niu S, Zhu C, Yang S, Chen A (2014) Highly sensitive colorimetric detection of 17β-estradiol using split DNA aptamers immobilized on unmodified gold nanoparticles. Sci Rep 4:7571
Zhao T, Liu R, Ding X, Zhao J, Yu H, Wang L, Xu Q, Wang X, Lou X, He M (2015) Nanoprobe-enhanced, split aptamer-based electrochemical sandwich assay for ultrasensitive detection of small molecules. Anal Chem 87(15):7712–7719
Zhu C, Zhao Y, Yan M, Huang Y, Yan J, Bai W, Chen A (2016) A sandwich dipstick assay for ATP detection based on split aptamer fragments. Anal Bioanal Chem 408(15):4151–4158
Li Q, Wang Y-D, Shen G-L, Tang H, Yu R-Q, Jiang J-H (2015) Split aptamer mediated endonuclease amplification for small-molecule detection. Chem Commun 51(20):4196–4199
Mirkin CA, Letsinger RL, Mucic RC, Storhoff JJ (1996) A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature 382(6592):607–609
Mairal T, Özalp VC, Sánchez PL, Mir M, Katakis I, O’Sullivan CK (2008) Aptamers: molecular tools for analytical applications. Anal Bioanal Chem 390(4):989–1007
Kent AD, Spiropulos NG, Heemstra JM (2013) General approach for engineering small-molecule-binding DNA split aptamers. Anal Chem 85(20):9916–9923
Schlosser K, Li Y (2010) A versatile endoribonuclease mimic made of DNA: characteristics and applications of the 8-17 RNA-cleaving DNAzyme. Chembiochem 11(7):866–879
Juewen L, Yi L (2007) A DNAzyme catalytic beacon sensor for paramagnetic Cu2+ ions in aqueous solution with high sensitivity and selectivity. J Am Chem Soc 129(32):9838–9839
Yong H, Jia C, Shulin Z, Ming S, Zhen-Feng C, Hong L (2013) Label-free colorimetric aptasensor based on nicking enzyme assisted signal amplification and DNAzyme amplification for highly sensitive detection of protein. Anal Chem 85(9):4423–4430
Gu J-A, Lin Y-J, Chia Y-M, Lin H (2013) Colorimetric and bare-eye determination of fluoride using gold;nanoparticle agglomeration probes. Microchim Acta 180(9–10):801–806
Chao L, Wei L, Liu X, Lin L, Li G (2014) Ultrasensitive detection of lead ion based on target induced assembly of DNAzyme modified gold nanoparticle and graphene oxide. Anal Chim Acta 831(23):60–64
Acknowledgements
This work was supported by the Science and Technology Development Foundation of Nanjing Medical University (2017NJMU175), The National Key Research and Development Program: The key technology of palliative care and nursing for cancer patients (ZDZX2017ZL-01) and The High Level Innovation Team of Nanjing Medical University (JX102GSP201727).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 133 kb)
Rights and permissions
About this article
Cite this article
Zhu, S., Wang, X., Jing, C. et al. A colorimetric ATP assay based on the use of a magnesium(II)-dependent DNAzyme. Microchim Acta 186, 176 (2019). https://doi.org/10.1007/s00604-019-3244-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3244-9