Abstract
Colorimetric methods are described for the determination of ascorbic acid (AA) and alkaline phosphatase (ALP). Both assays are based on the inhibition of the peroxidase (POx)-like activity of Prussian Blue nanocubes (PB NCs) capped with citric acid. They catalyze the oxidation of 3,3,5,5-tetramethylbenzidine (TMB) by H2O2 to produce a blue color with an absorption maximum at 652 nm. On addition of AA, the PB NCs are reduced to Prussian White (PW) which does not act as a POx mimic. This results in a decreased rate of the formation of the blue coloration whose intensity decreases with increasing concentration of AA. The assay allows AA to be quantified with a 35 nM detection limit (at 3σ/m). The hydrolysis of AA phosphate by ALP leads to the formation of AA which can be quantified by the above method. Based on this, the activity of ALP can be determined by measurement of the intensity of the blue coloration thus formed. The method can be used to determine the activity of ALP with a detection limit as low as 0.23 U·L−1.

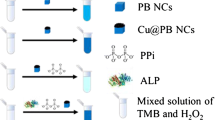
Schematic presentation of a method for colorimetric determination of ALP activity. AA obtained by ALP-catalyzed hydrolysis of ascorbic acid phosphate (AAP) inhibits the intrinsic peroxidase-like activity of PB NCs by reducing Prussian Blue nanocrystals (PB NCs) to form inactive Prussian White (PW).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Alkaline phosphatase (ALP) catalyzes the hydrolysis of phosphoryl esters in weakly alkaline media [1]. It plays a vital role in regulation of intracellular processes and acts as a biomarker for disease diagnosis [2]. Various methods for detection of ALP have been reported, including chromatography [3], electrochemistry [4], colorimetry [5], surface-enhanced Raman scattering [6], and chemiluminescence [7]. Among these methods, enzyme-based colorimetric analysis have drawn great attraction due to their cost effectiveness, convenience of visual observation and simplicity. Owing to the sensitivity and selectivity issues, it is still urgent to develop new materials possessing special property via simple preparation approach and utilize them in detection of the ALP activity based on the colorimetric method.
Exploring efficient mimetics of enzyme received great attention, which are widely applied in colorimetric analysis. A variety of nanomaterials, such as carbon nanomaterials [8,9,10], Cu2+ [11], Au@Pd [12], Au nanoclusters [13], CuO nanoparticles [14], 3-dimensional C/CeO2 hollow nanostructure frameworks [15] and MoS2 nanosheets [16], TiO2 nanotube arrays [17], have been demonstrated to possess peroxidase-like activity and can be used for H2O2 determination. Peroxidase mimetics have the superiority outperforming natural enzymes of robustness to harsh environments, low cost, ease of preparation and high stability, which have been intensively utilized in colorimetric sensing of H2O2 and its related molecules [18]. Prussian Blue (PB) based materials have been widely used in many fields of oxygen reduction reaction [19], lateral flow assay [20], fiber optic gas sensor [21], electrochemical sensor [22,23,24]. Recently, PB nanoparticles have been reported to possess highly intrinsic peroxidase-like activity owing to Fe3+/Fe2+ redox couple [25]. However, preparation of novel PB nanocrystals and developing PB-related sensing platform on the basis of the inhibiting the peroxidase-like activity of PB NCs in the presence of some special small molecules has seldom reported.
Herein, we describe the use of PB NCs as a novel nanoprobe for colorimetric sensing of AA and ALP activity, and the principle of this strategy was demonstrated in Scheme 1. The PB NCs can catalyze the oxidation of the peroxidase substrate 3,3,5,5-tetramethylbenzidine (TMB) to produce blue color accompanying with the increase of absorbance peak at 652 nm in the presence of H2O2. In the presence of AA, the catalytic process will be hampered. Similarly, owing to L-ascorbic acid-2-phosphate (AAP) can be converted into AA catalyzed by ALP through the hydrolysis reaction, the intrinsic peroxidase-like activity of PB NCs will be directly inhibited. By reduction of PB NCs into Prussian white (PW) [26, 27], the amount of oxTMB will decrease simultaneously with the color fading and decrease of the absorbance intensity at 652 nm. The practical application of the colorimetric sensor for the detection of ALP in the human serum samples were also investigated.
Experimental
Materials
Citric acid was purchased from Shanpu Co., Ltd. (Shanghai, china, http://www.aladdinreagent.com), iron chloride (FeCl3), potassium hexacyanoferrate (K4Fe(CN)6) were obtained from Hengxing chemical reagent Co., Ltd. (http://hxhxsj.com.cn). Ascorbic acid, HCl were purchased from Sinopharm Chemical Reagent Co., Ltd. (http://www.sinoreagent.com). Acetone was obtained from Chengdu KeLong Chemical Co., Ltd. (https://www.en-cphi.cn/company-kelong). Alkaline phosphate (ALP) and L-ascorbic acid-2-phosphate (AAP) were obtained from Sigma-Aldrich Chemical Co. (USA, http://www.bszh.com/Sigma-Aldrich.aspx). 0.2 M phosphate buffered saline (PBS) was used.
Apparatus
The UV-vis spectra were obtained on a UV-2450 spectrophotometer (Shimadzu, Japan). The morphologies and the composition of the nanocomposite were characterized by transmission electron microscopy (TEM, JEOL, Japan). FT-IR spectra (IR) were collected on a Nexus-670 Fourier transform-infrared spectrophotometer (Nicolet Instrument Co, USA) using KBr pressed pellet transmission mode.
Determination of AA
PB NCs were synthesized as literature with slightly modification (see Electronic Supporting Material) [28]. In a typically assay, 10 μL (0.1 mg·mL−1) PB NCs and 100 μL AA with different concentrations were added into 770 μL of PBS (0.2 M, pH 6.5) and mixed for 1 min at room temperature. Then, 10 μL of 0.5 mM TMB and 10 μL of 100 μM H2O2 were added to the mixture and incubated for 30 min at 30 °C for the UV-vis measurement by scanning from 500 to 800 nm, and the maximum absorbance wavelength of 652 nm is used for quantitative analysis.
Determination of ALP
In a typically assay, 10 μL of 10 mM AAP and 10 μL of different concentrations of ALP were sequentially added into 80 μL of 10 mM Tris-HCl buffer (pH 8.0). Then, the mixture was incubated at 37 °C for 15 min. The detection steps are similar to those of the detection of AA.
For the selectivity test, 3 U·L−1 ALP, 30 U·L−1 trypsin (Try), horseradish peroxide (HRP) and glucose oxidase (GOx); 3 mM of hemoglobin (Hb), bovine serum albumin (BSA), uric acid (UA), dopamine (DA) and cysteine (Cys); 300 mM of K+, Na+, Ca2+ and Mg2+ were added into detection system respectively and then recorded the UV-vis spectra.
Analysis of real samples
To study the performance of the proposed method in samples, human serum was collected from hospital and filtered through a 0.22 μm microporous membrane and immediately diluted 50 times. The spiked samples were prepared by adding ALP solution with different activities to the diluted serum samples. The procedure for the detection of ALP in serum samples was the same as described above, in “Determination of ALP” sub-section.
Results and discussion
Characterization of the PB NCs
TEM image (Fig. 1a) shows the uniformly dispersed PB NCs are nanocubes. And the TEM image of the PB after AA addition is also presented in Fig. S1, which keep in good shape. The FTIR spectrum shows sharp peaks at 2085 cm−1, 2085 cm−1 and 598 cm−1 (Fig. 1b), attributing to the -OH, C ≡ N and M-C (M = metal) stretching vibrations, respectively. Typically, the PB NCs with blue color show a broad UV-Vis absorption band with a maximum peak centering at 710 nm (Fig. 1c), which may be ascribed to the charge-transfer transition energy of Fe (II) and Fe (III) in PB NCs [29]. All the above evidences confirm the successful synthesis of the PB NCs.
TEM image (a) and FTIR spectra (b) of the PB NCs. c UV absorption of PB NCs and PBNCs + AA, inset shows the photos of the corresponding solutions. d UV absorption spectra of H2O2-TMB incubated with PB NCs in the presence of various concentrations of AA. the inset shows the corresponding photos of the solutions
The feasible and principle of the detection system
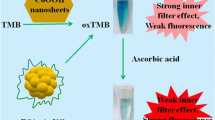
The principle of the colorimetric detection of ALP using PB NCs is depicted in Scheme 1, where TMB is employed to confirm the peroxidase-like activity of PB NCs. PB NCs can catalyze the oxidation of TMB into a blue oxidation product (oxTMB) by H2O2 with a significant absorbance peak at 652 nm (Fig. 1d). AAP converts into AA catalyzed by ALP through a hydrolysis reaction. AA can lead to the formation of PW from PB NCs and decrease the amount of oxTMB, thus the chromogenic reaction of TMB can be inhibited, accompanying with a sharp decrease of the absorbance at 652 nm (Fig. 1d). To this end, the change of the absorbance of oxTMB can reflect the peroxidase-like activity of the PB NCs. Therefore, we can use the TMB-H2O2-PB NCs system for the colorimetric detection of AA and ALP activity.
UV-Vis spectroscopy is used to investigate the mechanism of AA for its inhibition of TMB oxidation. Figure 1c shows that the characteristic peak of the PB NCs at 707 nm decreases with the addition of AA, suggesting the decrease of PB NCs. This can also be proved by the different addition order of the regents. In the system of TMB-H2O2-PB NCs, the first incubation of PB NCs and AA, and then addition of TMB did not result obvious color change; while blue color occurs if the AA was added after incubation the mixture of PB NCs and TMB, indirectly suggested that AA-induced transformation of PB NCs make an important role in AA detection. According to the literature, PB NCs can be converted to Prussian white (PW) [26, 27], and therefore, here, on addition of AA, the PB NCs are reduced to Prussian white (PW) which does not act as a POx mimic. The decrease of PB will decrease the peroxidase-like activity and the amount of oxTMB will decrease simultaneously.
Optimization of detection conditions
The effects of temperature, incubation time, pH, concentration of PB NCs, TMB and H2O2 on the absorbance is important. We utilized ΔA (ΔA = A0-A) as the criterion to optimize the detection conditions, where A0 and A are the absorbance at 652 nm in the absence and presence of AA or ALP, respectively. It is found that the pH strongly effects the peroxidase activity of PB NCs and the maximum ΔA is observed at pH 6.5 (Fig. 2a). It may result from that the structure of PB NCs become unstable and can be damaged at higher pH (pH > 8) [28]. The experimental results show that the best condition for the detection appears at pH 6.5, temperature of 30 °C, incubation time of 20 min, the volume of 10 μL PB NCs, the concentrations of TMB and H2O2 of 0.5 mM and 0.1 M (Fig. 2b–f).
Analytical performance of the method for AA and ALP detection
Under the optimum condition, AA is determined using PB NCs based nanoprobe. As indicates in Fig. 3a, the absorbance at 652 nm decreases with the increasing concentration of AA from 0.4 μM to 4.5 μM. Figure 3b shows the plot of ΔA against the concentration of AA. A linear correlation exists between the value of ΔA and the concentration of AA in the range of 0.4–4.5 μM. The linear regression equation is ΔA = 0.1422 [AA] – 0.0411 (R2 = 0.9945) with the detection limit as low as 35 nM (at 3σ/m, where σ is the standard deviation of the blank and m is the slope of the calibration plot). Moreover, with the increasing concentration of AA, the color of the solutions changed from dark blue to colorless (Fig. 3a, inset). Therefore, the developed method is applicable for visually detection of AA.
a UV-Vis spectra of H2O2-TMB incubated with PB NCs in the presence of different concentration of AA, and the inset shows the photos of the corresponding solutions. b Calibration plot of (a) with the analytical wavelength at 652 nm. c UV-vis absorbance spectra of H2O2-TMB with PB NCs in the presence of ALP with different activity in the presence of AAP incubated, the inset photos show the corresponding solutions colors. d Calibration plot of (c) with the analytical wavelength at 652 nm
For ALP detection, the absorbance at 652 nm decreases as the ALP concentration increased (Fig. 3c). These results suggest the hydrolysis of AAP to AA by ALP. Figure 3d displays a good linear relationship between ΔA and the ALP concentration in the range from 0.6 to 6 U·L−1, with the linear regression equation of ΔA = 0.1339 [ALP] – 0.0471 (R2 = 0.9973) and the limit detection of 0.23 U·L−1 (at 3σ/m). The detection performance of the PB NCs based nanoprobe is comparable to many reports on the basis of colorimetric methods for ALP activity detection [30,31,32], which suggests that the performance of the developed sensing platform is good (Table 1).
Selectivity and reversibility of the method
To demonstrate the selectivity of this assay for ALP, the possible interferences (30 U·L−1 of Try, HRP, GOx; 3 mM of Hb, BSA, UA, DA, Cys and 300 mM of K+, Na+, Ca2+ and Mg2+) were performed. As shown in Fig. 4, the addition of larger amount (10 times or higher) of other enzymes and proteins and even 100 times of the investigated metal ions into the sensing system do not result in remarkable change in the absorbance spectra. In contrast, upon the addition of ALP (3 U·L−1), the absorbance at 652 nm is significantly changed. These results substantially suggest that the method has high selectivity toward ALP. In addition, the reversibility of the PB NCs based nanoprobe was conducted. As shown in Fig. S2, the PB NCs are reduced to PW in the presence of AA with the fading of blue color, while introduction of oxidants such as KMnO4, the PW is oxidized to PB. The results show that the PB NCs has good reversibility.
(A) UV absorption spectra of TMB-H2O2-PB NCs in the presence of ALP and other biologically relevant reactive species. From left to right: a) ALP (3 U·L−1); 30 U·L−1 of b) Try, c) HRP, d) GOx; 3 mM of e) Hb, f) BSA, g) UA, h) DA, i) Cys; 300 mM of j) K+, k) Na+, l) Ca2+, m) Mg2+. The error bars demonstrate the standard deviations of three independent measurements
Real sample assay
The PB-based nanoprobe detection system is also applied for ALP detection in diluted human plasma, the serum samples were firstly filtered, diluted with phosp—hate buffered saline, and spiked with different concentrations of ALP (0.7, 2, and 5 U·L−1). The detection results are displayed in Table 2. The recoveries of the spiked serum samples ranged from 94.3% to 106.5%, which was satisfactory for detection of ALP. Taken together, the above results suggest that the method can be employed for the detection of ALP in biological system.
Conclusions
It is demonstrated that PB NCs can be utilized as a colorimetric probe for selective and sensitive visual monitoring of AA and activity of ALP. The peroxidase activity of PB NCs can be inhibited by AA owing to the PB NCs are reduced to PW in the presence of AA, and causing the absorption maximum at 652 nm to decrease. Therefore, the assay based on the peroxidase activity of PB NCs demonstrates as an effective way for some small molecules or enzyme activity detection. On the basis of the inhibition of peroxidase-like activity of PB or other nanomaterials, newly biosensing platforms can be developed, which will be expanded in analytical and disease diagnosis. Though the detection method is effective and sensitive, the reversibility needs to be further improved since the selection of the oxidant for the conversion of PW to PB NCs is important. It is believed that the sensor based on the inhibition of the peroxidase activity will show great promise in the future.
References
Deng JJ, Yu P, Wang YX, Mao LQ (2015) Real-time ratiometric fluorescent assay for alkaline phosphatase activity with stimulus responsive infinite coordination polymer nanoparticles. Anal Chem 87:3080–3086
Song HW, Wang HY, Li X, Peng YX, Pan JM, Niu XH (2018) Sensitive and selective colorimetric detection of alkaline phosphatase activity based on phosphate anion-quenched oxidase-mimicking activity of Ce(IV) ions. Anal Chim Acta 31:154–161
Takuya H, Masaru S, Kohei T, Hirotaka M, Tomonari U, Hiroki H (2006) Assay of alkaline phosphatase in salmon egg cell cytoplasm with fluorescence detection of enzymatic activity and zinc detection by ICP-MS in relation to metallomics research. B Chem Soc Jpn 79:1211–1214
Ino K, Kanno Y, Arai T, Inoue KY, Takahashi Y, Shiku H, Matsue T (2012) Novel electrochemical methodology for activity estimation of alkaline phosphatase based on solubility difference. Anal Chem 84:7593–7598
Wei H, Chen CG, Han BY, Wang EK (2008) Enzyme colorimetric assay using unmodified silver nanoparticles. Anal Chem 80:7051–7055
Ingram A, Moore BD, Graham D (2009) Simultaneous detection of alkaline phosphatase and beta-galactosidase activity using SERRS. Bioorg Med Chem Lett 19:1569–1571
Zhu XH, Zhao TB, Nie Z, Liu Y, Yao SZ (2015) Non-redox modulated fluorescence strategy for sensitive and selective ascorbic acid detection with highly Photoluminescent nitrogen-doped carbon nanoparticles via solid-state synthesis. Anal Chem 87:8524–8530
Qian J, Yang XW, Yang ZT, Zhu GB, Mao HP, Wang K (2015) Multiwalled carbon nanotube@reduced graphene oxide nanoribbon heterostructure: synthesis, intrinsic peroxidase-like catalytic activity, and its application in colorimetric biosensing. J Mater Chem B 3:1624–1632
Song YJ, Chen Y, Feng LY, Ren JS, Qu XG (2011) Selective and quantitative cancer cell detection using target-directed functionalized graphene and its synergetic peroxidase-like activity. Chem Commun 47:4436–4438
Zheng AX, Cong ZX, Wang JR, Li J, Yang HH, Chen GN (2013) Highly-efficient peroxidase-like catalytic activity of graphene dots for biosensing. Biosens Bioelectron 49:519–524
Chang YQ, Zhang Z, Hao JH, Yang WS, Tang JL (2016) A simple label free colorimetric method for glyphosate detection based on the inhibition of peroxidase-like activity of Cu(II). Sensors Actuators B Chem 228:410–415
Dehghani Z, Hosseini M, Mohammadnejad J, Bakhshi B, Rezayan AH (2018) Colorimetric aptasensor for Campylobacter jejuni cells by exploiting the peroxidase like activity of Au@Pd nanoparticles. Microchim Acta 185:448
Wang GL, Jin LY, Dong YM, Wu XM, Li ZJ (2015) Intrinsic enzyme mimicking activity of gold nanoclusters upon visible light triggering and its application for colorimetric trypsin detection. Biosens Bioelectron 64:523–529
Chen W, Chen J, Feng YB, Hong L, Chen QY, Wu LF (2012) Peroxidase-like activity of water-soluble cupric oxide nanoparticles and its analytical application for detection of hydrogen peroxide and glucose. Analyst 137:1706–1712
Wang N, Duan JZ, Shi WJ, Zhai XF, Guan F, Yang LH, Hou BR (2018) A 3-dimensional C/CeO2 hollow nanostructure framework as a peroxidase mimetic, and its application to the colorimetric determination of hydrogen peroxide. Microchim Acta 185:417
Liu HM, Wang BC, Li DH, Zeng XY, Tang X, Gao QS, Cai JY, Cai HH (2018) MoS2 nanosheets with peroxidase mimicking activity as viable dual-mode optical probes for determination and imaging of intracellular hydrogen peroxide. Microchim Acta 185:287
Zhang LL, Han L, Hu P, Wang L, Dong SJ (2013) TiO2 nanotube arrays: intrinsic peroxidase mimetics. Chem Commun 49:10480–10482
Ni PJ, Sun YJ, Dai HC, Lu WD, Jiang S, Wang YL, Li Z, Li Z (2017) Prussian blue nanocubes peroxidase mimetic-based colorimetric assay for screening acetylcholinesterase activity and its inhibitor. Sensors Actuators B Chem 240:1314–1320
Jia SP, Zang JB, Li W, Tian PF, Zhou SY, Cai HX, Tian XQ, Wang YH (2018) A novel synthesis of Prussian blue nanocubes/biomass-derived nitrogen-doped porous carbon composite as a high-efficiency oxygen reduction reaction catalyst. Electrochim Acta 289:56–64
Zhao BX, Huang Q, Dou LN, Bu T, Chen K, Yang QF, Yan LZ, Wang JL, Zhang DH (2018) Prussian blue nanoparticles based lateral flow assay for high sensitive determination of clenbuterol. Sensors Actuators B Chem 275:223–229
Muthusamy S, Charles J, Renganathan B, Sastikumar D (2018) In situ growth of Prussian blue nanocubes on polypyrrole nanoparticles: facile synthesis, characterization and their application as fiber optic gas sensor. J Mater Sci 53:15401–15417
Han J, Zhuo Y, Chai YQ, Yuan R, Xiang Y, Zhu Q, Liao N (2013) Multi-labeled functionalized C60 nanohybrid as tracing tag for ultrasensitive electrochemical aptasensing. Biosens Bioelectron 46:74–79
Zhuo Y, Yuan PX, Yuan R, Chai YQ, Hong CL (2009) Bienzyme functionalized three-layer composite magnetic nanoparticles for electrochemical immunosensors. Biomaterials 30:2284–2290
Cui L, Hu J, Li CC, Wang CM, Zhang CY (2018) An electrochemical biosensor based on the enhanced quasi-reversible redox signal of Prussian blue generated by self-sacrificial label of Iron metal-organic framework. Biosens Bioelectron 122:168–174
Zhang WM, Ma D, Du JX (2014) Prussian blue nanoparticles as peroxidase mimetics for sensitive colorimetric detection of hydrogen peroxide and glucose. Talanta 120:362–367
Xing HH, Zhang XW, Zhai QF, Li J, Wang EK (2017) Bipolar electrode-based reversible fluorescence switch using Prussian blue/Au nanoclusters nanocomposite film. Anal Chem 89:3867–3872
Karyakin AA, Karyakina EE (1999) Prussian blue-based 'artificial peroxidase'as a transducer for hydrogen peroxide detection. Application to biosensors. Sensors Actuators B Chem 57:268–273
Huang W, Liang Y, Deng YQ, Cai YH, He Y (2017) Prussian blue nanoparticles as optical probes for visual and spectrophotometric determination of silver ions. Microchim Acta 184:2959–2964
Fu GL, Liu W, Feng SS, Yue XL (2012) Prussian blue nanoparticles operate as a new generation of photothermal ablation agents for cancer therapy. Chem Commun 48:11567–11569
Hu Q, He MH, Mei YQ, Feng WJ, Jing S, Kong JM, Zhang XJ (2017) Sensitive and selective colorimetric assay of alkaline phosphatase activity with Cu(II)-phenanthroline complex. Talanta 163:146–152
Hu Q, Zhou BJ, Dang PY, Li LZ, Kong JM, Zhang XJ (2017) Facile colorimetric assay of alkaline phosphatase activity using Fe(II)-phenanthroline reporter. Anal Chim Acta 950:170–177
Yang JJ, Zheng L, Wang Y, Li W, Zhang JL, Gu JJ, Fu Y (2016) Guanine-rich DNA-based peroxidase mimetics for colorimetric assays of alkaline phosphatase. Biosens Bioelectron 77:549–556
Liu HJ, Li M, Xia YN, Ren XQ (2016) A turn-on fluorescent sensor for selective and sensitive detection of alkaline phosphatase activity with gold nanoclusters based on inner filter effect. ACS Appl Mater Interfaces 9:120–126
Liu JJ, Tang DS, Chen ZT, Yan XM, Zhong Z, Kang LT, Yao JN (2017) Chemical redox modulated fluorescence of nitrogen-doped graphene quantum dots for probing the activity of alkaline phosphatase. Biosens Bioelectron 94:271–277
Mei YQ, Hu Q, Zhou BJ, Zhang YH, He MH, Xu T, Li F, Kong JM (2017) Fluorescence quenching based alkaline phosphatase activity detection. Talanta 176:52–58
Acknowledgments
This work was supported by the National Natural Science Foundation of China (21645008, 21305042, 21375037), Scientific Research Fund of Hunan Provincial Education Department (14B116), Science and Technology Department (14JJ4030), the construct program of the key discipline in Hunan province and the Aid Program for Science and Technology Innovative Research Team in Higher Educational Institutions of Hunan Province.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 3.36 mb)
Rights and permissions
About this article
Cite this article
Wu, T., Hou, W., Ma, Z. et al. Colorimetric determination of ascorbic acid and the activity of alkaline phosphatase based on the inhibition of the peroxidase-like activity of citric acid-capped Prussian Blue nanocubes. Microchim Acta 186, 123 (2019). https://doi.org/10.1007/s00604-018-3224-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-3224-5